Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Alcohol-associated liver disease (ALD) remains a challenging enigma for basic scientists and clinicians. Despite extensive research and clinical trials since the 1940s, many important facets of this disease have yet to be resolved. Paramount among these important questions are the following: (1) Why does cirrhosis develop in only a small fraction of heavy alcohol abusers? (2) What is the pathogenesis of severe ALD? (3) What are the most effective treatments for patients with severe ALD?
A systematic analysis of the global burden of disease in 2016 evaluated 195 locations around the world and showed that alcohol use was the seventh leading risk factor for both deaths and disability-adjusted life years. Globally, 32.5% of people between 15 and 95 years of age were current drinkers. Another study found that between 1999 and 2016, annual deaths from cirrhosis in the USA increased by 65%, and rates of HCC doubled. Alcohol was a major contributing factor to these events. Moreover, mortality trends from chronic liver disease from 2007 to 2016 in the USA showed that HCV-related mortality had begun to decline due to the use of DAAs, whereas that for ALD continued to increase.
Alcohol abuse is the most common etiology of cirrhosis in the developed world (see Chapter 74 ). It is the underlying cause of 44% of liver disease deaths in the USA (approximately 13,000 deaths annually), exceeding those for hepatitis C, the second most common fatal liver disease in this country. In Europe and the USA combined, ALD and its complications account for approximately 50,000 deaths each year. Unfortunately, although addiction and mental disorders have increasingly gained support from medical care and social support systems, it is remarkable that alcohol addiction has remained severely stigmatized.
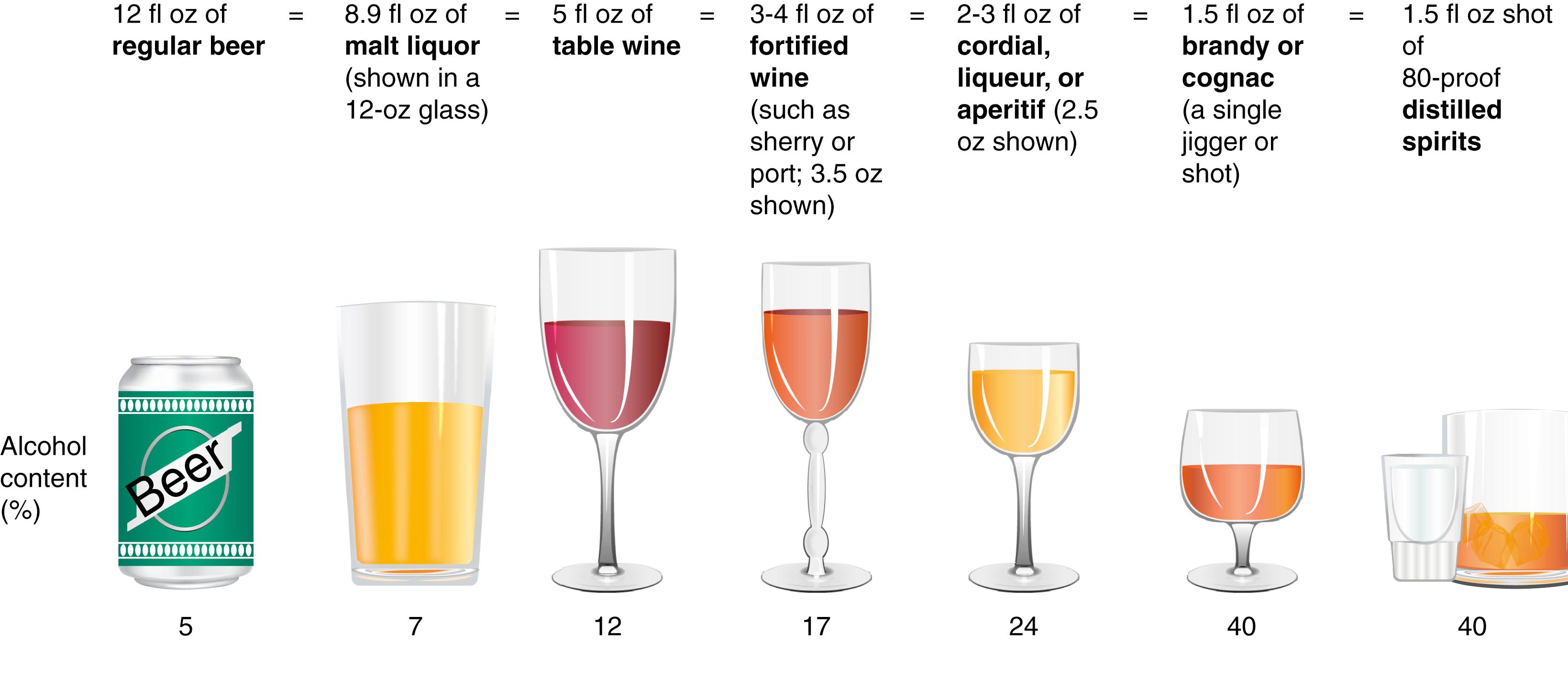
Numerous studies have shown that ALD develops in women after a shorter duration of drinking and with a lower daily alcohol intake than in men. , Population-based surveys have documented that men must usually drink 40 to 80 g of alcohol daily and women 20 to 40 g daily for 10 to 12 years to achieve a significant risk of liver disease. Fig. 86.1 illustrates the alcohol content of various beverages sold in the USA, their typical serving sizes, and the definition of a “standard drink.” A useful website for drinking information is https://www.rethinkingdrinking.niaaa.nih.gov/ . The standard drink in the USA is 14 g of alcohol, and this standardization provides a common definition for reporting alcohol intake. Data from the USA and Europe indicate that subjects diagnosed with alcohol-associated hepatitis or cirrhosis are often drinking about 15 standard drinks/day.
