Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
First used in neurosurgery for the treatment of hydrocephalus in the early 20th century, endoscopy has had an expansion of its roles in skull base and other pathologies.
The endoscope has opened new options for treatment of pituitary tumors via the endonasal transsellar approach and extended approaches, hydrocephalus via the endoscopic third ventriculostomy, intraventricular lesions via minimally invasive techniques, and single-suture cranial synostosis via earlier treatment and less morbidity.
Collaborations of neurosurgeons with otolaryngologists specialized in skull base pathology have facilitated great advancements in endoscopic skull base surgery.
The vascularized pedicled nasoseptal flap has reduced the rates of cerebrospinal fluid leak that previously limited the use of the endoscope in the anterior skull base and parasellar region.
Comparison of endoscopic and microscopic techniques for similar pathologies remains limited because of the heterogeneity of provider techniques, the clinical features of each patient, and the institutional availability of endoscopic equipment.
The earliest use of the endoscope intracranially was reported by Victor D. Lespinasse in 1910 for the treatment of hydrocephalus. Dandy used the technique more extensively, initially for the treatment of hydrocephalus in infants and removal of the choroid plexus, which was limited by high perioperative mortality. Dandy became known as the father of neuroendoscopy. Interest in cranial endoscopy was driven by the lack of alternative treatments for hydrocephalus in the first half of the 20th century. Endoscopic techniques evolved from fulguration or obliteration of the choroid plexus to fenestration techniques involving the ventricles and subarachnoid planes. The first endoscopic third ventriculostomy was reported by Mixter in 1923. In 1934, Putnam demonstrated development of a coagulating ventriculoscope for choroid plexus cauterization in hydrocephalus. Limited development in the technology for endoscopes, in combination with the placement of the first shunt with a valve by Nulsen and Spitz in 1949, decreased the interest in cranial endoscopy. Improvement in the rod-lens rigid endoscopes, which were initially used for urologic procedures, offered improved illumination and resolution that enabled use for cranial surgery. ,
Gerard Guiot, a pioneer in intracranial and skull base endoscopy, performed the first endoscopic approach to a pituitary tumor in 1962, although he later abandoned the technique because it provided inadequate visualization. , , In 1973, Fukushima and colleagues introduced the modern endoscope, which could be used for the biopsy of intraventricular lesions, cyst fenestration, and treatment of hydrocephalus. Bushe and Halves were the first to report the use of a modern endoscope in pituitary surgery in 1978. , A few other reports emerged in the 1970s describing the use of an endoscope as an adjunct to the microscope in transsphenoidal approaches.
Application of the endoscope to surgery in the sella turcica did not become popular until the mid-1990s, when endoscopic sinus surgery had virtually replaced open techniques by otolaryngologists and functional endoscopic sinus surgery was advanced before being adapted for skull base surgery. , Jankowski and associates reported experience with a purely endoscopic transsphenoidal approach for 3 cases of sellar lesions. Sethi and Pillay, a combined otolaryngology/neurosurgery team, used an endonasal transnasal/transseptal approach, while Rodziewicz and coworkers described a septum-saving endonasal approach. Jho and Carrau, a neurosurgeon and an otolaryngologist, helped to pioneer modern endoscopic endonasal approaches for the treatment of pituitary adenomas. Cappabianca and de Divitiis helped to further the development of endoscopic instrumentation and technical innovations in endoscopic approaches, coining the term functional endoscopic pituitary surgery. Frank and Pasquini, another neurosurgery/otolaryngology team, developed the ethmoid-pterygoid-sphenoid endoscopic approach for resection of cavernous sinus lesions. The neurosurgeon Kassam and otolaryngologists Carrau and Snyderman further expanded the role of endoscopic skull base approaches and use of the Haddad-Bassagasteguy nasoseptal flap for prevention of cerebrospinal fluid (CSF) leak. Modern endoscopic cranial surgery developed from the growth in popularity of minimally invasive surgery through keyhole approaches in combination with the development of purely endoscopic endonasal approaches to the skull base.
