Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The prevalence of allergic disease, particularly food allergy, has increased in recent years throughout the world, especially in developed countries.
Allergen-specific immunoglobulin E (IgE) antibody is the most important trigger molecule for allergic inflammation.
Laboratory tests for IgE antibody that support a high likelihood of allergic disease are widely available and clinical practice guidelines advocate their use. A positive IgE antibody test result is an indicator of allergic sensitization and a risk factor that provides support for a clinical history-based diagnosis of allergic disease.
Treatments for allergic diseases include avoidance, pharmacotherapy, immunotherapy, and therapeutic monoclonal anti-IgE antibody that requires demonstration of a total serum IgE level between 30 and 700 kU/L in serum prior to use.
Clinical investigations of the natural history of allergic diseases in children have shown a reproducible sequence of sensitization to allergens known as the allergy march that makes it important to establish the diagnosis of allergy at an early age to identify allergen triggers, optimize treatment in order to ameliorate progression of serious diseases such as asthma, and prevent the development of anaphylaxis.
Tests for total IgE and allergen-specific IgE antibodies are useful in the diagnostic workup of a patient with a high pretest suspicion of having allergic disease.
Measurement of total serum IgE in children and adults is of limited value in the diagnosis of allergic disease due to the overlap between atopic and nonatopic populations. An elevated total serum IgE level carries a high predictive value for allergic disease, but a normal level does not exclude allergic disease. Measurement of total serum IgE is diagnostically useful in patients suspected of having allergic bronchopulmonary aspergillosis.
Measurement of allergen-specific IgE antibodies is diagnostically useful and can be recommended in the following clinical situations:
Evaluation of children with clinical signs and symptoms of allergy, including eczema, gastrointestinal signs, rhinitis, or wheezing.
Evaluation of children and adults suspected of having allergic respiratory disease to establish the diagnosis and to define the specificity of allergen sensitization to pollens, animal proteins, dust mites, fungal antigens, and foods. Results are useful to identify allergen specificities for immunotherapy treatment regimens.
Confirmation of sensitization to food allergens and aid in determining the presence of food allergy in children and adults with signs of immediate hypersensitivity following food ingestion.
Evaluation of sensitization to insect venom allergens, particularly as an aid in defining venom specificity when skin tests are equivocal or there is sensitization to multiple venoms.
Confirmation of the clinical history-based diagnosis of drug hypersensitivity in patients with a clinical history of anaphylactic sensitivity.
Confirmation of sensitization to occupational allergens (e.g., natural rubber latex).
Testing for allergen-specific IgE, IgG, and IgG4 antibodies is not routinely recommended for evaluating the effects of immunotherapy, or for excluding the diagnosis of anaphylactic sensitivity to insect venoms in treated patients.
Tests for IgE antibodies are indicated only in patients who have had a thorough medical history and physical examination and their history provides suspicion of allergic disease.
The prevalence of allergic disease has increased significantly in the United States and throughout the developed world ( ). Longitudinal epidemiologic studies have shown an increased prevalence of sensitization to common allergens and increased prevalences of asthma, atopic dermatitis, food allergy, and allergic rhinitis in children. More than 40% of children age 6 years and older and more than 35% of children age 1 to 5 years have serologic evidence of sensitization to one or more common allergens ( ; ). Allergic sensitization, which is defined by the presence of immunoglobulin E (IgE) antibodies to one or more allergens, is a significant risk factor for allergic disease. Allergic sensitization is also a distinguishing characteristic of atopy, the inherited tendency to develop allergic diseases.
Estimates of the prevalences of common allergic diseases in the United States vary with disease, patient age, and geographic location. The prevalence of asthma has increased steadily from the 1950s to the present and currently is estimated at between 5% and 10% among adults and children in different states (Centers for Disease Control and Prevention ). More than 31 million persons in the United States suffer from asthma, with more than 7 million being children ( , ). The prevalence of allergic rhinitis has increased in the United States from about 5% in 1970 to approximately 9% in 2004, thus affecting more than 25 million Americans ( ).
The prevalence of food allergies has also increased. The cumulative prevalence of sensitization to common food allergens in children may be as high as 24% in the United States. In contrast, the cumulative prevalence of food allergies in children is 4% to 8% (see later discussion), and many sensitized children do not display signs and symptoms of systemic food allergy ( ). Given the high overall prevalence of allergic disease in the United States, estimates of the total yearly cost of diagnosing and managing patients with allergic diseases run into the tens of billions of dollars. Simply stated, allergic diseases are a major public health concern.
