Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Anticipating the clinical course of the patient’s condition and assessing the likelihood of deterioration are crucial to the decision to intubate, especially if the patient is to leave the emergency department (ED) for a period of time (e.g., interfacility transfer, diagnostic testing).
Although videolaryngoscopy (VL) has reduced the chance of a failed intubation attempt caused by difficult anatomic features that often thwart direct laryngoscopy (DL), an assessment of the patient for potential difficult intubation, bag-mask ventilation (BMV), ventilation using an extraglottic device (EGD), and cricothyrotomy is an essential step before a neuromuscular blocking agent (NMBA) is administered. The mnemonics LEMON, ROMAN, RODS, and SMART can serve as useful aids.
Physiologic derangement can contribute to morbidity and mortality during emergency airway management. Cardiovascular optimization with fluids, blood, and pressor agents should be undertaken, when time allows, to reduce the risk of circulatory collapse and cardiac arrest.
In the absence of a crash patient (agonal, unresponsive to laryngoscopy) or difficult airway, rapid sequence intubation (RSI) is the airway management method of choice for ED patients.
Tube placement confirmation using end-tidal carbon dioxide (ET co 2 ) is essential after intubation; failure to detect adequate quantities of exhaled CO 2 is evidence of esophageal intubation until proven otherwise.
VL increases first-attempt intubation success, even when compared with DL combined with various optimization techniques. First-attempt success (FAS) is associated with fewer adverse events and better outcomes. Emergency airway managers should learn, and adopt, VL as the method of choice for emergency intubation.
Cricothyrotomy is indicated in the “can’t intubate, can’t oxygenate” failed airway situation and should be performed once this has been identified. Delays may increase the likelihood or severity of hypoxic injury to the patient.
Etomidate is used in more than 90% of all RSIs. Either rocuronium or succinylcholine is a reasonable NMBA for use during RSI. Rocuronium has less potential for adverse effects but a longer duration of action.
EGDs are rarely used in ED airway management but offer additional options for rescue oxygenation of the failed airway and are used in many prehospital systems.
Airway management is the cornerstone of resuscitation and is a defining skill for the specialty of emergency medicine. The emergency clinician has primary airway management responsibility, and all emergency airway techniques lie within the domain of emergency medicine. Although rapid sequence intubation (RSI) is the most commonly used method for emergent tracheal intubation, emergency airway management includes various intubation techniques and devices, approaches to the difficult airway, and rescue techniques when intubation fails.
The decision to intubate should be based on careful patient assessment and appraisal of the clinical presentation with respect to three essential criteria: (1) failure to maintain or protect the airway; (2) failure of ventilation or oxygenation; and (3) the patient’s anticipated clinical course and likelihood of deterioration.
A patent airway is essential for adequate ventilation and oxygenation. If a patient is unable to maintain a patent airway, patency should be established by using airway maneuvers such as repositioning, chin lift, jaw thrust, or insertion of an oral or nasal airway. Likewise, the patient must be able to protect against the aspiration of gastric contents, which carries significant risks for morbidity and mortality. Historically, the presence of a gag reflex has been advocated as a reliable indicator of the patient’s ability to protect the airway, but this has been definitively proven to be unreliable because the gag reflex is absent in 12% to 25% of normal adults, and there is no evidence that its presence or absence corresponds to airway protective reflexes or predicts the need for intubation. The patient’s ability to swallow or handle secretions is a more reliable indicator of airway protection. The recommended approach is to evaluate the patient’s level of consciousness; ability to phonate in response to voice command or query, which provides information about the integrity of the upper airway and level of consciousness; and ability to manage his or her own secretions (e.g., pooling of secretions in the oropharynx, absence of swallowing spontaneously or on command). In general, a patient who requires a maneuver to establish a patent airway or who easily tolerates an oral airway requires intubation for airway protection, unless there is a temporary or readily reversible condition, such as an opioid overdose.
Gas exchange, both oxygenation and removal of carbon dioxide, is required for vital organ function. Ventilatory failure that is not easily reversible or persistent hypoxemia despite maximal oxygen supplementation is a primary indication for intubation. This assessment is clinical and includes an evaluation of the patient’s general status, oxygen saturation by pulse oximetry, and ventilatory pattern. Continuous capnography also can be helpful, but is not essential if oximetry readings are reliable. Arterial blood gases (ABGs) are neither required to determine the patient’s need for intubation, nor practical to obtain before an emergency intubation. In addition, ABGs may be misleading, causing a false sense of security and delay in intubating a deteriorating patient. If obtained, they should be interpreted in the context of the patient’s clinical status. Patients who are clinically improving despite severe or apparently worsening ABG alterations may not require intubation, whereas a rapidly tiring asthmatic, for example, may require intubation, even though ABG values are only modestly disturbed. In most cases, clinical assessment, including pulse oximetry with or without capnography, consideration of the timeline of the patient’s respiratory emergency, and observation of improvement or deterioration in the patient’s clinical condition will lead to a correct decision.
The need for prolonged mechanical ventilation generally mandates intubation. An external mask device, continuous positive airway pressure (CPAP), and bilevel positive airway pressure (BiPAP) have all been used successfully to manage patients with exacerbations of chronic obstructive pulmonary disease (COPD) and congestive heart failure, obviating the need for intubation (see Chapter 2 ), but, despite these advances, many patients who need assisted ventilation or positive pressure to improve oxygenation require intubation.
