Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
High-altitude illness represents a spectrum of clinical entities with neurologic and pulmonary manifestations that overlap in their presentations and share common elements of pathophysiology. Acute mountain sickness (AMS) is the relatively benign and self-limited presentation, whereas high-altitude pulmonary edema (HAPE) and high-altitude cerebral edema (HACE) have potentially life-threatening manifestations.
Often overlooked by travelers as high-altitude destinations are cities such as La Paz, Bolivia (3,700 meters, approximately 12,100 feet), Lhasa, Tibet (3,600 m, ~11,800 ft), Cusco, Peru (3,400 m, ~11,200 ft), and Quito, Ecuador (2,850 m, ~9,350 ft), which reside at an elevation where altitude illness is likely to develop. In 2014, >15 million people visited Lhasa, a 6-fold increase over 8 yr since the opening of the Qinghai-Tibet Railway. In the western United States, 35 million people visit alpine resorts each year. Over 40% of those who stay above 3,300 m (~11,000 ft) have been found to suffer from AMS. In Colorado alone, approximately 150,000 children <12 yr old visit the mountains annually on ski holidays with their families. In 2014, AMS symptoms were severe enough to result in 1,350 visits to emergency departments (EDs) throughout Colorado. Because of the increasing family travel to high altitude, thousands of children are likely to develop AMS symptoms.
The altitude threshold where clinical illness may begin to occur is 1,500 m (~4,900 ft). At this altitude a mild impairment in oxygen transport begins, although altitude illness is relatively rare until higher elevations are reached. Children with underlying medical problems that impair oxygen transport may be predisposed to developing altitude illness at these lower levels. At moderate high altitude, 2,500-3,500 m (~8,000-11,500 ft), arterial oxygen saturation (Sa o 2 ) is generally well maintained; however, mild tissue hypoxia may occur as a result of low arterial oxygen partial pressure (Pa o 2 ), and altitude illness becomes common after rapid ascent above 2,500 m (~8,200 ft). This is the altitude range that most people visit and the elevation of many popular U.S. ski resorts and thus the most common range to find the greatest number of altitude illness cases. Very high altitude, 3,500-5,500 m (~11,500-18,000 ft), is associated with the most serious altitude illness; Sa o 2 falls below 90%, on the steep portion of the oxyhemoglobin dissociation curve, and marked desaturation may occur with relatively small increases in altitude. At these heights, severe hypoxemia is seen with sleep, exercise, and illness. HAPE and HACE are most common in this environment. Extreme high altitude, above 5,500 m (~18,000 ft), generally results in severe altitude illness during acute ascent without supplemental oxygen. Acclimatization at intermediate altitudes is required to reach extreme altitudes. Complete acclimatization is not possible, and long visits result in progressive deterioration.
The partial pressure of oxygen (P o 2 ) in the atmosphere decreases logarithmically as geographic altitude rises, but oxygen remains a constant 20.93% of the barometric pressure. Sa o 2 falls with increasing altitude, eventually triggering central chemoreceptor responses to produce hyperventilation in an attempt to normalize Sa o 2 ; relative hypoventilation exacerbates the hypoxemia of high-altitude exposure. During sleep, periodic breathing associated with high-altitude exposure may result in periods of apnea, causing further arterial oxygen desaturation. Fluid homeostasis often shifts at altitude, resulting in a generalized fluid retention and redistribution into intracellular and interstitial spaces, manifested by peripheral edema, decreased urine output, and impaired gas exchange.
Gradual ascents allowing for acclimatization over several weeks have allowed successful summiting of many of the world's highest peaks without supplemental oxygen. Without this gradual approach, rapid exposure to extreme altitude results in loss of consciousness and asphyxia in minutes. Children acclimatize as well as, if not better than, adults when comparing heart rate and Sa o 2 of 7-9 yr olds to their parents during a slow ascent.
Some of the responses to hypoxia are mediated at the molecular level by hypoxia-inducible factor (HIF). This transcriptional activator orchestrates the expression of hundreds of genes in response to both acute and chronic hypoxic conditions. Acclimatization begins at the altitude that causes Sa o 2 to fall below sea-level values. Most healthy, unacclimatized visitors to high altitude will not experience a significant drop in Sa o 2 (<90%) until they reach elevations above 2,500 m (~8,200 ft). Children with preexisting conditions that reduce oxygen transport may have altitude intolerance and hypoxic stress at lower levels. Of particular importance are both acute and chronic cardiac and respiratory illnesses. An individual's inherent ability to acclimatize is also important. Previous successful acclimatization may be predictive of future responses for adults in similar conditions but may not be the case for children. Some acclimatize easily without developing clinical symptoms; others may transiently develop AMS during acclimatization; and a few have marked reactions to altitude exposure, fail to acclimatize, and develop severe altitude illness.