Recent attempts to explain the increased prevalence of allergic diseases have focused on both genetic and environmental influences. A large number of genes and genetic polymorphisms contribute to the development of allergic diseases (see later discussion), but they fail to account for the risk of developing a particular allergic disease and do not explain the rapid changes in disease prevalences noted previously. Current theories postulate a causative role for environmental influences to account for the increased prevalence of allergic diseases. Several investigators have proposed that diminished exposure to microorganisms during early childhood in developed countries may lead to aberrant development of the adaptive immune system and may predispose to both allergic disease and certain autoimmune diseases ( ). This theory, called the hygiene hypothesis , postulates that early exposure to bacteria and other microorganisms promotes development of mature antigen-presenting dendritic cells and regulatory T (Treg) cells, whereas diminished exposure leads to high levels of T helper 1 (Th1) and Th2 effector T cells that participate in the pathogenesis of immune-mediated diseases. The proposed mechanism for the beneficial effect of early exposure to microorganisms and environmental antigens includes a role for enhanced release of the anti-inflammatory cytokines interleukin (IL)-10, IL-12, and transforming growth factor-β by dendritic cells, which induces antigen-specific Treg cells ( ). This effect may be mediated by interactions between bacterial products (e.g., bacterial endotoxin) and Toll-like receptors (TLR2 and TLR4) on dendritic cells, monocytes, and macrophages of the innate immune system. Longitudinal studies in large cohorts of allergic patients and experimental studies in animal models continue to investigate the epidemiology and biological mechanisms of allergic disease development and to validate the hygiene hypothesis.
The signs and symptoms of allergic diseases can be difficult to distinguish from those of other disorders ( ). Clinical manifestations of allergy may be relatively mild and self-limited, as in seasonal allergic rhinitis, or they can manifest with severe symptoms that have marked morbidity, such as in allergic asthma. Laboratory tests are useful in the evaluation of patients with allergic disease, but awareness of the basic mechanisms of immediate-type hypersensitivity and knowledge of the empirical relationships between test results and their diagnostic predictive values are required to translate testing results into a clinical diagnosis. This chapter addresses these subjects, beginning with a review of the biological mechanisms of immediate-type hypersensitivity and the “allergic phenotype.” It then discusses laboratory tests as they may be applied to the diagnostic evaluation of children and adults with suspected allergic disease based on their clinical history and physical examination.
The distinction between sensitization to an allergen or allergenic component and clinical allergy is a key concept emphasized throughout this chapter. The authors identify the chemical components of common allergens, including species-specific and cross-reactive allergenic components. The minimum number of tests needed to achieve high positive and negative predictive values for allergic disease is overviewed for different clinical conditions. The discussion mentions clinical situations and testing schemes in which laboratory testing is not likely to be cost-effective or may lead to erroneous diagnoses. Recent developments in the laboratory evaluation of allergy include an expanding repertoire of tests for IgE antibodies to putative allergens, including tests for antibodies to highly purified native allergenic proteins and recombinant allergen components. Analytic methods that utilize chip-based microarrays to test for over 100 allergen-specific IgE antibodies simultaneously have proven useful in research studies. These methods lend themselves to interpretive reporting with patient-specific comments that aid in identifying clinically significant patterns of sensitization to allergenic components.
IgE is the principal trigger molecule for allergic inflammation. In the late 1960s, investigators working independently in two laboratories described a new isotype of human immunoglobulin from patients with allergic diseases that was responsible for the skin-sensitizing activity of serum ( ; ). This immunoglobulin, originally called IgND, was later named IgE ( ). In the years that followed, investigators have focused on discovering the basic biological mechanisms of immediate hypersensitivity. These include mechanisms that control IgE production by immunocompetent B-lymphocytes and that promote the recruitment of inflammatory cells and release of vasoactive mediators from IgE-sensitized effector cells at sites of allergic inflammation. Recent studies have focused on investigating genetic determinants of the allergic phenotype and on identifying genes responsible for regulating IgE production and for influencing tissue responses to allergic inflammation.
The term atopic (from the word atopy ) describes those individuals who are genetically predisposed to produce specific IgE antibodies following exposure to allergens. These individuals are highly likely to develop allergic diseases. Nevertheless, while atopy is characteristic of allergic disease, signs and symptoms of allergic diseases, notably asthma and eczema, may also occur in the absence of atopy. This finding demonstrates that the genetic predisposition to allergic diseases is complex and fails to account entirely for development of clinical allergy. It is useful to consider two principal domains of genetic influence: genes that encode molecules directly involved in immune responses that strongly influence IgE synthesis and determine atopy, and genes that affect other complementary biological functions. These include tissue structure and repair, tissue permeability to allergens, cell migration, and fibrinolysis, which determine the susceptibility of end organs to inflammation and modulate exposure of the immune system to environmental allergens ( ; ).