Chronic alcohol abuse can result in a spectrum of liver injury that ranges from mild fatty infiltration to steatohepatitis, steatohepatitis with fibrosis, cirrhosis, and HCC ( Fig. 86.2 ). Fat accumulation in liver cells, the earliest and most predictable response to alcohol ingestion, is seen in 90% to 100% of heavy drinkers. , Although fatty liver is considered to be a benign condition that reverses quickly with abstinence, cirrhosis can develop within 5 years in 10% of patients who continue to drink heavily. Much more important than steatosis is the development of necroinflammation and fibrosis (alcohol-associated hepatitis) that occurs in approximately 10% to 35% of heavy drinkers. On liver histology, steatosis, steatohepatitis with or without fibrosis, and alcohol-associated cirrhosis represent the spectrum of ALD. It is unclear, however, whether the histologic findings always correlate with the clinical presentation. For example, alcohol-associated steatohepatitis on a liver biopsy specimen can be present in patients with minimal symptoms (moderate alcohol-associated hepatitis) or with severe clinical manifestations of the disease (severe alcohol-associated hepatitis). Moderate alcohol-associated hepatitis is diagnosed less often than severe alcohol-associated hepatitis because these patients may not seek medical care or visit emergency departments with only nonspecific symptoms of nausea, diarrhea, or fatigue.
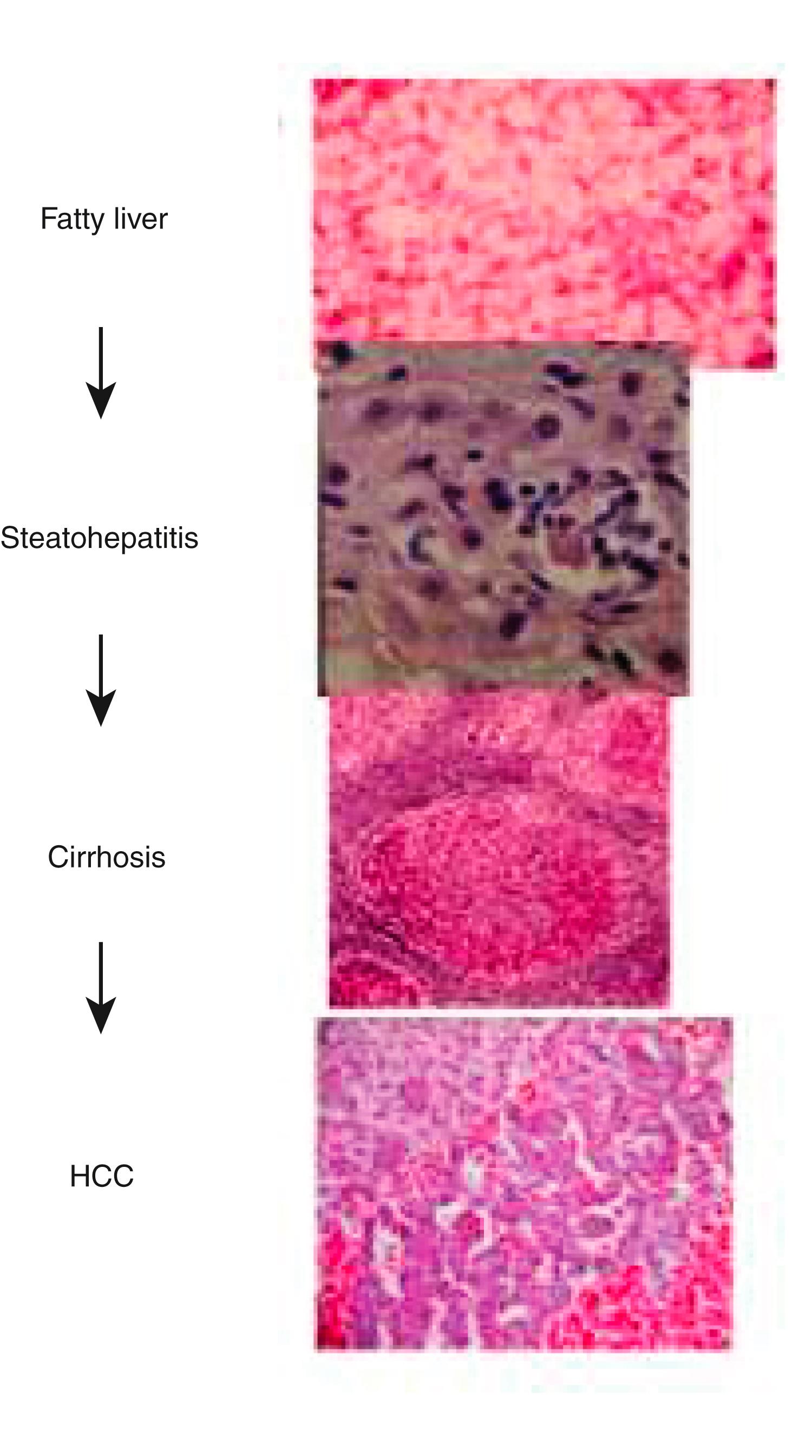
Alcohol-associated hepatitis is an important clinical entity for several reasons: (1) patients with severe alcohol-associated hepatitis have extremely high short-term mortality rates; (2) patients with severe alcohol-associated hepatitis can develop portal hypertension in the absence of cirrhosis; and (3) alcohol-associated hepatitis is a well-documented precursor of cirrhosis, with an elevated long-term mortality rate 9 times higher than that for patients with fatty liver alone. , With continued alcohol abuse, a fine mesh-like pattern of fibrosis (micronodular cirrhosis) develops in 8% to 20% of heavy drinkers. Over time this lesion can evolve to include broad bands of fibrosis that separate large nodules of liver tissue (macronodular cirrhosis). HCC typically develops in this setting.