Certain conditions indicate the need for intubation, even without an immediate threat to airway patency or adequacy of ventilation and oxygenation. These conditions are characterized by a moderate to high likelihood of predictable airway deterioration, worsening physiologic derangement, or the need for intubation to facilitate a patient’s evaluation and treatment. Intubation may be indicated relatively early in the course of certain overdoses. Although the patient initially may be protecting the airway and exchanging gas adequately, intubation is advisable to guard against the strong likelihood of clinical deterioration, which can occur after the initial phase of care when the patient is no longer closely observed. Patients with septic shock have high metabolic demand, myocardial depression, increased peripheral oxygen extraction, and vascular permeability. The combination of ventilatory fatigue, depressed pump function, and the need for directed fluid resuscitation predictably results in the need for intubation as pulmonary vascular congestion, hypoxia, and the work of breathing worsen. A patient who has sustained significant multiple traumatic injuries may require intubation, even if the patient is ventilating normally through a patent airway and has adequate oxygen levels. For example, a multiple trauma patient with hypotension, an open femur fracture, and diffuse abdominal tenderness warrants early intubation, even if the patient is initially awake and alert, without airway injury or hypoxemia. Active resuscitation, pain control, need for invasive procedures and imaging outside of the emergency department (ED), and inevitable operative management dictate the need for early airway control. In addition, a patient with penetrating neck trauma may have a patent airway and adequate gas exchange. Nevertheless, early intubation is advisable when there is evidence of vascular or direct airway injury, because these patients tend to deteriorate and increasing hemorrhage or swelling in the neck will compromise the airway and confound later attempts at intubation.
The common thread among these indications for intubation is the anticipated clinical course. In each case, it can be anticipated that future events may compromise the patient’s ability to maintain and protect the airway or ability to oxygenate and ventilate. Waiting until these occur may result in a difficult airway.
In most patients, intubation is technically straightforward. Although early ED-based observational registries reported cricothyrotomy rates of approximately 1% for all intubations, more recent studies have shown a lower rate, less than 0.5%. As would be expected with an unselected, unscheduled patient population, the ED cricothyrotomy rate is greater than in the operating room, which occurs in approximately 1 in 200 to 2000 elective general anesthesia cases. Bag-mask ventilation (BMV) is difficult in approximately 1 in 50 general anesthesia patients and impossible in approximately 1 in 600. However, BMV is difficult in up to one-third of patients in whom intubation failure occurs, and difficult BMV makes the likelihood of difficult intubation 4 times higher and the likelihood of impossible intubation 12 times higher. The combined failure of intubation, BMV, and oxygenation in elective anesthesia practice is estimated to be exceedingly rare, approximately 1 in 30,000 patients. These numbers cannot be extrapolated to populations of ED patients who are acutely ill or injured and for whom intubation is urgent and unavoidable. Although patient selection cannot occur, as with a preanesthetic visit, a preintubation analysis of factors predicting difficult intubation gives the provider the information necessary to formulate a safe and effective plan for intubation.
Preintubation assessment should evaluate the patient for anatomic features that would herald a difficult airway. This includes an assessment for potential difficulty with laryngoscopy and intubation, BMV, placement of and ventilation with an extraglottic device (EGD; see later discussion), and cricothyrotomy. Knowledge of all four domains is crucial to successful planning. A patient who exhibits difficult airway characteristics is highly predictive of a challenging intubation, although the emergency clinician should always be ready for a difficult to manage airway because some difficult airways may not be identified by a bedside assessment.
Airway difficulty exists on a spectrum. Some patients may have a single minor anatomic or pathophysiologic reason for airway difficulty, whereas others may have numerous difficult airway characteristics, complicating laryngoscopy, bag ventilation, use of an EGD, and cricothyrotomy. Although both sets of patients represent potential intubation challenges, the latter group, especially if obstructing upper airway pathology is part of the problem, more often has crossed a threshold of difficulty beyond which neuromuscular blockade would be avoided because a “can’t intubate, can’t oxygenate” (CI:CO) failed airway may ensue. In these cases, the preferred approach is to use topical anesthesia methods, with titrated parenteral sedation, to achieve intubation without the use of a neuromuscular blocking agent (NBMA). This is particularly true when intubation is undertaken with conventional laryngoscopy (versus use of a video laryngoscope or flexible endoscope) or when use of NBMAs would result in immediate physiologic deterioration and instability. Patients with refractory hypoxemia or severe metabolic acidosis may be intolerant to even brief periods of apnea. In such patients, an awake approach is preferred, particularly if anatomic difficulty coexists. Airways predicted to be anatomically difficult when using a traditional laryngoscope may not prove difficult when a videolaryngoscope is used (see later discussion). Occasionally, RSI remains the preferred method, despite assessment that the patient has a difficult airway, as part of a planned approach to airway management. This may include physiologic optimization, use of videolaryngoscopy (VL), and a double setup, in which a rescue approach, such as cricothyrotomy, is fully prepared for immediate use in the event of intubation failure. Regardless of the results of a reassuring bedside assessment for airway difficulty, significant challenges may be encountered with intubation and BMV, and the clinician must be prepared for unanticipated difficulty with every intubation.