Knowledge of genes that predispose to allergic diseases comes from several sources. Population-wide linkage analysis studies have identified the chromosomal locations of candidate genes and positional cloning studies support the relationship of putative allergy genes to known genes for molecules involved in regulating the immune response. Genome-wide association studies examine the relationships between candidate genes for atopy (IgE synthesis) and disease susceptibility in populations with asthma and other common allergic diseases ( ; ).
Among the various candidate genes, some of the strongest evidence for a role in allergic disease exists for genes encoding molecules that promote IgE synthesis. Included in this group are the following: genes for IL-4, IL-13, IL-4 receptor α chain (IL4R) and signal transducer and activator of transcription 6 (STAT6) ( ; ). As described later, the products of these genes collectively influence the development of Th2 cells, and promote the expression and deletional class switching of ε immunoglobulin heavy-chain genes. Class II human leukocyte antigen (HLA) loci and alleles on chromosome 6p strongly influence the immune response and sensitization to specific allergens and allergenic component proteins. For example, responsiveness to the common aeroallergens Derp p and Derp f peptides from dust mites ( Dermatophagoides pteronyssinus and Dermatophagoides farinae ) is found in linkage with other loci associated with bronchial hyperresponsiveness in asthmatic individuals ( ). A similar linkage has been proposed for an atopy gene closely linked to the IgE Fc receptor α-chain locus on chromosome 1q23 ( ). Studies suggest that additional genes may predispose to allergic disease, including genes for the interleukin 6 receptor (IL6R) and GATA-binding protein 3 involved in the production of Th2 lymphocytes, and genes for TL4R that influence the innate immune response to environmental antigens. Cytokine genes, including the genes for IL-3, IL-5, and IL-33, influence the development, proliferation, and recruitment of allergic effector cells, including mast cells, basophils, and eosinophils.
Other genes influence allergic disease susceptibility by affecting biological functions that facilitate exposure to allergens or determine the response of tissue to inflammation. Mutations in the gene for filaggrin on chromosome 1q21 predispose to atopy and atopic dermatitis ( ). Filaggrin is an important protein component of the cutaneous epidermal barrier. Null mutations in the filaggrin gene may promote allergy development by weakening this barrier ( ). The products of several genes determine the response of tissues to chronic inflammation. ADAM33 is an exemplary gene. This gene encodes a metalloproteinase that influences airway remodeling and smooth muscle hyperactivity associated with asthma susceptibility and progression ( ). Examples of several genes that predispose to atopy and allergic disease are listed Table 56.1 . While the genes mentioned earlier influence susceptibility to allergic disease, and mutations and polymorphisms of several candidate genes have been identified, there is no clinical evidence at this time to support routine testing for genetic polymorphisms in the evaluation of patients with allergic disease.
| Chromosome | Genetic Locus | Phenotype |
|---|---|---|
| 6p21-23 | HLA D region (HLA-DRB1 and DQB1) | Responder or nonresponder to common allergens |
| 11q12 | Fc ε receptor α chain | Enhanced responsiveness to aeroallergens; high total serum immunoglobulin (Ig) E |
| 5q31 | Cytokines interleukin (IL)-3, IL-4, IL-5, IL-9, IL-13, and IL-33 | High total serum IgE |
| 16 | IL-4 receptor | High total serum IgE |
| 20p13
1q21 |
ADAM33 gene
Filaggrin |
Enhanced airway responsiveness, airway remodeling Diminished epithelial barrier to allergens |
As noted earlier, class II genes of the HLA D region strongly influence individual responses to exogenous antigens ( ; ). Gene products of the highly polymorphic HLA D region—DR, DQ, and DP molecules expressed on the plasma membranes of antigen-presenting dendritic cells and B cells—bind processed antigen for presentation to immunocompetent T cells, initiating the immune response ( ). B cells act as antigen-presenting cells by binding antigen (allergen) through membrane-associated immunoglobulin, that is, IgE-Fc ε RI, ( ). The cellular and humoral responses that follow antigen presentation are complex and include interactions mediated by cell-to-cell contacts and secretion of stimulatory cytokines. Production of mature IgE antibodies by B cells exemplifies these mechanisms ( Fig. 56.1 ).