The liver is the main organ responsible for ethanol metabolism; other organs such as the stomach contribute to much lesser degrees. Ethanol is metabolized by 3 major systems in the liver: alcohol dehydrogenase (ADH), cytochrome P450 2E1 (CYP2E1), and, of least importance, catalase. ADH is the primary enzyme system responsible for metabolism of ethanol at low concentrations, whereas CYP2E1 contributes to ethanol metabolism at higher tissue concentrations of ethanol (>10 mM). Furthermore, CYP2E1 activity is up-regulated by exposure to ethanol, thereby leading to faster metabolism with chronic excessive alcohol use. Both ADH and CYP2E1 convert ethanol to acetaldehyde, which is then converted by aldehyde dehydrogenase (ALDH) to acetate. Acetaldehyde is a highly reactive and potentially toxic compound that is responsible for many of the systemic toxic effects of alcohol, such as nausea, headaches, and flushing.
Acetaldehyde is also postulated to play an etiologic role in ALD by forming adducts with reactive residues on proteins or small molecules (e.g., cysteines). These chemical modifications can alter or interfere with normal biologic processes, exert cellular toxicity, and stimulate the host’s immune response and cause autoimmune-like manifestations. Antibodies against such oxidatively modified proteins have been found in both human and animal models of ALD. An example is the hybrid adduct of malondialdehyde and acetaldehyde, unique to alcohol exposure, which induces an immune reaction in human alcoholics and in animal models. Acetaldehyde has also been shown to impair mitochondrial glutathione transport and to sensitize hepatocytes to TNF-α‒mediated killing. Lastly, acetaldehyde disrupts intestinal barrier function, contributing to endotoxemia and proinflammatory cytokine production.
In addition to forming cytotoxic metabolites such as acetaldehyde, ethanol metabolism can alter the cellular oxidation-reduction (redox) state, thereby modulating liver injury. Specifically, the oxidation of ethanol uses nicotinamide-adenine dinucleotide (NAD + ) as an electron acceptor and thereby causes a shift in the ratio of reduced NAD (NADH) to NAD + to a more reduced state. This change in the redox state can impair normal carbohydrate and lipid metabolism; multiple effects ensue, including a decrease in the supply of ATP to the cell and an increase in hepatic steatosis.
Oxidative stress is an imbalance between pro-oxidants and antioxidants. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are products of normal metabolism and can be beneficial to the host (e.g., by contributing to bacterial killing). Overproduction of ROS and RNS or inadequate antioxidant defenses (e.g., low levels of vitamins, selenium, mitochondrial glutathione), or both, can lead to liver injury. Oxidative stress in ALD is usually documented by detection of one of several indirect markers: (1) protein oxidation (e.g., protein thiol or carbonyl products); (2) lipid oxidation (e.g., isoprostanes, malondialdehyde); (3) DNA oxidation (e.g., oxodeoxyguanosine); or (4) depletion or induction of antioxidant defenses (e.g., vitamin E, glutathione, thioredoxin).
The stimulus for oxidative stress in the liver comes from multiple sources. In hepatocytes, CYP2E1 activity increases after alcohol consumption—in part because of stabilization of messenger RNA. The CYP2E1 system leaks electrons to initiate oxidative stress. CYP2E1 is localized in the hepatic lobule in areas of alcohol-induced liver injury. Moreover, overexpression of CYP2E1 in mice and in HepG2 cells (a human hepatoma cell line) in vitro leads to enhanced alcohol hepatotoxicity. , Nonparenchymal cells and infiltrating inflammatory cells (e.g., polymorphonuclear leukocytes [neutrophils]) are another major source of pro-oxidants that are used for normal cellular processes, such as killing invading organisms. Major enzyme systems for pro-oxidant production in Kupffer cells and infiltrating macrophages in the liver include NAD phosphate (NADPH) and inducible nitric oxide synthase. Mice deficient in NADPH oxidase or mice treated with the drug diphenyleneiodonium sulfate, which blocks NADPH oxidase, are resistant to ethanol-induced liver injury. A critical subunit of the NADPH oxidase complex, p47phox, has been shown to play a role in liver parenchymal cells in producing ALD in mice. Infiltrating neutrophils use enzyme systems such as myeloperoxidase to generate hypochlorous acid (HOCl − , a halide species that causes oxidative stress) and RNS. One study found that in neutrophils the p47phox oxidative pathway is regulated by microRNA-223. Oxidative stress can mediate liver injury through at least 2 major pathways: direct cell injury and cell signaling. Direct cell injury is indicated by markers such as lipid peroxidation and DNA damage. An even more important role is played by signaling pathways; for example, activation of transcription factors such as nuclear factor kappa B (NF-κB) plays a critical role in the production of pro-inflammatory cytokines such as TNF.