Endoscopes are generally classified as either rod-lens endoscopes or fiberoptic endoscopes (fiberscopes), according to the technology used. Rod-lens endoscopes transmit images through a series of lenses and are always rigid. They provide a clearer image and better illumination than do fiberscopes. Fiberscopes transmit images through fiberoptic threads and can be maneuvered without image distortion. The resolution of fiberscopes is proportional to the number of fibers in the endoscope. Because of the nature of optic fibers, which may be flexed without breaking, fiberscopes can be fixed or flexible. However, rigid fiberscopes allow the presence of more pixel fibers than do flexible or steerable fiberscopes.
Various fiberscopes have been used in neurosurgery. Flexible fiberscopes have the smallest diameter, and one can be used as a stylet within a ventricular catheter. They do not have a working channel but are appropriate for visualizing catheter placement and ensuring an intraventricular position during the treatment of hydrocephalus. With a steerable fiberscope, the tip of the endoscope can be bent in varying degrees. A working channel is present, and its orientation is adjustable, which enables the instruments to reach all of the structures visualized. Such devices have been utilized in choroid plexus cauterization with endoscopic third ventriculostomy procedures, bronchoscopy, and procedures of the nasopharynx. The diameter of the fiberscope varies with the number of optic fibers; the larger fiberscopes provide better image quality. Rigid fiberscopes are available in a variety of lengths and diameters. A working channel is present, but targets can be used only on a straight line from the bur hole. The quality of vision remains inferior to that of rod-lens endoscopes.
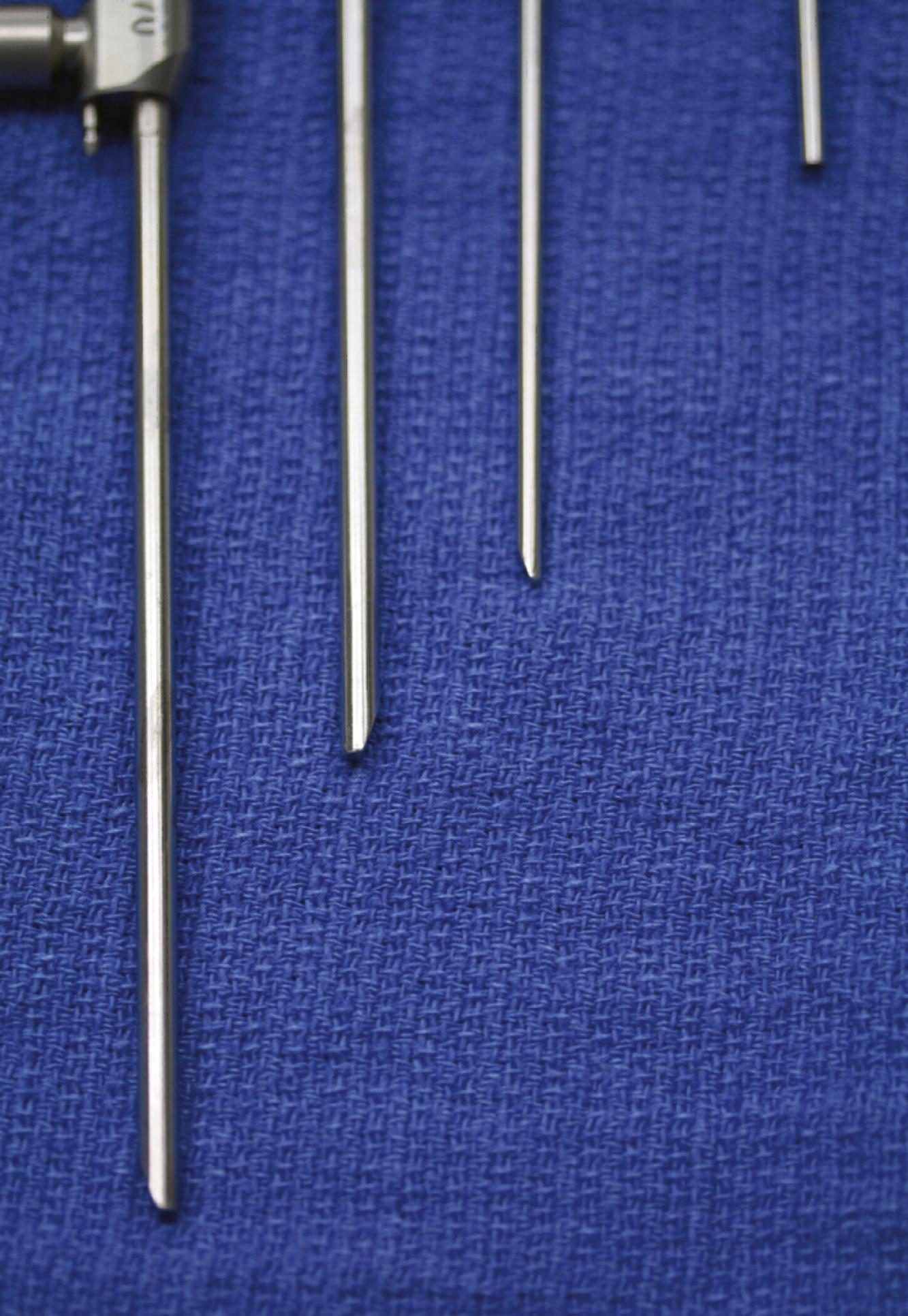
Rod-lens endoscopes are used overwhelmingly in cranial endoscopy because of the superior quality of the image obtained. They are heavier because of the mandatory attachment of the camera and fiberoptic cable for the light source. Assorted viewing angles are available (e.g., 0-, 30-, 45-, 70-, and 120-degree angled endoscopes; Fig. 34.1 ). Endoscopes range between 3 and 6 mm in size, depending on the manufacturer