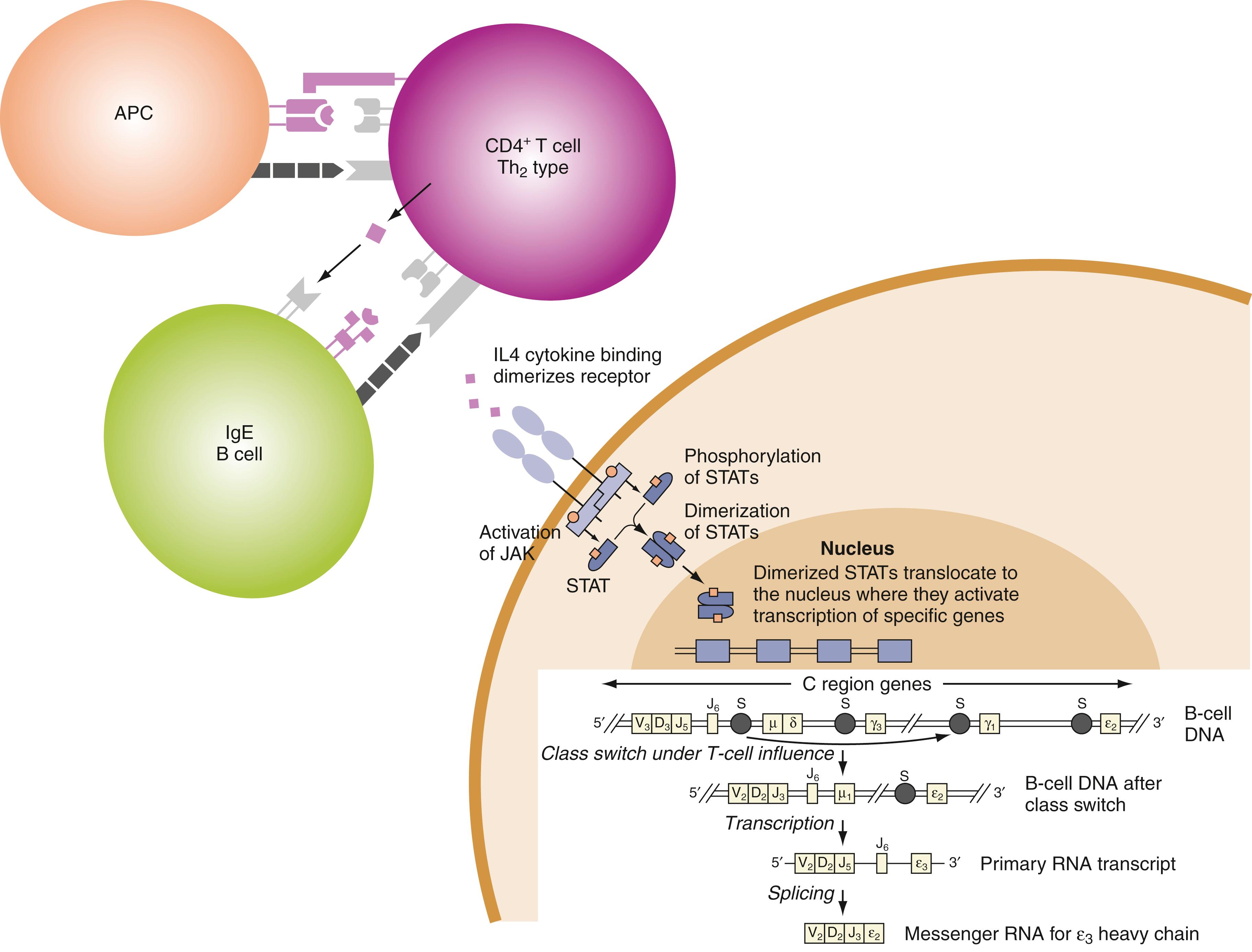
All B cells initially produce IgM antibodies. Rearrangement of their V, D, and J exons that encode portions of the Ig variable region create a variable region motif (VDJ) that encodes the antigen binding (Fab) portion of a mature Ig heavy chain. The rearranged VDJ motif determines specificity for antigen and is located upstream from exons that encode the constant regions of the various Ig heavy chains, including the α-, γ-, and ε-exons. Production of mature transcripts for complete Ig molecules that bear these heavy chains requires additional rearrangements at the heavy-chain (IgH) locus. These rearrangements result in a change in expression of heavy-chain constant region domains from μ to another isotype (e.g., γ). This process is referred to as deletional switch recombination because endonucleases excise exons encoding domains of the μ-chain in an irreversible process ( ; ). Two pathways involving rearrangement of Ig heavy-chain genes lead to the production of IgE antibodies: a direct pathway from IgM to IgE and a sequential pathway from IgM to an IgG intermediate to IgE ( ).