Mitochondria are the major consumers of molecular oxygen and major generators of ROS in the liver. Mitochondrial dysfunction is well documented in ALD and contributes to oxidative stress. Mitochondrial abnormalities in ALD include megamitochondria observed on light and electron microscopy and functional mitochondrial abnormalities as documented by an abnormal 13C ketoacid breath test result (ketoacids are metabolized by mitochondria). Short-term administration of alcohol enhances hepatic superoxide generation in liver mitochondria, thereby increasing the flow of electrons along the respiratory electron transport chain. The increased NADH/NAD + ratio caused by ethanol intake favors superoxide generation. Because hepatic mitochondria lack catalase, glutathione plays a critical role in protecting mitochondria against oxidative stress. Mitochondria do not make glutathione but instead import it from the cytosol. In ALD, the transport of glutathione into mitochondria is impaired, and selective mitochondrial glutathione depletion is observed. Glutathione depletion also sensitizes the liver to the toxic effects of TNF, which also impairs mitochondrial function.
Normal mitochondrial function requires continuous exchange of substrate between the cytosol and the mitochondrial matrix, and this is catalyzed by specific exchangers within the inner mitochondrial membrane. By contrast, exchange of most water-soluble metabolites between the cytosol and the intermembrane space occurs through the voltage-dependent anion channel in the mitochondrial outer membrane. Alcohol-mediated closure of the voltage-dependent anion channel limits free diffusion of metabolites into the intermembrane space and causes mitochondrial dysfunction. This process is likely a cause of global alterations in mitochondrial function related to alcohol abuse and ALD.
In mammals, the liver plays a central role in methionine metabolism; nearly half of the daily intake of methionine is metabolized in the liver ( Fig. 86.3 ). The first step in methionine metabolism is the formation of S-adenosylmethionine (SAMe) in a reaction catalyzed by methionine adenosyltransferase. Activity of this enzyme is depressed in ALD. SAMe is the principal biological methyl donor through the transmethylation pathway, the precursor of aminopropyl groups used in polyamine biosynthesis, and a precursor of glutathione through its conversion to cysteine along the transsulfuration pathway.
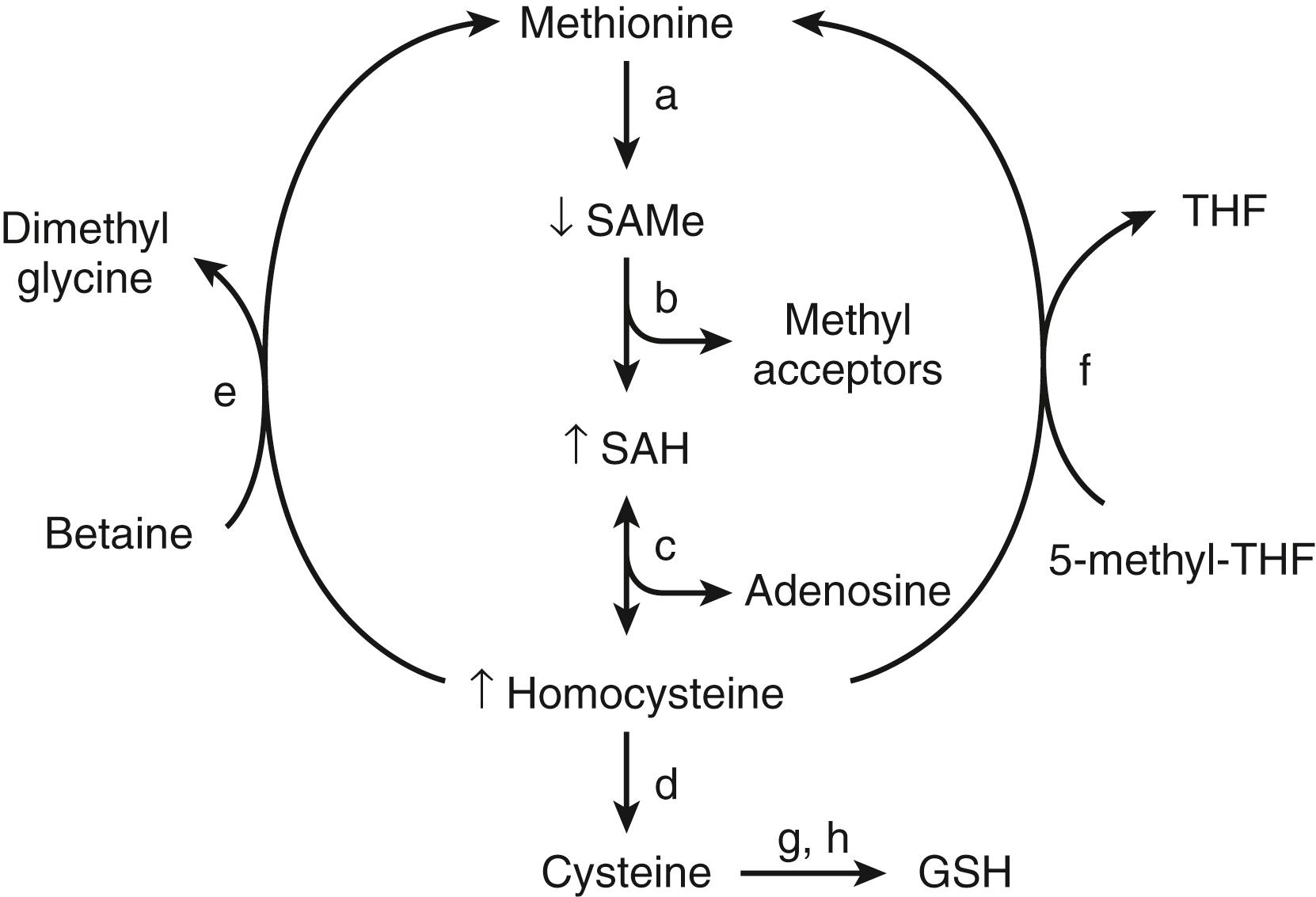
Deficiency of SAMe in patients with ALD was first noted in the early 1980s, when it was observed that alcoholic subjects had delayed clearance of an oral bolus of methionine (presumably because of blocked conversion of methionine to SAMe). , Functional methionine adenosyltransferase activity was subsequently shown to be subnormal in liver biopsy specimens from alcoholic subjects. Exogenous administration of SAMe corrects the deficiency and attenuates the severity of many experimental forms of liver injury.