and lens used. Certain types of rod-lens endoscopes have up to two working channels through which instrumentation can be used. These working channels can also be used in addition to a trocar sheath, which enables the surgeon to use endoscopes with different viewing angles without the need to reinsert them through brain tissue. Different sheaths are available with one to multiple channels for inserting instruments, providing irrigation, or supplying suction. Instrumentation in the forms of varying forceps, scissors, suction, or coagulation has been developed specifically to work with these endoscopes. The 0- and 30-degree endoscopes are the most widely used. The 0-degree endoscope minimizes the risk of disorientation, but the view through instruments inserted through the working channel remains in the periphery of the field of vision. The 30-degree endoscope allows for a better control of the instruments and, with simple rotation, provides an angle of view with a surface area twice as large as that provided by a 0-degree endoscope.
The optimal use of the endoscope requires the use of a light source combined with a camera and monitor. , Halogen, mercury vapor, and xenon light sources are available. The light source is connected to the endoscope via a fiberoptic cable, and its intensity can be modulated. Xenon light sources provide the best illumination for neuroendoscopy. The camera is connected to the endoscope via an adapter and transmits the image to a video monitor for viewing by the rest of the surgical team. Cameras are available as a single-chip charge-coupled device (CCD) or a three-chip CCD. Most systems use a single-chip CCD because of its lower cost and lighter weight, despite lower image quality. A monitor with the highest possible quality should be selected, but its resolution should not exceed that of the camera. The size of the screen should not be excessive, as a larger screen limits the quality of the image obtained. The loss of quality of the image is most pronounced in screens exceeding 33 cm and when a fiberscope is used. Irrigation is useful in optimizing visualization and should be used in clearing debris from the lens. Lactated Ringer solution is the substance of choice for irrigation.