Glottic visualization is paramount in emergency airway management. With direct laryngoscopy (DL), if the vocal cords can be seen (Cormack and Lehane [CL] grade I or II view; Fig. 1.1 ), the chance of intubation success is high. However, when the glottic aperture cannot be visualized (CL grade III or IV), intubation success is less likely. Very few of the difficult airway markers thought to limit DL access have been scientifically validated, yet applying them in combination can provide a reasonable assessment of anticipated airway difficulty. On the other hand, VL rarely fails to provide adequate laryngeal visualization but may introduce difficulty with indirect tube placement. Characterization of difficult VL predictors is not well studied, and although mnemonics exist that attempt to cover predictors of both difficult direct and VL, the components are too broad to be clinically useful (see discussion later). Like DL, adequate video views are highly correlated with intubation success, although the strength of this association can depend on the device used and operator experience. Whether DL or VL is planned, a standard screening process for difficulty should be undertaken with every patient. Our recommended approach uses the mnemonic LEMON ( Box 1.1 ), which has been shown to have reasonable sensitivity and high negative predictive value.

L ook externally for signs of difficult intubation (by gestalt)
E valuate 3-3-2 rule
M allampati scale
O bstruction or obesity
N eck mobility
The patient first should be examined for external markers of difficult intubation, which are determined based simply on the intubator’s clinical impression or initial gestalt. For example, the severely bruised and bloodied face of a combative trauma patient, immobilized in a cervical collar on a spine board, should (correctly) invoke an immediate appreciation of anticipated difficulty. Subjective clinical judgment can be highly specific but insensitive and so should be augmented by other evaluations whether or not the airway appears to be challenging.
The second step in the evaluation of the difficult airway is to assess the patient’s airway geometry to determine suitability for DL. Glottic visualization with a direct laryngoscope necessitates that the mouth opens adequately, the submandibular space is adequate to accommodate the tongue, and the larynx be positioned low enough in the neck to be accessible. These relationships have been explored in various studies by external measurements of mouth opening, oropharyngeal size, neck movement, and thyromental distance. The 3-3-2 rule is an effective summary of these assessments. The 3-3-2 rule requires that the patient be able to place three of his or her own fingers between the open incisors, three of his or her own fingers along the floor of the mandible beginning at the mentum, and two fingers from the laryngeal prominence to the underside of the chin ( Fig. 1.2 ). A patient with a receding mandible and high-riding larynx is exceptionally difficult to intubate using DL because the operator cannot adequately displace the tongue and overcome the acute angle for a direct view of the glottic aperture. In practice, the operator compares the size of his or her fingers with the size of the patient’s fingers and then performs the three tests.

Oral access is assessed with the Mallampati scale ( Fig. 1.3 ). Visibility of the oral pharynx ranges from complete visualization, including the tonsillar pillars (class I), to no visualization at all, with the tongue pressed against the hard palate (class IV). Classes I and II predict adequate oral access, class III predicts moderate difficulty, and class IV predicts a high degree of difficulty. A meta-analysis has confirmed that the four-class Mallampati score performs well as a predictor of difficult laryngoscopy (and, less so, of difficult intubation), but the Mallampati score alone is not a sufficient assessment tool. A Mallampati score necessitates an awake compliant patient to perform the assessment in the way in which it was originally described. Nearly 50% of ED patients requiring intubation cannot cooperate with this assessment, but it can be improvised by using a direct laryngoscope blade as a tongue depressor in obtunded or uncooperative patients.

Upper airway (supraglottic) obstruction may make visualization of the glottis, or intubation itself, mechanically impossible. Conditions such as epiglottitis, head and neck cancer, Ludwig angina, neck hematoma, glottic swelling, or glottic polyps can compromise laryngoscopy, passage of the endotracheal tube (ETT), BMV, or all three. Examine the patient for airway obstruction and assess the patient’s voice to satisfy this evaluation step. Although obesity alone may not be an independent marker of difficult DL, it likely contributes to challenges in other areas of airway management. Nevertheless, obese patients generally are more difficult to intubate than their nonobese counterparts, and preparations should account for this and for the more rapid oxyhemoglobin desaturation and increased difficulty with ventilation using BMV or an EGD (see later).
Neck mobility is desirable for any intubation technique and is essential for positioning the patient for optimal DL. Neck mobility is assessed by flexion and extension of the patient’s head and neck through a full range of motion. Neck extension is the most crucial motion but placing the patient in the full sniffing position provides the optimal laryngeal view by DL. Modest limitations of motion do not seriously impair DL, but severe loss of motion, as can occur in ankylosing spondylitis or rheumatoid arthritis, may make DL impossible. Cervical spine immobilization in trauma patients artificially reduces cervical spine mobility, but DL is still highly successful in this group of patients.