In models of alcohol-induced hepatotoxicity, SAMe has been shown to maintain mitochondrial glutathione levels. Depletion of mitochondrial glutathione is thought to be one pathogenic factor in the development of ALD, and SAMe, but not other glutathione prodrugs, prevents mitochondrial glutathione depletion in experimental ALD (possibly by protecting mitochondrial glutathione transport systems). The antioxidant response element (ARE) is an essential component of upstream regulatory sequences present on many genes that provide hepatoprotection, including most phase II detoxification enzymes (see Chapter 88 ). NF-E2-related factor 2 (Nrf2) is a critical transcription factor that binds to the ARE and plays a key role in cellular responses to stress via the Keap1-Nrf2-ARE pathway. In experimental cholestatic liver disease, Nrf2 binding decreases, and this is partially prevented with SAMe therapy. Therefore, SAMe therapy may help maintain GSH levels as well as induce other antioxidant pathways through maintenance of appropriate Nrf2 binding. , SAMe also decreases lipopolysaccharide (LPS)-stimulated TNF release and increases interleukin (IL)-10 release in a monocyte cell line. Similarly, in rats fed a diet to induce SAMe deficiency, serum TNF levels increase and sensitivity to endotoxin-induced hepatotoxicity, which can be blocked by injection of SAMe, increases markedly. These data support the concept that SAMe may have direct hepatoprotective functions and may modify LPS-stimulated cytokine production.
Although serum SAMe levels are decreased in patients with ALD, levels of the downstream products S-adenosylhomocysteine (SAH) and homocysteine are elevated. , , Elevated SAH levels have been shown to sensitize hepatocytes to TNF-mediated destruction, and SAH may be a critical physiologic sensitizer of TNF-mediated killing in liver injury. Homocysteine and SAH can be removed by giving betaine, which facilitates regeneration of methionine from homocysteine. Folic acid also can play a critical role in the regeneration of homocysteine to methionine by means of 5-methyltetrahydrofolate (5-MTHF). Folic acid deficiency enhances the development of alcohol-induced liver injury in micropigs, and alcohol feeding interferes with normal folic acid metabolism in several different pathways—from impaired intestinal uptake to increased renal excretion. Collectively, the data support a role for altered methionine-transmethylation-transsulfuration metabolism in ALD and link these pathways to TNF hepatotoxicity.
The centrilobular area of the hepatic lobule (the functional unit of the liver) has the lowest oxygen tension and greatest susceptibility to hypoxia (see Chapter 71 ). Chronic alcohol intake increases oxygen uptake by the liver and increases the lobular oxygen gradient. A chronic intragastric feeding model in rats has been used to define the mechanisms underlying hepatic hypoxia and the association of these mechanisms with cycling of urinary alcohol levels (UALs). At high UALs, hepatic hypoxia is observed, along with reduced ATP levels; the NADH/NAD + ratio is shifted to the reduced state; and, hypoxia-inducible factor (HIF) genes are up-regulated. When UALs fall, reperfusion injury occurs, with free radical formation and peak liver enzyme release from hepatocytes. Hepatocyte-specific HIF-1α has been shown to be up-regulated in alcohol-fed mice and to play a role in hepatic lipid accumulation. One study has shown that the increase in HIF-1α in ALD is regulated by microRNA-122, a negative regulator of HIF-1α. Levels of microRNA-122, the most abundant microRNA in hepatocytes, are increased in the circulation but significantly decreased in the liver in ALD in mice and in humans. The low miR-122 levels in ALD are due to a direct inhibitory effect of alcohol on the transcription of this microRNA. Whereas HIF and HIF-regulated proteins appear to be up-regulated in the liver during alcohol feeding, intestinal HIF is markedly down-regulated. This down-regulation appears to contribute to increased intestinal permeability with subsequent endotoxemia and liver injury. Indeed, one mechanism of the beneficial action of the probiotic Lactobacillus GG in experimental ALD appears to be maintenance of intestinal HIF. ,
The endoplasmic reticulum (ER) stress response is induced by the accumulation of unfolded or misfolded proteins. To deal with the ER stress response, cells activate a series of signaling pathways termed the unfolded protein response, which can either be protective (usually in the short term) or detrimental (usually in the long term). One of the effects of a prolonged unfolded protein response can be increased production of TG and cholesterol, which can lead to fatty liver. Some potential inducers of ER stress in ALD include elevated homocysteine levels, acetaldehyde and acrolein adducts, and oxidative stress. Moreover, the cascade of ER stress and activation of the ER-associated molecule, STING, that triggers phosphorylation of the interferon regulatory factor 3 has been identified as an important mechanism for alcohol-induced hepatocyte damage. Phosphorylated interferon regulatory factor 3 interacts with mitochondrial apoptotic proteins to result in hepatocyte damage, apoptosis, and the release of damage-associated molecular patterns (DAMPs), such as ATP and uric acid, that then contribute to inflammasome activation (see Chapter 88 ).