Synthesis of mature IgE molecules occurs in a series of steps that begins with expression of germline (sterile) transcripts of the ε heavy chain. Germline transcription must occur before deletional switch recombination can proceed. At the molecular level, this process involves interaction with a promoter exon upstream from the four exons that encode domains of the ε heavy chain (see later discussion and Fig. 56.1 ). The ε-promoter contains binding sites for the transcription factors STAT6 and nuclear factor κB (NF-κB), which drives transcription by activating the promoter. Mutations at binding sites for these transcription factors block isotype switching to IgE. Because germline transcripts are “sterile” and do not encode a functional heavy chain, further metabolism of the germline transcript by endonucleases is required to create a functional heavy chain molecule. Endonucleases excise Cμ and ligate the now adjacent VDJ and Cε sequences. The process of deletional switch recombination involves rearrangement of double-stranded deoxyribonucleic acid (DNA) at specific sites called S regions by enzymatically catalyzed breakage, cleavage, and recombination of DNA to create the functional heavy chain.
At the cellular level, IgE synthesis is initiated by cell-to-cell interactions and is driven by soluble cytokine mediators ( ; ). Cytokines produced by T helper/inducer cells of the Th2 phenotype are required for the production of IgE heavy-chain transcripts. The stimulatory cytokines IL-4 and IL-13 provide essential signals for synthesis of IgE. Th2 cells produce several cytokines that act as growth factors for B cells, including IL-4 and IL-13, which have been shown to induce production of germline ε-transcripts in human B cells ( ; ). These cytokines exert their effects by interacting with multimeric cell surface receptors composed of α and γ chains. The functional receptors for IL-4 and IL-13 are of 2 types that share a common ligand-binding IL-4R α chain. Type 1 receptors expressed on Th2 cells and mast cells bind only IL-4 and are heterodimers of the IL-4R α chain and γ chain. Type 2 receptors are widely expressed on T cells, bind both IL-4 and IL-13, and are heterodimers of the IL-4R α chain and IL-13R α chain. Binding of IL-4 and IL-13 to these receptors induces a series of phosphorylation reactions, which result in DNA transcription driven by phosphorylated transcription factors. Tyrosine kinases of the Janus family (JAK1, JAK2, and JAK3) are associated with intracellular domains of the IL-4 and IL-13 receptors and catalyze phosphorylation of the receptors, enabling them to bind intracellular transcription factors. The transcription factors, including STAT6 and NF-κB, once phosphorylated, translocate to the nucleus, where they bind to the ε-promoter and induce expression of germline ε-transcripts ( ; ).
Coincident with the interaction of antigen-presenting cells and T cells described earlier, which takes place primarily through the T-cell antigen receptor (TCR) and leads to production of IL-4 and IL-13, a second signal provided by cell-to-cell interaction promotes switch recombination from μ to ε heavy-chain expression ( ). This signal is provided by the interaction of CD40 (constituitively expressed on B cells) and CD40 ligand (CD154) expressed only on activated T cells. Engagement of the TCR results in upregulation of CD40 ligand on the T-cell surface. CD40 ligand interacts with CD40 on the surface of B cells to catalyze activation of NF-κB, which, in turn, facilitates activation of the ε-promoter through STAT6 and other activation-induced proteins ( , ). CD40 is a member of the tumor necrosis factor (TNF) receptor superfamily; engagement of CD40 by CD40 ligand antagonizes apoptosis of B cells. Engagement of CD40 by CD40 ligand also upregulates CD80-CD86 expression on B cells, which interacts with CD28 on T cells to strongly amplify cytokine transcription and secretion of IL-4 and IL-13. The importance of CD40 ligand (CD154) to the process of class switching is illustrated by patients with X-linked hyper-IgM syndrome who are deficient in CD40 ligand and are unable to produce IgG, IgA, or IgE antibodies ( ).
IgE-producing B cells at mucosal sites and in lymph node follicles typically differentiate into short-lived plasmablasts and plasma cells while a small number of long-lived plasma cells persist in bone marrow ( ). This finding helps to account for the rapid decline in IgE antibody levels that occur when antigen stimulation is withdrawn. Initial IgE responses to allergen occur primarily at extrafollicular sites where low-affinity antibodies are produced by direct class switching from IgM to IgE. Persistent or recurrent antigenic stimulation promotes development of higher-affinity IgE antibodies by a process of somatic mutation called affinity maturation .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here