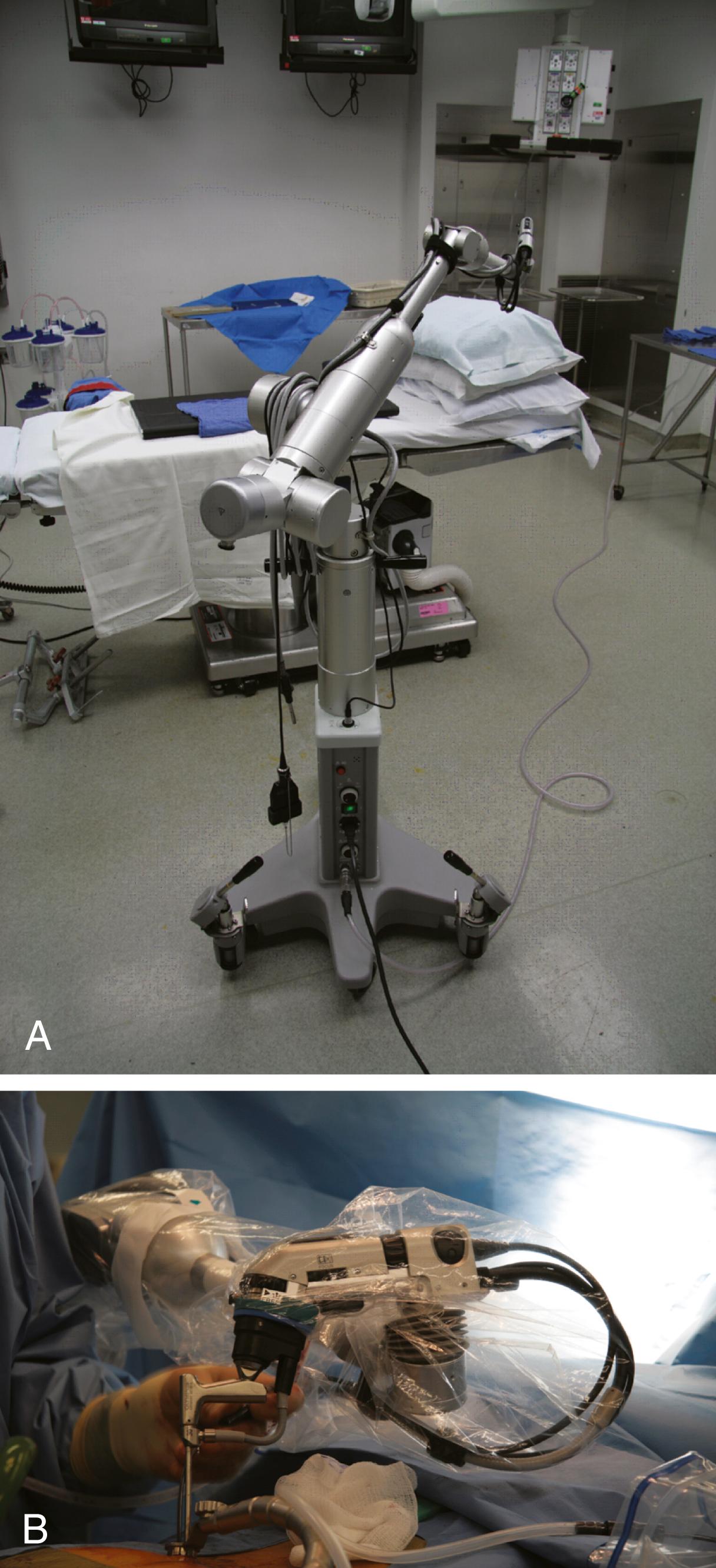
Rigid endoscope holders may increase the surgeon’s comfort during lengthy procedures, but they restrict the surgeon’s freedom of movement. Holders have been developed with a combination of pneumatic and electromagnetic brakes, combining the advantages of freehand movements with the possibility of very secure and firm positioning. Most endoscope holders need continuous manual adjustment at each of their joints, which limits their usefulness. They are usually mounted directly to the side of the operating room table, which limits the endoscope’s range of movement. One type of device is the Olympus EndoArm (Olympus, Melville, NY), a pneumatic endoscope holder mounted on its own base ( Fig. 34.2 ). The EndoArm consists of an arm with several ball-and-socket joints that enable movement in all planes. Movement of the joints in either direction is controlled by a single button, which gives the surgeon more fluidity in moving the endoscope. Regardless of current advances, a recent survey of 52 international surgeons demonstrated that most (54%) do not use an endoscopic holder because of deficits such as crude movements, downward drift, loss of depth perception, lack of flexibility, iatrogenic injury, cost, and bulky construction.