Both of the major pathways that degrade most cellular proteins in eukaryotic cells, the ubiquitin-proteasome system and autophagy, are affected in ALD. The ubiquitin-proteasome pathway is the primary proteolytic pathway of eukaryotic cells (see Chapter 72 ). It controls the levels of numerous proteins involved in gene regulation, cell division, and surface receptor expression, as well as the stress response and inflammation. The proteasome system is now considered a cellular defense mechanism because it also removes irregular and damaged proteins generated by mutations, translational errors, or oxidative stress.
Animal studies have demonstrated that chronic ethanol feeding results in a significant decrease in proteolytic activity of the proteasome. This decreased activity can lead to abnormal protein accumulation, including accumulation of oxidized proteins. The decrease in proteasome function correlates significantly with the level of hepatic oxidative stress. Hepatocytes from alcoholics contain large amounts of ubiquitin in the form of cellular inclusions, or Mallory (or Mallory-Denk) bodies, which accumulate because they are not degraded efficiently by the proteasome. When hepatocytes die as a result of proteasome inhibition, they inappropriately release cytokines such as IL-8 and IL-18. IL-8 recruits neutrophils and probably plays a role in neutrophil infiltration in alcohol-associated hepatitis, whereas IL-18 sustains inflammation in the liver.
Evidence for the effects of alcohol on autophagy, a process responsible for degradation of long-lived or aggregated proteins and cellular organelles, is emerging (see Chapter 72 ). Studies in rats have shown that alcohol consumption inhibits multiple key steps in autophagy.
It is now generally accepted that the gut flora and gut-derived toxins play a critical role in the development of ALD and its complications ( Fig. 86.4 ). Indeed, in the 1960s, it was shown that germ-free rodents or rodents treated with antibiotics to “sterilize the gut” were resistant to nutritional and toxin-induced liver injury. Early studies showed that rats fed a choline-deficient diet developed cirrhosis, which could be prevented by oral neomycin. When endotoxin was added to the water supply, however, neomycin no longer prevented the development of liver injury and fibrosis. Subsequently, antibiotics, prebiotics, and probiotics have all been used to prevent experimental alcohol-induced liver injury. Numerous clinical studies also have demonstrated that plasma endotoxin levels are significantly elevated in patients with different stages of ALD—fatty liver, hepatitis, and cirrhosis—when compared with healthy control subjects. Ethanol-induced endotoxemia observed in experimental rodent models of ALD has also provided support for the essential role of LPS in the development of liver injury. Drastic reduction in gut microbiota with antibiotics in mice has been shown to result in significant attenuation of alcohol-induced inflammation not only in the liver, but also in the intestine and in the brain. Despite prevention of an alcohol-induced increase in LPS in the circulation and elimination of liver inflammation, however, alcohol still induced serum ALT elevations in mice, thereby suggesting a direct effect of alcohol on hepatocyte damage.