A modified mnemonic, LEMONS , has been described, with the “S” referring to the patient’s oxygen saturation. Although not a direct contributor to difficulty with DL, a low starting oxygen saturation will result in a shorter period of safe apnea and a reduced time to perform laryngoscopy and achieve ETT placement. Some providers may prefer “LEMONS” over “LEMON,” but we consider oxygen status (and overall clinical status) a part of preintubation assessment that is distinct from difficult airway assessment. An alternative mnemonic, “HEAVEN” ( H ypoxemia, E xtremes of size, A natomic challenges, V omit/blood/ fluid in the airway, E xsanguination, and N eck mobility) has been shown, in a retrospective review of aeromedical RSIs, to predict difficulty with both video and DL. However, the components of HEAVEN are a broad mixture of physiologic and anatomic attributes, some of which are either vague (anatomic challenges) or self-evident (blood/vomit in the airway). The “HEAVEN” mnemonic lacks sufficient detail and specificity required to apply it at the bedside reliably, and we recommend use of the LEMON mnemonic. As noted, identification of a difficult intubation does not preclude use of an RSI technique. The crucial determination is whether the emergency clinician judges that the patient has a reasonable likelihood of intubation success, despite the difficulties identified, and that ventilation with BMV or an EGD will be successful in case intubation fails (hence the value of the BMV and EGD assessments; see Boxes 1.2 and 1.3 ).
R adiation or resistance to ventilation
O bstruction, obesity and obstructive sleep apnea
M allampati, male, mask seal
A ged
N o teeth
R estricted mouth opening or R esistance to ventilation
O bstruction, obesity, or obstructive sleep apnea
D istorted anatomy
S hort thyromental distance
Attributes of difficult BMV have largely been validated and can be summarized with the mnemonic ROMAN (see Box 1.2 ).
R esistance/ R adiation—Resistance to ventilation (requiring high ventilation pressures) caused by intrinsic pulmonary disease such as asthma, COPD, adult respiratory distress syndrome [ARDS]), or a history of directed head and neck radiation are strong predictors of difficult BMV.
O bstruction/ O besity/ O bstructive sleep apnea—Obstruction of the airway, particularly supraglottic obstruction, or presence of obesity, which results in redundant upper airway tissues, increased chest wall weight, and resistance of abdominal mass, similarly are predictors of difficult BMV.
M allampati/ M ask seal/ M ale—High Mallampati classification, inability to achieve a good mask seal (e.g., because of facial trauma or presence of a beard), and male gender all have associations with challenging rescue mask ventilation.
A ge—This refers to advanced age and is best judged by the physiologic appearance of the patient, but age older than 55 years increases risk.
N o teeth —Identifies the edentulous patient. Lack of teeth, which form a strut to support the mask for ventilation and also support the upper and lower lips, independently interferes with mask seal and hence successful BMV. The difficulty with BMV of the edentulous patient is the basis of the advice often cited for patients with dentures: “teeth out to intubate, teeth in to ventilate.” Another approach involves placing the mask inside the patient’s lower lip. This may limit air leaks in patients without teeth and eliminates the risk of aspiration associated with dental prosthetics or rolled gauze ( Fig. 1.4 ).

Difficult BMV is common in the ED and out-of-hospital patients, but, with proper technique, BMV is usually successful. In patients undergoing elective anesthesia, impossible B MV is exceptionally rare (<0.5%) and is associated with the ROMAN mnemonic factors. The likelihood of difficult or impossible BMV increases proportionately to the number of these factors present.
Placement of an EGD, such as a laryngeal mask airway (LMA), Combitube, or similar upper airway device, often can convert a CI:CO situation to a “can’t intubate, can oxygenate” situation, which allows time for rescue of a failed airway (see following section). Difficulty achieving placement or ventilation with an EGD can be predicted by the mnemonic RODS (see Box 1.3 ) .
Fortunately, if the emergency clinician has already performed the LEMON and ROMAN assessments, only the “D ” for distorted anatomy remains to be evaluated (see Box 1.3 ). EGDs are placed blindly and have a mask or balloon structure that, when inflated, obstructs the oropharynx proximally and esophageal inlet distally, permitting indirect ventilation. Distorted upper airway anatomy can result in a poor seal and ineffective ventilation. Short thyromental distance, the “S ” in RODS, is identified as part of the 3-3-2 measurement during the LEMON assessment. Patients with receded mandibles have tongues that sit more posteriorly in the oral cavity creating an anatomic hurdle that the EGD must traverse to get to its final resting position relative to the glottis.
Difficult cricothyrotomy can be anticipated whenever there is limited access to the anterior neck or the laryngeal landmarks are obscured. This can be assessed using the mnemonic SMART ( Box 1.4 ). Prior surgery, hematoma, tumor, abscess, scarring (as from radiation therapy or prior injury), local trauma, obesity, edema, or subcutaneous air each has the potential to make cricothyrotomy more difficult. Perform an examination for the landmarks needed to perform cricothyrotomy as part of the preintubation difficult airway assessment of the patient. Point-of-care ultrasound can be used at the bedside to locate the cricothyroid membrane, thereby allowing the emergency clinician to mark the location on the surface of the neck in high-risk cases. The emergency clinician should not avoid performing a rescue cricothyrotomy when necessary, even in the presence of predicted difficulty. Prediction of the difficulty and identification of the factors causing the difficulty help the clinician to work through the problem to achieve success.
S urgery
M ass (abscess, hematoma)
A ccess/anatomy problems (obesity, edema)
R adiation
T umor
The actual degree to which an intubation is difficult is highly subjective, and quantification is challenging. The CL system is the most widely used system for grading a laryngoscopic view of the glottis, which grades laryngoscopy according to the extent to which laryngeal and glottic structures can be seen (see Fig. 1.1 ). In grade 1 laryngoscopy, all or nearly all of the glottic aperture is seen; in grade 2, the laryngoscopist visualizes only a portion of the glottis (arytenoid cartilages alone or arytenoid cartilages plus part of the vocal cords), in grade 3 only the epiglottis is visualized, and, in grade 4, not even the epiglottis is visible.