Cranial endoscopy was first used in the setting of hydrocephalus before the advent of shunt systems, when the condition was commonly fatal. Interest in the use of endoscopy has resumed because of the high rate of long-term morbidity associated with the use of shunts, most commonly shunt malfunctions and infections. In some cases, to avoid placement of a shunt system, the endoscope can be used to perform a third ventriculostomy with or without choroid plexus cauterization. Third ventriculostomy has become an important part of the treatment of hydrocephalus, and its long-term success has varied greatly, depending on the cause of hydrocephalus. Most long-term studies cite success rates of 65% to 75% for third ventriculostomies in the treatment of hydrocephalus, with scoring systems designed to predict outcome. Endoscopy for the treatment of hydrocephalus can also be performed in patients of a very young age, including newborns.
The endoscope can also be used as an adjunct to a shunt technique to localize proper placement of a ventricular catheter. The endoscope has been used extensively in cases of multiloculated hydrocephalus to lower the high rates of shunt infections and revisions needed in the setting of multiple shunts. One goal of endoscopic surgery in multiloculated hydrocephalus is to fenestrate fluid pockets into uniloculated hydrocephalus that may necessitate fewer shunts. , Fenestration of the septum pellucidum with the use of an endoscope may be performed to treat an isolated lateral ventricle , ; in some cases, fenestration of multiple intraventricular membranes may be required, , as may aqueductoplasty or stent placement in cases of fourth ventricular outlet obstruction. ,
Endoscopic approaches for colloid cysts were described as early as the 1980s. Complete lesion resections have been described more commonly in cases of colloid cysts than for other types of intraventricular tumors. Colloid cysts are successfully treated in 60% to 90% of cases, and such treatment seems to carry lower morbidity rates than does open craniotomy; however, few endoscopic series have reported any long-term follow-up past 5 years. One meta-analysis showed that endoscopic resection of colloid cysts had a higher rate of incomplete resection, cyst recurrence, and reoperation than did microsurgical resection. This may be because bimanual microdissection of the cyst wall from the choroid plexus attachment and internal cerebral vein cannot be achieved with the endoscopic technique.
Endoscopic resection of intraventricular tumors has been well described as an alternative to open microsurgical resection for a variety of cases. Tumors that have been described as suitable for successful endoscopic resection have included subependymal giant cell astrocytoma, exophytic low-grade gliomas extending into the ventricles, central neurocytoma, small choroid plexus tumors, and intraventricular craniopharyngiomas. Factors favorable for complete tumor resection include a soft tumor consistency, tumor diameter of less than 2 cm, moderate to low vascularity, associated hydrocephalus, histologically low-grade tumor, and tumor location in the third or lateral ventricle. Tumor resection can be time consuming because it is performed in a piecemeal manner through the endoscope’s working channel, which in most endoscopes is limited to 2.4 mm. The endoscope can be used effectively in treating intraventricular mass lesions, such as thalamic gliomas and lesions of the pineal region, especially when they cause hydrocephalus. , , In pineal tumors, the endoscope can be used to obtain samples of CSF for tumor markers and cytologic study, to inspect the intraventricular cavities to detect gross intraventricular nodules not visible on magnetic resonance imaging, to obtain a biopsy specimen of the tumor, and to perform a third ventriculostomy to address the hydrocephalus. Good results in controlling hydrocephalus have been obtained. Advantages of the endoscopic approach over a stereotactic needle biopsy include direct visualization of the tumor, a larger biopsy specimen, and the ability to stop the bleeding. , Endoscopy can also be used as a palliative measure to treat the cystic components of certain inoperable tumors and to implant an Ommaya reservoir in locations more remote from the ventricular system.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here