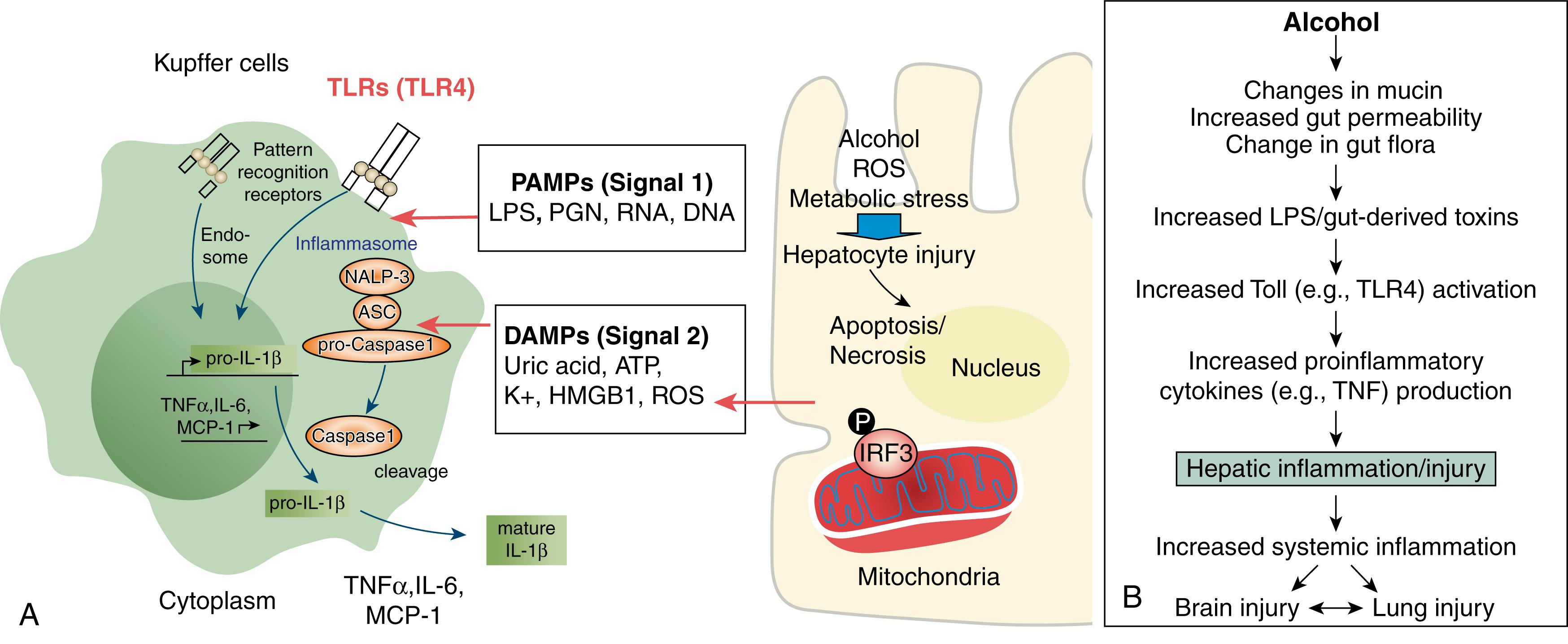
LPS is a prototype PAMP that represents a strong inflammatory signal for the host through recognition by Toll-like receptor 4, a pattern recognition receptor expressed on immune cells and many other cell types in the liver. With increased gut leakiness, many different PAMPs can translocate to the liver via the portal circulation and contribute to inflammatory cell activation. Alcohol-induced gut barrier dysfunction and endotoxemia are multifactorial events, with altered microflora and impaired intestinal integrity among the causal factors (see Fig. 86.4 ). Alcohol promotes the overgrowth of Gram-negative bacteria in the intestines of patients with chronic alcohol abuse. Studies on gut flora from alcoholics in an inpatient treatment program have demonstrated altered microflora composition, with decreased numbers of Bifidobacteria spp. and Lactobacilli spp. One human study found depletion of Akkermansia muciniphila in humans with ALD and showed that replacement of Akkermansia spp. can ameliorate alcohol-induced liver disease in mice. Another study found that after a 10-day alcohol feeding binge, a decrease in intestinal A. muciniphila was an early change in the gut microbiota related to alcohol. Multiple animal studies have documented altered gut flora with chronic alcohol feeding. Intestinal bacterial overgrowth of both aerobic and anaerobic bacteria after 3 weeks of intragastric alcohol feeding has been demonstrated in an animal model of ALD. Hepatic steatosis and steatohepatitis occurred at the same time as translocation of live bacteria into the systemic circulation. Importantly, prebiotic therapy attenuated liver injury. In another study, mice were fed alcohol for 8 weeks, and major changes in gut flora occurred relatively late in the disease process, whereas changes in gut barrier function and endotoxemia occurred much earlier. Fecal pH increased in association with altered gut flora, and probiotic therapy for the final 2 weeks effectively treated the liver disease (with a decrease in serum liver enzyme levels, reduction in endotoxemia, and correction of intestinal trefoil factor and tight-junction proteins). In these studies, alcohol intake decreased the levels of critical gut antimicrobial peptides.
Alcohol and its metabolite, acetaldehyde, induce intestinal permeability to various macromolecules, including LPS, in both human subjects and animal models of ALD. Translocation of LPS across the gut epithelial barrier has been attributed to the disruption of the intestinal barrier integrity. Indeed, decreased tight-junction (ZO-1) protein levels were observed in sigmoid colonic biopsies of human alcoholics compared with healthy controls. The decrease was attributed to an increase in miRNA-212 expression observed in alcoholic subjects compared with controls. Alcohol-induced oxidative stress and generation of nitric oxide in the intestines of experimental animals leading to loss of tight junction integrity, gut leakiness, endotoxemia, hepatic inflammation, and liver injury have also been reported. A number of laboratories have also reported increased intestinal permeability in experimental animal models of ALD due to redistribution and decreased expression of intestinal tight-junction proteins. Increased intestinal production of proinflammatory cytokines, such as TNF and IL-6, can also contribute to alcohol-associated endotoxemia by altering tight-junction morphology and distribution, thereby creating a self-perpetuating vicious cycle that can amplify bacterial translocation. Finally, emerging evidence suggests that intestinal fungi also contribute to ALD. Alcohol-dependent patients have been shown to display reduced intestinal fungal diversity and overgrowth of Candida spp. Consistent with this finding, antifungal therapy reduced the alcohol-related intestinal fungal overgrowth and increased serum β-glucan levels.