Fewer than 1% of stable patients undergoing DL during elective anesthesia yield a grade 4 laryngoscopy, a finding associated with an extremely difficult intubation. Grade 3 laryngoscopy, which represents highly difficult intubation, is found in less than 5% of patients. Grade 2 laryngoscopy, which occurs in 10% to 30% of patients, can be subdivided further into grade 2a, in which the arytenoids and a portion of the vocal cords are seen, and grade 2b, in which only the arytenoids are seen. Intubation failure occurs in up to two-thirds of grade 2b cases but in less than 1 in 20 grade 2a cases. Approximately 80% of all grade 2 laryngoscopies are grade 2a; the rest are grade 2b. First-attempt intubation success drops off significantly as the glottic view transitions from a grade 2a to 2b; however, a grade 1 view is associated with virtually 100% intubation success. Outside of the operating room, the rate of difficulty may be higher. In one review of emergency adult inpatient intubations, as many as 10% were considered difficult (grade 3 or 4 CL direct view or more than three attempts required). The incidence of difficult ED intubations is unknown but is likely much higher. An alternative system of grading laryngeal view, percentage of glottic opening (POGO), also has been proposed and validated but has not been widely used or studied. The incidence of difficult intubation and the predictors thereof are primarily based on the use of conventional DL and do not apply to VL. In a single-center assessment of ED intubations at an academic ED using VL exclusively, 80% of intubations predicted to be difficult were managed with NBMAs, with a 90% first-attempt success (FAS) rate. Thus predictors of difficult DL do not impact VL to the same degree. Nonetheless, a bedside assessment should still be performed in all patients so potential pitfalls can be identified and avoided.
Immediately after intubation, the operator should apply an end-tidal carbon dioxide (ET co 2 ) detection device to the ETT and assess it through six manual ventilations. Disposable colorimetric ET co 2 detectors are highly reliable, convenient, and easy to interpret, indicating adequate CO 2 detection by color change ( Figs. 1.5 and 1.6 ) and determining tracheal and esophageal intubation in patients with spontaneous circulation. The persistence of detected CO 2 after six manual breaths indicates that the tube is within the airway, although not necessarily within the trachea. CO 2 is detected with the tube in the mainstem bronchus, trachea, or supraglottic space. Correlation of ET co 2 detection with the depth markings on the ETT, particularly important in pediatric patients, confirms tracheal placement. Rarely, BMV before intubation or ingestion of carbonated beverages may lead to the release of CO 2 from the stomach after esophageal intubation, causing a transient false indication of tracheal intubation. Washout of this phenomenon universally occurs within six breaths.


Although colorimetric ET co 2 measurement is highly sensitive and specific for detecting esophageal intubation, caution is required for patients in cardiopulmonary arrest. In general, patients in early cardiopulmonary arrest (as long as cardiopulmonary resuscitation [CPR] is being performed) still produce sufficient CO 2 (2%) to cause a positive color change. Persistent color change is definitive evidence of correct ETT placement, and lack of color change is indicative of a misplaced (likely esophageal) tracheal tube. Although unlikely, insufficient gas exchange during prolonged cardiac arrest, or with inadequate CPR, might prevent CO 2 detection in the exhaled air, even when the tube is correctly placed within the trachea. However, the absence of color change (i.e., the absence of CO 2 in expired air), even if the patient is in complete or prolonged cardiac arrest, should prompt a careful evaluation to ensure that an esophageal intubation has not occurred. Newer resuscitation guidelines have suggested continuous quantitative measurement of ET co 2 during cardiac arrest to gauge the efficacy of CPR. This circumstance arises in approximately 25% to 40% of intubated cardiac arrest patients.
When ET co 2 detection is not possible, tracheal tube position can be confirmed using other techniques. One approach involves point-of-care ultrasound. In live patient and cadaver studies, ultrasonography performed over the cricothyroid membrane or upper trachea has accurately confirmed ETT position in the trachea, especially during intubation.
Another method of tube placement confirmation is the aspiration technique, based on the anatomic differences between the trachea and esophagus. The esophagus is a muscular structure with no support within its walls and is therefore collapsible when negative pressure is applied. The trachea is held patent by cartilaginous rings and thus is less likely to collapse when negative pressure is applied. Vigorous aspiration of air through the ETT with the ETT cuff deflated results in occlusion of the ETT orifices by the soft walls of the esophagus, whereas aspiration after tracheal placement of the tube is easy and rapid. Although once quite common, these devices are now rarely used and generally only in austere environments.
A gum elastic bougie can be placed through the center of an ETT to further corroborate tube location. Passing the bougie deeply through the tube, with little or no resistance, suggests an esophageal intubation because the bougie has likely passed beyond the tube and into the esophagus and stomach. If the ETT is in the trachea, the tip of the bougie will encounter resistance after emerging only a couple of inches from the tracheal tube, as it abuts the wall of the right mainstem bronchus. Another technique involves sliding the bougie in an upward and downward motion over a few inches distal to the tracheal tube. A vibration from contact of the deflected tip of the bougie with the anterior tracheal rings may be transmitted to the operator’s fingertips.