The most important response to acute hypoxia is an increase in minute ventilation. Peripheral chemoreceptors in the carotid bodies respond to hypoxia by signaling the respiratory control center in the medulla to increase ventilation. This decreases alveolar carbon dioxide partial pressure (P aco 2 ), resulting in a corresponding increase of P ao 2 and arterial oxygenation. This increased ventilation, known as the hypoxic ventilatory response (HVR) , varies in magnitude among individuals, may be genetically predetermined, and is related to the ability to acclimatize. Changes in the HVR and the onset of AMS with ascent to high altitude have been found to be remarkably similar between children and their fathers. Additional research has demonstrated that familial clustering of AMS accounts for up to 50% of the variability of AMS onset among children. A low HVR and relative hypoventilation are implicated in the pathogenesis of both AMS and HAPE, whereas a strong HVR enhances acclimatization. As ventilation increases, a respiratory alkalosis occurs, exerting negative feedback on central respiratory control and limiting further ventilation increase. The kidneys excrete bicarbonate in an effort to compensate for the alkalosis. As the pH normalizes, ventilation rises slowly, reaching a maximum after 4-7 days. This process is enhanced by acetazolamide, which induces a bicarbonate diuresis.
Increased sympathetic activity and catecholamine release on ascent result in elevation of heart rate, blood pressure, cardiac output, and venous tone. Except at extreme altitudes, acclimatization results in the resting heart rate gradually returning to near sea-level values. Resting relative tachycardia is evidence of poor acclimatization.
Hematopoietic acclimatization consists of an increase in hemoglobin (Hb) and red blood cells (RBCs) and in 2,3-diphosphoglycerate (DPG). After acute ascent, an early increase of up to 15% occurs in Hb concentration primarily from fluid shifting into the extravascular space. Acclimatization leads to an increase in plasma volume and total blood volume. Erythropoietin is secreted in a HIF-mediated response to hypoxemia within hours of ascent, stimulating the production of new RBCs, which begin to appear in the circulation in 4 or 5 days. Hypoxemia also increases 2,3-DPG, resulting in a rightward shift of the oxyhemoglobin dissociation curve, favoring release of oxygen from the blood to the tissues. This is counteracted by the leftward shift of the curve caused by the respiratory alkalosis from hyperventilation. The result is a net null change in the oxyhemoglobin dissociation curve and an increase in O 2 -Hb binding in the lung, raising Sa o 2 . Climbers at extreme altitude respond with marked hyperventilation, alkalosis, and a leftward shift that favors oxygen loading in a hypoxic environment and increases Sa o 2 .
The incidence of high-altitude illness depends on several variables, including the rate of ascent, previous altitude exposure, and individual genetic susceptibility. Sleeping altitude, final altitude reached, and duration of stay at altitude are also clear risk factors for AMS development. AMS is very common with rapid ascent . Climbers around the world who ascend quickly (1 or 2 days) from sea level to altitudes of about 4,300-6100 m (14,000-20,000 ft) have a very high incidence of AMS (27–83%). The rapid ascent profile associated with air travel to high-altitude locations also results in high AMS attack rates. Trekkers who fly into the Khumbu region to explore the Mt. Everest area have a higher incidence of AMS (47%) compared with those who walk (23%). Skiers who visit resorts in the western United States from sea level generally fly or drive to the region but sleep at relatively moderate altitudes (2,000-3,000 m, 6,300-9,700 ft). Among this population, AMS occurs in approximately 25%.
Children have the same incidence of AMS as adults. Individual (genetic) susceptibility for the development of AMS plays a significant role in risk assessment. Most individuals with a previous history of AMS after acute ascent are likely to experience similar symptoms with repeated visits to altitude. Gender does not affect the incidence of AMS.
The symptoms of AMS develop several hours after arrival at high altitude, whereas the development of HAPE and HACE generally requires several days of altitude exposure. Because hypoxemia occurs within minutes of arrival, it cannot be the direct cause of high-altitude illness, but rather the initiating factor.