Inflammasomes are intracellular multiprotein complexes that sense danger signals from damaged cells and pathogens and assemble to mediate the caspase-1 activation that results in piroteolytic cleavage of pro-IL-1β and IL-18 into bioactive forms. Inflammasome activation is present in the liver in ALD, and the NLRP3 inflammasome seems to play a central role (see Fig. 86.4 ). The levels of DAMPs, including uric acid and ATP, are increased in ALD, and inhibition of these sterile danger signals can prevent inflammasome activation in mice. Metabolic DAMPs have been shown to mediate inflammation and crosstalk between damaged hepatocytes and to mediate immune-cell activation in ALD. Inflammasome activation in chronic alcohol use is not limited to the liver, and NLRP3 inflammasome activation and increased IL-1β levels have also been found in the brains of alcohol-fed mice. Inflammasome activation requires 2 signals; the first, usually a TLR-mediated signal, induces pro-IL-1β production, and the second, usually a DAMP-induced signal, results in inflammasome and capase-1 activation that releases bioactive IL-1β. Secreted IL-1β quickly binds to its receptor, thereby amplifying inflammation, increasing hepatocyte damage, and promoting liver fibrosis. Inhibition of the actions of IL-1β with a recombinant IL-1 receptor antagonist has been shown to improve ALD in mice and accelerate liver regeneration after chronic alcohol exposure. ,
Inflammasome activation can also lead to pyroptosis , a form of cell injury and death. Pyroptosis has been reported in alcohol-associated hepatitis in mice. Caspase-11 is up-regulated in hepatocytes in alcohol-associated hepatitis, and gasdermin D, which is downstream of caspase 11, contributes to pyroptosis.
In addition to ATP and uric acid, experimental evidence suggests a role for other DAMPs in ALD. For example, high mobility group box-1 (HMGB-1) is highly induced in ALD in the liver, where hepatocytes have been shown to secrete this normally intracellular and intranuclear protein. Other DAMPs—IL-33 and its decoy receptor soluble ST2 (IL-1 receptor like 1)—have been shown to play different roles in early and late stages of ALD in mice.
Increased plasma and hepatic concentrations of proinflammatory cytokines (e.g., TNF) are consistently observed in rodent models of ALD and are stimulated in large part by gut-derived toxins (see Fig. 86.4 ). TLR-4 activation by endotoxin results in recruitment of the adaptor molecules, MyD88, and Toll/IL-1 receptor domain-containing adapter inducing interferon-β (TRIF), which each activate separate downstream signaling cascades. Data suggest that the MyD88-independent pathway TRIF is more important in the development of experimental ALD, whereas NASH appears to signal through the MyD88-dependent pathway.
Dysregulated cytokine metabolism in human ALD has been recognized since the 1980s, with the initial observation that peripheral blood monocytes from patients with alcohol-associated hepatitis significantly increases basal and LPS-stimulated TNF production. , Serum concentrations of TNF-inducible cytokines and chemokines, such as IL-6, IL-8, IL-18, monocyte chemoattractant protein 1, and others, are elevated in patients with alcohol-associated hepatitis or cirrhosis, and the levels often correlate with markers of an acute-phase response, reduced liver function, and poor clinical outcomes.
This enhanced cytokine response to a physiologic stimulus such as LPS is termed priming . Increased serum or urinary levels of neopterin and other markers indicate that monocytes and Kupffer cells are primed in ALD. This priming for LPS-stimulated TNF production has been reproduced in vitro by culturing monocyte cell lines with relevant concentrations of alcohol. This response appears to be mediated, at least in part, by induction of CYP2E1 and oxidative stress. Not only are levels of proinflammatory cytotoxic cytokines increased in ALD, but also monocyte and Kupffer cell production of protective anti-inflammatory cytokines, such as IL-10, is decreased.
Several strategies have been devised to decrease cytokine production or activity in an attempt to block or attenuate liver injury. Examples include antibiotics to modulate intestinal flora and LPS, gadolinium chloride to destroy Kupffer cells, and antioxidants such as glutathione prodrugs to inhibit cytokine production. Each of these strategies has been successful in attenuating alcohol-induced liver injury in rats. Prebiotics, such as oat bran, and probiotics have also been shown to decrease endotoxemia in experimentally induced alcohol-associated liver injury. Moreover, anti-TNF antibody has been used to prevent liver injury in alcohol-fed rats, and alcohol-associated liver injury does not develop in mice that lack the TNF type I receptor.
Hepatocytes normally are resistant to TNF killing. Hepatocytes from rats fed alcohol or hepatocytes incubated in alcohol are sensitized to TNF killing, however. , Some potentially relevant mechanisms for such sensitization include mitochondrial depletion of glutathione, accumulation of SAH, and proteasome inhibition, among others. Therefore, in ALD, monocytes and Kupffer cells are primed to increase production of TNF, and hepatocytes are sensitized to TNF killing. These processes are closely intertwined with previously described mechanisms such as oxidative stress, mitochondrial dysfunction, abnormal metabolism of methionine, and dysfunction of proteasomes.
Alcohol-associated hepatitis may persist histologically for many months after exposure to ethanol has ceased, suggesting an ongoing immune or autoimmune response. Autoimmune reactions are well documented in patients with ALD, with autoantibodies directed against phospholipids, ADH, heat shock protein, and other potential antigens. Patients with ALD are at increased risk for the development of immune responses directed at neoantigens generated from the interactions of metabolites of alcohol (e.g., acetaldehyde or hydroxyethyl radicals) with liver proteins. Some studies also have linked genetic susceptibility and autoimmunity in ALD.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here