Quantitative or qualitative ET co 2 detection, with ultrasound or the bougie technique as backup, is the primary means of ETT placement confirmation. Secondary means include physical examination findings, oximetry, and radiography. The examiner should auscultate both lung fields and the epigastric area but should not rely on these findings alone. Pulse oximetry is indicated as a monitoring technique in all critically ill patients, not just those who require intubation. Oximetry is useful in detecting esophageal intubation but may not show a decreasing oxygen saturation for several minutes after a failed intubation because of the oxygen reservoir (preoxygenation) created in the patient before intubation. Although chest radiography is universally recommended after ETT placement, its primary purpose is to ensure that the tube is well positioned below the cords and above the carina. Because the esophagus lies directly behind the trachea, a single anteroposterior chest radiograph is not sufficient to confirm tracheal intubation, although esophageal intubation may be detected if the ETT is clearly outside the air shadow of the trachea. In cases in which doubt persists, a fiberoptic scope can be passed through the ETT to identify tracheal rings, another “gold standard” for confirmation of tracheal placement.
Algorithms for emergency airway management have been developed and provide a useful guide for planning intubation and rescue in case of intubation failure. The algorithms are applied after the decision to intubate, and the approach is predicated on two key determinations that are to be made before active airway management is initiated ( Fig. 1.7 ). The first determination is whether the patient is in cardiopulmonary arrest or a state of near arrest and is likely not to resist attempts at laryngoscopy. Such a patient—agonal, near death, in circulatory collapse—is deemed a “crash” airway patient for the purposes of emergency airway management and is treated using the crash airway algorithm by an immediate intubation attempt without use of drugs; this can be supplemented by a single large dose (2.0 mg/kg intravenous [IV]) of succinylcholine if the attempt to intubate fails and the patient is thought not to be sufficiently relaxed ( Fig. 1.8 ). In a crash scenario, larger doses of succinylcholine are recommended because poor circulation impairs drug delivery, resulting in paralysis that may be slower in onset and incomplete. A higher dosing can help to compensate for this impaired distribution. If a crash airway is not present, the LEMON, ROMAN, RODS, and SMART evaluations are made to determine if a difficult airway is present, and, if so, the difficult airway algorithm is used ( Fig. 1.9 ).


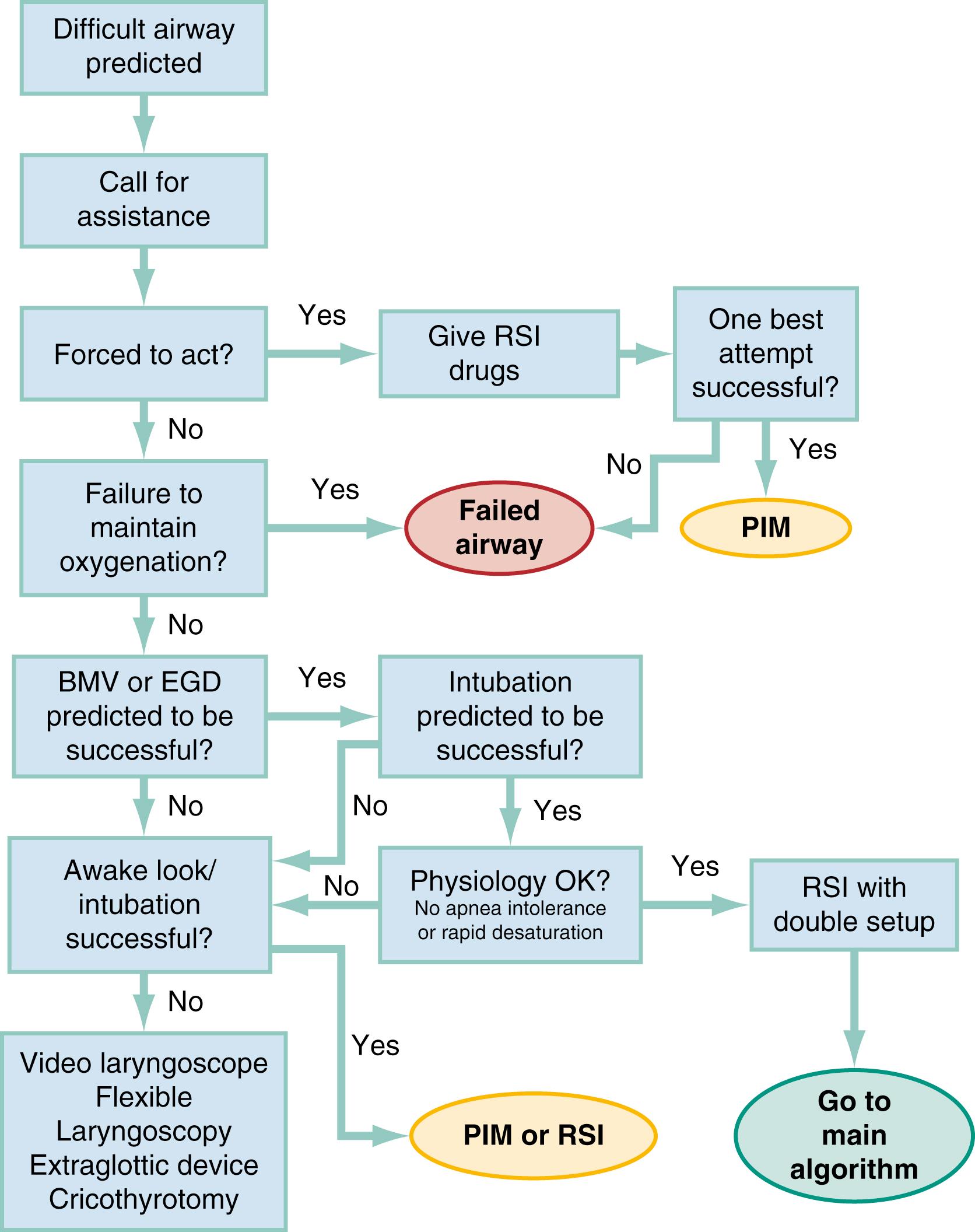
For patients who require emergency intubation but who have neither a crash airway nor a difficult airway, RSI is indicated. RSI provides the safest and quickest method of achieving intubation in such patients. After administration of RSI drugs, intubation attempts are repeated until the patient is intubated or a failed intubation is identified. If more than one intubation attempt is required, oxygen saturation is monitored continuously and, if saturations decrease to 92% or less, the laryngoscopic attempt is aborted (unless the operator feels he or she is on the cusp of successful tube placement) and BMV is performed until saturation is sufficiently recovered for another attempt. If the oxygen saturation continues to fall, despite optimal use of BMV or EGD, a failed airway exists. This is referred to as a CI:CO type of failed airway. A second form of failed airway is present when there have been three unsuccessful “best attempts” at laryngoscopy, because subsequent attempts by the same clinician are unlikely to succeed. The three failed laryngoscopy attempts are defined as attempts by an experienced clinician using the best possible patient positioning, device, and technique. Three attempts by a trainee using a direct laryngoscope may not count as best attempts if an experienced emergency clinician is available or VL has not yet been attempted. In addition, the emergency clinician can identify a failed airway after even a single laryngoscopic attempt if it is judged that intubation likely will be impossible (e.g., grade 4 laryngoscopic view with DL, despite optimal patient positioning and use of external laryngeal manipulation) and no alternative device (e.g., videolaryngoscope, intubating LMA) is available. The failed airway is managed according to the failed airway algorithm ( Fig. 1.10 ).