The clinical manifestations of AMS/HACE are primarily the result of central nervous system (CNS) dysfunction caused by hemodynamic mechanical factors and biochemical mediators of permeability. The CNS vasodilatory response to hypoxemia causes an increase in cerebral blood flow and volume. Significant elevation of brain volume is observed in moderate to severe AMS and HACE but has not been demonstrated in mild AMS. Hypoxic alteration of CNS vascular autoregulation and hypertension from exercise may increase pressure transmission to the brain's capillary beds, resulting in transcapillary leakage and vasogenic edema. HIF-mediated vascular endothelial growth factor, the inducible form of nitric oxide synthase, reactive cytokines, and free radical formation may increase permeability. Both mechanical and biochemical activation of the trigeminovascular system have been proposed as the cause of high-altitude headache , the primary symptom of AMS. Vasogenic edema has been implicated in severe AMS and HACE, but MRI reveals signal changes in persons with and without clinical AMS. It has been well established that adults can have changes in cognitive function with acute exposure to high altitude. Investigation of cognitive dysfunction in healthy, lowlander European children found significant impairment in verbal short-term memory, episodic memory, and executive functions 24 hr after arrival at 3,450 m (11,400 ft). These impairments were attributed to hypoxia-induced dysfunction of the cerebral white matter. The neuropsychological changes were found to be reversible, since cognitive function returned to baseline on reevaluation 3 mo after returning to sea level.
Many of the responses to hypoxia and altitude exposure occur in both individuals who develop symptoms and those who remain free of AMS. To address the discrepancy in symptomatic illness, the “tight fit” hypothesis was proposed. This theory suggests that the development of AMS/HACE is the result of a lack of intracranial space to accommodate increasing volume from brain swelling and edema that develop at altitude. The adequacy of the intracranial and intraspinal space to buffer changes in brain and cerebrospinal fluid (CSF) volume is the central concept. Buffering occurs as the intracranial CSF is displaced by the foramen magnum into the space available in the spinal canal, followed by increased CSF absorption and decreased CSF production. Individuals with less CSF buffering capacity have less compliance and are hypothesized to become more symptomatic (develop AMS).
A comprehensive approach to travel to high altitude with children should focus on 3 phases: planning the ascent and assessment of risk, recognition and management of altitude-associated illness, and follow-up of any illness relative to future travel or diagnostic testing necessary.
Planning for travel to high altitude with children should consider rate of ascent, formulation of an emergency plan for communication and evacuation, and availability of medical care at the high-altitude destination. The availability of medical care and evacuation from altitude will influence the degree of personal preparation necessary. Slow ascent with time for acclimatization is the best prevention for all forms of altitude illness . Residing for a few days at moderate altitudes (2,000-3,100 m, 6,600-10,200 ft) followed by graded ascent before arriving at high altitude. One extra night of acclimatization (at the same sleeping altitude) should be taken for every 1,000 m (~3,300 ft) gained. Rapid ascent by air may be avoidable through alternate routes or alternate means of transportation. Exposure to hypobaric hypoxia (reduced barometric pressure with maintained O 2 of 20.9%) decreases end-tidal CO 2 and AMS score, while increasing Sa o 2 and exercise endurance on exposure to higher altitude. Staying over in Denver, Colorado, for 1 or 2 days before traveling to higher alpine destinations is an example of such a strategy and has been an effective technique of acclimatization; it has the advantage of reducing the likelihood of developing AMS, HACE, or HAPE. A similar trend in preacclimatization was found with preexposure to normobaric hypoxia (maintained barometric pressure with O 2 <20.9%) using commercially available low-O 2 tents or hypoxia breathing masks, although not as effective as preacclimatization with hypobaric hypoxia. Slow, gradual ascent is another effective means of acclimatization. The altitude at which someone sleeps is considered more important than the highest altitude reached during waking hours. Guidelines recommend that above 3,000 m (~9,800 ft), one should not increase sleeping elevation by >500 m (1,600 ft) per day and should include a rest day every 3-4 days with no ascent to a higher sleeping elevation. Acclimatization and slow ascent are by far the best ways to avoid AMS. The first few days at altitude, individuals should limit their activity and maintain adequate hydration.