The perception of a difficult airway is relative, and many emergency intubations rightly are considered “difficult.” Deciding whether to treat the airway as a typical emergency airway or whether to use the difficult airway algorithm is based on the degree of perceived difficulty, operator experience, armamentarium of airway devices, and individual circumstances of the case. The LEMON, ROMAN, RODS, and SMART assessments provide a systematic framework to assist in identifying the potentially difficult airway but are not meant to firmly determine whether any individual patient should, or should not, receive an NMBA.
When preintubation evaluation identifies a potentially difficult airway (see Fig. 1.9 ), the approach is based on the premise that NMBAs generally should not be used unless the emergency clinician believes that (1) intubation is likely to be successful, (2) oxygenation can be maintained via BMV or EGD should the patient desaturate during an intubation attempt, and (3) the patient will not experience cardiovascular catastrophe or arrest from precipitous desaturation or hemodynamic collapse following administration of RSI medications. This is particularly true when intubation is undertaken with a conventional laryngoscope which may result in prolonged laryngoscopy and a lower FAS, even if that attempt is augmented by laryngeal manipulation and use of a bougie. , In addition, anatomic features of a difficult airway should be considered in the context of deranged physiology, provider experience, and availability of VL. Patients with refractory hypoxemia, right heart failure, or severe metabolic acidosis may be best managed with an awake intubation, especially if anatomic challenges exist, because the possibility of prolonged or repeated laryngoscopy combined with rapid physiologic decline with the onset of hypopnea can result in rapid arrest and anoxic injury. In these cases, if RSI is still deemed the best approach, then all other elements should be optimized to increase FAS. This includes (ideally) use of VL, robust preoxygenation and cardiovascular optimization with fluids, blood, and pressor agents, as necessary. The one exception to this recommendation occurs in the “forced to act” scenario.
A forced to act imperative permits RSI, even in a highly difficult airway situation in which the operator is not confident of the success of laryngoscopy or of sustaining oxygenation. This usually occurs in the setting of a rapidly deteriorating patient with an obviously difficult airway and a presumed clinical trajectory of imminent arrest or airway obstruction. Although this is not yet a crash airway situation, the operator is forced to act—that is, there is a need to act immediately to intubate before orotracheal intubation quickly becomes impossible or the patient arrests. The patient retains sufficient muscle tone and voluntary effort (including combative behavior induced by hypoxia) to require administration of drugs before intubation can be attempted. Consider an agitated patient with rapidly advancing anaphylaxis or angioedema, a morbidly obese patient in severe, end-stage status asthmaticus, or an intensive care unit (ICU) patient with inadvertent or premature extubation, respiratory failure, and difficult airway. Within seconds to minutes, perhaps before a full difficult airway assessment can be done or preparations can be completed for an alternative airway approach (e.g., flexible endoscopy), the patient’s rapid deterioration signals impending respiratory arrest. This is a unique situation in which the operator may be compelled to take the one best chance to secure the airway by rapidly administering RSI drugs, despite obvious airway difficulty, and attempting intubation before the airway crisis has advanced to the point that intubation is impossible or delay has caused hypoxic arrest. If laryngoscopy fails, the RSI drugs have optimized patient conditions for cricothyrotomy or insertion of an alternative airway device, depending on the operator’s judgment.
Therefore, in the difficult airway algorithm, the first determination is whether the operator is forced to act . If so, RSI drugs are given, a best attempt at laryngoscopy is undertaken, and, if intubation is not successful, the airway is considered failed, and the operator moves immediately to the failed airway algorithm. However, in the vast majority of difficult airway situations, the operator is not forced to act, and the first step is to ensure that oxygenation is sufficient to permit a planned orderly approach to airway management. If oxygenation is inadequate, oxygenation cannot be made adequate by supplementation with BMV, and anatomic challenges are significant, then the airway should be considered a failed airway. Although inadequate oxygenation should be defined on a case-by-case basis, oxygenation saturation decreasing to less than 93% is the accepted threshold, because this represents the point at which hemoglobin undergoes a conformational change, more readily releases oxygen, and increases the pace of further desaturation. Oxyhemoglobin saturations in the mid-80s, if holding steady, might be considered adequate in some circumstances, particularly if the patient is chronically hypoxemic. When oxygenation is inadequate or dropping, the failed airway algorithm should be used because the predicted high degree of intubation difficulty, combined with failure to maintain oxygen saturation, is analogous to the CI:CO scenario.
However, when oxygenation is deemed adequate, the next consideration is whether RSI is appropriate, on the basis of the operator’s assessment of the likelihood of (1) successful ventilation with BMV or EGD in case intubation is unsuccessful, (2) the likelihood of successful intubation by laryngoscopy, and (3) the severity of physiologic derangement. If the operator is not confident of successful intubation or rescue oxygenation and time allows, an awake technique can be used. In this context, awake means that the patient continues to breathe and, although IV sedation and analgesia may be administered, can cooperate with caregivers. If the operator judges that anatomic challenges are minimal and would not significantly adversely affect laryngoscopy or rescue oxygenation, then the patient’s physiologic vulnerability is considered. If the patient is deemed both hemodynamically stable and not at risk for immediate desaturation, then RSI is performed. However, if the patient is thought to be intolerant of apnea because of severe metabolic acidosis or anticipated precipitous desaturation or exhibits profound, refractory shock such that the vasoplegic effects of sedative/induction agents might precipitate circulatory collapse, then an awake technique is preferred. If RSI is performed on a patient with significant difficult airway attributes identified, then we recommend a double setup, with preparations simultaneously undertaken for rescue cricothyrotomy or another immediate rescue technique.
During an awake intubation, the patient is prepared by applying topical anesthesia with atomized or nebulized lidocaine, ideally preceded by a drying agent such as glycopyrrolate. Titrated doses of sedative and analgesic agents (or ketamine, which provides both actions) may be required for the patient to tolerate the procedure. Once this is accomplished, any of a number of different devices can then be used to attempt glottic visualization, with device selection most often dictated by patient anatomy and pathology. Regardless of the route taken to the airway (nasal or oral), VL, whether flexible or rigid, is preferable to DL. If the glottis is adequately visualized, the patient can be intubated at that time or, in a stable difficult airway situation, the emergency clinician may proceed with planned RSI, now assured of intubation success. If the awake laryngoscopy is unsuccessful, the patient can be intubated with any of numerous techniques shown in the last box in Fig. 1.9 . For each of these methods, the patient is kept breathing but is variably sedated and anesthetized. The choice among these methods depends on clinician experience and preference, device availability, and patient attributes.
Management of the failed airway is dictated by whether the patient can be oxygenated. If adequate oxygenation cannot be maintained with rescue BMV, the rescue technique of first resort is cricothyrotomy (see Fig. 1.10 ). Multiple attempts at other methods in the context of failed oxygenation only delay cricothyrotomy and place the patient at increased risk for hypoxic brain injury. However, if an alternative device (i.e., an EGD such as an LMA or King LT airway) is readily available and the operator judges it to be an appropriate device for the patient’s anatomy, a single attempt can be made to use it simultaneously with preparations for immediate cricothyrotomy as long as initiation of cricothyrotomy is not delayed. If early indications are that the EGD is effective and oxygenation improves, cricothyrotomy can wait; however, the operator must continuously reassess EGD function and oxygenation status. If the EGD subsequently fails, cricothyrotomy must begin without delay.
If adequate oxygenation is possible, several options are available for the failed airway. In almost all cases, cricothyrotomy is the definitive rescue technique for the failed airway if time does not allow for other approaches (i.e., oxygenation cannot be maintained) or if they fail. We distinguish the difficult and failed airway as follows: the difficult airway is something one anticipates; the failed airway is something one experiences. The fundamental difference in philosophy between the difficult and failed airway is that the difficult airway is planned for, and the standard is to place a definitive airway (cuffed ETT) in the trachea. The failed airway is not planned for, and the standard is to achieve any airway that provides adequate oxygenation to avert hypoxic brain injury. Some devices used in the failed airway (e.g., EGDs) are temporary and do not provide definitive airway protection, so are not generally used in the planned management of the difficult airway.
Although many techniques are available for intubation of the emergency patient, four methods are the most common, with RSI being the most frequent approach. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here