Medical risk assessment encompasses consideration of age, previous altitude-associated illness, and possible predisposing circumstances to altitude illness. Very young infants (<4-6 wk) may not have completed the postnatal circulatory transition and may be more vulnerable to altitude-associated desaturation with periodic breathing, right-to-left shunting across the foramen ovale, and hypoxic pulmonary vasoconstriction. Infants who required supplemental oxygen during the neonatal period, especially for pulmonary hypertension, may be at risk for hypoxemia with prolonged altitude exposure. History and physical examination are useful to identify conditions predisposing to HAPE, including recent viral infections, cardiac malformations, or obstructive sleep apnea. Children are known to have greater pulmonary vascular reactivity than adults. Thus, respiratory illnesses such as otitis media, pneumonia, or bronchiolitis that cause release of inflammatory mediators will increase capillary permeability; although normally tolerated at sea level, when superimposed on hypoxia at high elevations, it may predispose children to serious altitude illness. If a child has had a recent upper or lower respiratory infection or otitis media, careful consideration should be given to rapid ascent above 2,000 m (~6,600 ft).
Children with chronic lung disease (e.g., cystic fibrosis, bronchopulmonary dysplasia) and obstructive sleep apnea (OSA) are at increased risk of hypoxia at altitude and development of HAPE. Therefore they should undergo S o 2 monitoring during altitude travel. Similarly, children with cardiac lesions involving an increase in pulmonary blood flow or pulmonary hypertension are at greater risk of developing HAPE. Children with trisomy 21 have increased pulmonary vascular reactivity and a higher risk of pulmonary hypertension, and are also more likely to have OSA and hypoventilation. Children with sickle cell anemia who live at sea level should reconsider travel to altitude, or else ascend carefully because sickle cell crisis may occur at as low as 1,500 m (~5,000 ft). Those with sickle cell trait may become symptomatic at altitudes above 2,500 m (~8,200 ft).
Acetazolamide is commonly prescribed as prophylaxis against AMS because of its ability to stimulate respiration and increase alveolar and arterial oxygenation. It acts as a carbonic anhydrase inhibitor that induces a renal bicarbonate diuresis, causing a metabolic acidosis that increases ventilation and arterial oxygenation. However, prophylactic pharmacologic therapy with acetazolamide in children is generally not recommended because preacclimatization with slow ascent achieves the same effect. Exceptions include children with previous susceptibility to AMS and an unavoidably rapid ascent, such as flying to La Paz, Bolivia (3,700 m, 12,100 ft), or Cusco, Peru (3,400 m, 11,200 ft), from sea level. The pediatric dose of acetazolamide is 2.5 mg/kg (maximum, 125 mg/dose) every 12 hr ( Table 90.1 ). In adults, it is recommended that prophylaxis begin 24 hr before arriving at altitude and be continued for 48 hr at altitude, or until the final destination high altitude is reached. The respiratory stimulation caused by acetazolamide also improves sleep by eradicating periodic breathing. Side effects are common and include paresthesias, polyuria, lightheadedness, dry mouth, and metallic taste with carbonated beverages. Acetazolamide is a nonbacteriostatic sulfonamide drug, so a history of anaphylactic reaction to sulfa medications is a contraindication to its use. Acetazolamide should be avoided in breastfeeding mothers and pregnant women. Dexamethasone is another agent that has been used for AMS prophylaxis in the adult population. However, it should not be used for prophylaxis in children because of the potential for side effects; pancreatitis, pseudotumor cerebri, and interference with normal growth. Low-risk children should not need medications for prophylaxis and should use gradual ascent to prevent illness.
| MEDICATION | CLASSIFICATION | INDICATION | DOSE AND ROUTE | ADVERSE EFFECTS |
|---|---|---|---|---|
| Acetazolamide | Carbonic anhydrase inhibitor | AMS prevention * | 2.5 mg/kg PO every 12 hr; max 125 mg/dose | Collateral effects include paresthesias and taste alteration |
| AMS treatment | 2.5 mg/kg PO every 12 hr; max 250 mg/dose | |||
| Dexamethasone | Steroid | AMS prevention † | Risk of adverse effects precludes prophylactic use | |
| AMS HACE treatment ‡ | 0.15 mg/kg PO/IM/IV every 6 hr; max 4 mg/dose | Hypertension, gastrointestinal hemorrhage, pancreatitis, growth inhibition | ||
| Nifedipine | Calcium channel blocker | HAPE treatment (small children) § | 0.5 mg/kg PO every 4-8 hr; max 20 mg/dose | Hypotension |
| HAPE treatment (>60 kg) § | 30 mg SR PO every 12 hr or 20 mg SR PO every 8 hr | |||
| Reentry HAPE prevention | Same dose as HAPE treatment | |||
| Sildenafil | Phosphodiesterase-5 inhibitor | HAPE ¶ | 0.5 mg/kg/dose PO every 4-8 hr; max 50 mg/dose every 8 hr |
* AMS prophylaxis is not routinely recommended in children. It is indicated when rapid ascent profile is unavoidable or with previous altitude illness in child about to undergo similar ascent profile. Doses as low as 1.25 mg/kg every 12 hr have been successful in some children.
† Use not warranted due to risk of adverse effects. Use slow, graded ascent or acetazolamide.
‡ Oxygen and descent are the treatment of choice for severe AMS. If acetazolamide is not tolerated, dexamethasone may be used. Oxygen, descent, and dexamethasone should be used in HACE.
§ In emergency settings where oxygen and descent are not an option, nifedipine is indicated.
¶ In emergency settings where oxygen and descent are not an option, if nifedipine is not well tolerated, sildenafil may provide an alternative.
AMS is easily identified in older children and adolescents using the Self-Report Lake Louise AMS Scoring System . The criteria require that the individual be in the setting of a recent gain in altitude, be at the new altitude for at least several hours, and report a headache plus at least 1 of the following symptoms: gastrointestinal (GI) upset (anorexia, nausea, or vomiting), general weakness or fatigue, dizziness or lightheadedness, or difficulty sleeping. Shortness of breath on exertion may also be a part of the clinical picture, although if occurring at rest, the presence of HAPE should be considered in the absence of other causes. The headache may vary from mild to severe; anorexia and nausea, with or without vomiting, are common. Sleep disturbance caused by periodic breathing is common in all visitors to high altitudes but is exacerbated in the setting of AMS. All the symptoms of AMS can range in severity from mild to incapacitating. Symptoms develop within a few hours after ascent and generally reach maximum severity between 24 and 48 hr, followed by gradual resolution. Most adults become symptom free by the 3rd or 4th day. The vague nature of this presentation has resulted in many misdiagnoses and morbidity among adults. In the setting of recent altitude exposure, these symptoms warrant a presumptive diagnosis of AMS and limitation of further ascent. Any evidence of CNS dysfunction, such as mild ataxia or altered mentation, is early evidence of HACE.
In nonverbal young children and infants, recognition of AMS symptoms is more challenging. AMS is often a diagnosis of exclusion and is characterized by nonspecific signs: fussiness, lack of playfulness, anorexia, nausea, vomiting, and disordered sleep. In most cases of AMS in nonverbal young children and infants, all these symptoms are present. Fussiness is defined as a state of irritability that is not easily explained by a cause, such as tiredness, wet diaper, hunger, teething, or pain from an injury. Fussy behavior may include crying, restlessness, or muscular tension. Decreased playfulness may be profound. Alterations of appetite may progress to frank vomiting. Sleep disturbance can manifest with either increased or decreased sleep compared to normal patterns. Most often, decreased sleep and the inability to nap are noted.
The Children's Lake Louise Score (CLLS) has been successfully tested in preverbal children <4 yr old by parents briefed on the use of the scoring system. The CLLS combines a score for the amount and intensity of unexplained fussiness with a symptom score of how well the child has eaten, played, and slept in the past 24 hr. Evaluating for headache is done by asking if the head hurts or by using a “faces” pain scale. GI symptoms are evaluated by asking children if they are “hungry” rather than trying to evaluate their appetite. A combined score of ≥7 is indicative of AMS ( Fig. 90.1 ). Many of the symptoms manifested by AMS in children may also result from the disruption of normal routine with travel. A change in environment, sleeping accommodation, or eating options can result in a fussy child. The threshold scores for AMS diagnostic criteria are modified to account for these baseline variations. Supplemental oxygen may serve as a diagnostic aid; 2-4 L/min by nasal cannula (27–33% O 2 ) for 15-20 min should significantly improve headache and other symptoms.
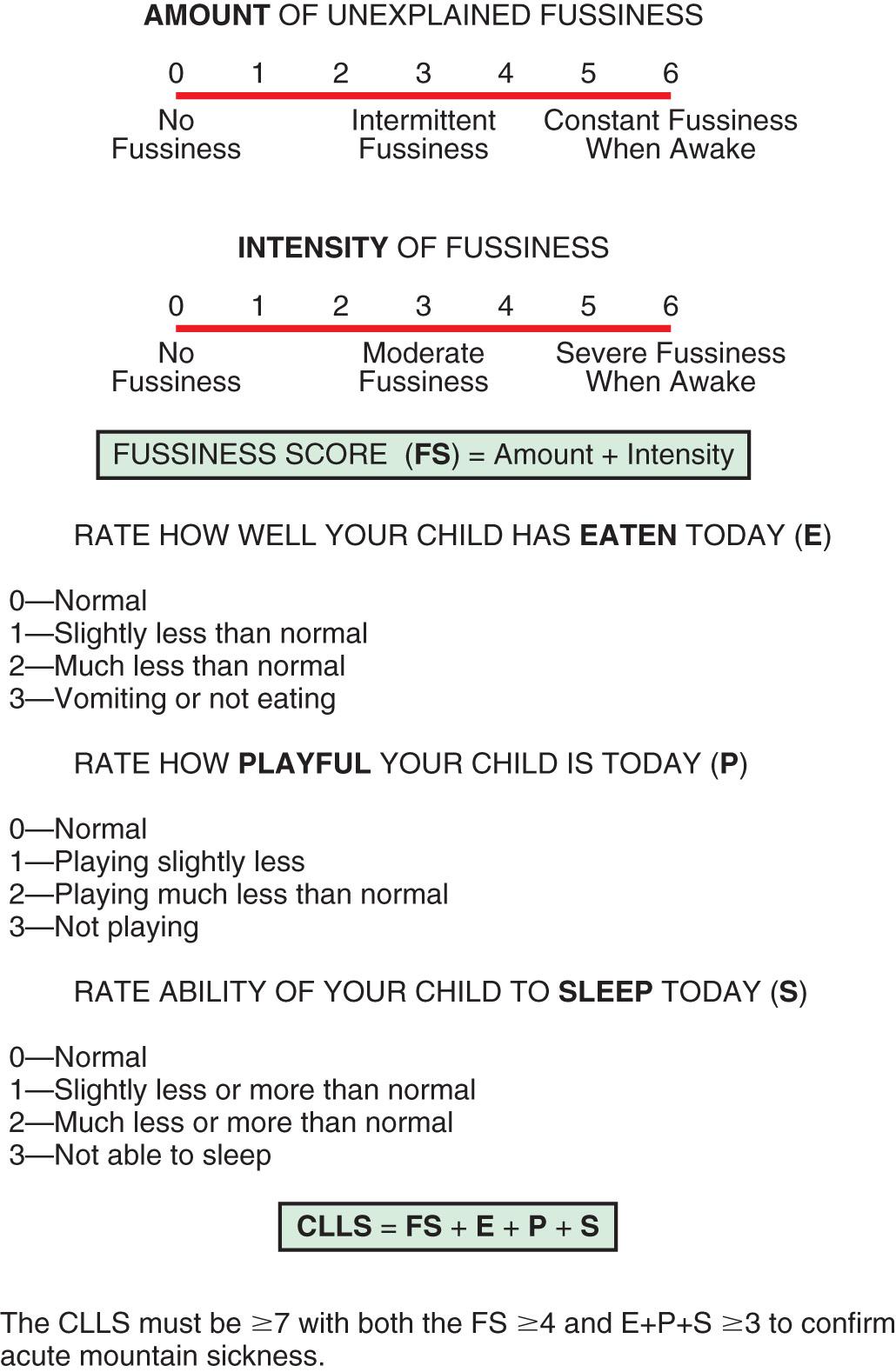
If symptoms occur >2 days after arrival at altitude and headache and dyspnea at rest are absent, and if the child fails to improve with supplemental oxygen, an alternative diagnosis should be sought. It must be emphasized that altered mental status, neurologic abnormalities, breathing difficulty, or cyanosis are not part of uncomplicated AMS. Any of these signs warrants immediate medical attention. If serious bacterial illness, a surgical condition, or another problem meriting specific intervention is suspected in a child, descent to lower altitude is recommended to eliminate the confounding variable of altitude illness.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here