Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
During stress response, the central nervous system (CNS) induces activation of both the sympathoadrenergic system (by release of catecholamines) and the hypothalamic-pituitary-adrenal (HPA) axis (by release of steroid hormones, glucocorticoids [GCs], and mineralocorticoids), with the target of maintaining homeostasis by influencing metabolic, cardiovascular, immunologic, and endocrine functions. In this context, the adrenal gland plays a key role, combining the location for synthesis and expression of catecholamines, GCs, androgenic hormones, and factors of the renin-angiotensin-aldosterone (RAA) system. Acute and chronic inflammatory diseases include stimulation of the HPA axis by the immune system, thereby leading to morphologic and functional changes, especially of the adrenal cortex. This phenomenon has been described for acute infectious diseases and for sepsis and septic shock.
Over 60 years ago, the seminal observation was made that administering an adrenal cortical steroid extract to a patient with progressive, active rheumatoid arthritis slowed progression of the disease. This soon led to the development of synthetic adrenal cortical steroids, which gained a remarkable reputation in the treatment of a wide range of inflammatory and autoimmune disorders. However, it soon became apparent that this efficacy did not come without a cost in terms of potentially serious adverse effects. In patients with sepsis and septic shock, negative results of trials with high doses of GCs evoked skepticism over the years. Meanwhile, several randomized trials revealed contradictory results with low doses of corticosteroids in patients with septic shock. Hence, there is still controversy about which patients profit best from this therapy and how to define and evaluate adrenal gland disorders.
The two paired adrenal glands are located in the retroperitoneal soft tissue near the top of each kidney. In neonates, the adrenal glands are relatively large (approximately one-third of the kidney’s size) compared with other organs. In the postnatal period, the cortex portion shrinks, leading not only to a relatively but also an absolutely smaller size of the organ. In adults, each adrenal gland weighs 4–5 g, has a flat form with a sagittal diameter of less than 1 cm, a transverse diameter of 3 cm, and a craniocaudal diameter of 4–5 cm. The right gland has a triangle/pyramid-like shape, whereas the left gland has a half-moon shape.
Circulatory supply to the adrenals, with a flow rate of about 5 mL per minute, is maintained by up to 50 arterial branches from the aorta, renal arteries, and inferior phrenic arteries for each gland. Blood flow is directed from the capsule into the subcapsular arteriolar plexus through the cortex toward the medulla, where a single vein drains the blood entering the vena cava or the renal vein. Direct blood supply to the medulla is maintained by medullary arteries.
The adrenal cortex receives afferent and efferent innervation. Direct contact of nerve terminals with adrenocortical cells has been suggested, and chemoreceptors and baroreceptors present in the adrenal cortex infer efferent innervation. Diurnal variation in cortisol secretion and compensatory adrenal hypertrophy are influenced by adrenal innervation. Splanchnic nerve innervation has an effect in regulating adrenal steroid release. The adrenal medulla secretes the catecholamines epinephrine and norepinephrine, both of which affect blood pressure, heart rate, sweating, and other activities regulated by the sympathetic nervous system. The adrenal cortex is divided into three layers: (1) the zona glomerulosa, just under the capsule; (2) the zona fasciculata, the middle layer; and (3) the zona reticularis, the innermost, netlike patterned area with reticular veins draining into medullary capillaries. The zona glomerulosa exclusively produces the mineralocorticoid aldosterone; the zonae fasciculata and reticularis produce GCs and androgens.
The adrenal glands are part of a complex system that produces interacting hormones to maintain physiologic integrity, especially during the stress response. , This system, the HPA axis, includes the hypothalamic region that produces corticotropin-releasing hormone (CRH), which stimulates the pituitary gland. The pituitary gland is composed of two major structures: the adenohypophysis (anterior pituitary) and neurohypophysis (posterior pituitary). The anterior pituitary is responsible for the secretion of corticotropin (adrenocorticotropic hormone [ACTH]), thyroid-stimulating hormone (TSH), growth hormone (GH), beta-lipotropin, endorphins, prolactin, luteinizing hormone (LH), and follicle-stimulating hormone (FSH). The posterior pituitary secretes vasopressin (antidiuretic hormone [ADH]) and oxytocin. Corticotropin regulates the production of corticosteroids by the adrenal glands. Hypothalamic neurons receive input from many areas within the CNS; they integrate these inputs and initiate an output to the anterior pituitary via the median eminence. The median eminence secretes releasing hormones into a hypophyseal portal network of capillaries that connect the median eminence with the pituitary hormones.
The anterior pituitary gland secretes adrenocorticotropin (ACTH) under stimulation from hypothalamic CRH. ACTH, in turn, stimulates the synthesis and release of GCs, mineralocorticoids, and androgenic steroids from the adrenal gland. In terms of a feedback loop, ACTH release is inhibited by GCs, which act on both the pituitary corticotropic cells and hypothalamic neurons. ACTH is also released during stress, independent of the circulating serum cortisol level. CRH, vasopressin, and norepinephrine act synergistically to increase ACTH release during stress. Endorphinergic pathways also play a role in ACTH regulation. Acute administration of morphine stimulates release of ACTH, whereas chronic administration blocks secretion of ACTH. ACTH and cortisol are secreted normally in a diurnal pattern, with the lowest concentrations between 10:00 pm and 2:00 am and highest levels around 8:00 am. Samples obtained at different times can provide useful dynamic information regarding HPA function. Loss of diurnal rhythm may indicate hypothalamic dysfunction.
The HPA axis is stimulated not only by physical or psychic stress but also by peptides such as ADH and cytokines. Thus the HPA axis plays an important role during infections and immunologic disorders. , Via interaction with the RAA system regulating fluid and salt balance, synthesis of androgens (e.g., dehydroepiandrosterone) with a possible impact on immunomodulation, and the sympathoadrenergic system, the HPA axis is probably the most important organ of the stress response. Stimulation of the immune system by infections induces the release of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, or IL-6. After a cascade, these cytokines stimulate both the hypothalamus and the anterior pituitary gland, which ultimately leads to the release of GCs. IL-6 is also able to induce a steroid release directly from the adrenal gland. The adequate increase of GC levels during inflammation is a crucial factor for an appropriate stress response. In acute infections, this release maintains metabolic and energy integrity. If the process is chronic, the HPA axis develops an adaptation, which induces typical clinical manifestations such as hypercatabolic states; hyperglycemia; and suppression of androgens, growth, and thyroid hormones. These changes, however, may increase the risk of secondary infections. Increased cortisol levels suppress higher regulatory levels of the HPA axis in terms of a negative feedback loop. Hence, after major surgery or during sepsis and septic shock, high cortisol and low ACTH levels are detectable. , Even the infusion of dexamethasone or CRH is not able to suppress increased cortisol levels in these patients. , Several investigations have demonstrated that adrenal cortisol synthesis in critically ill patients is not regulated by ACTH, but by paracrine pathways via endothelin, atrial natriuretic peptide, or cytokines such as IL-6. IL-6 directly induces the adrenal cortex to release cortisol, which, in chronic courses, can worsen the prognosis.
Cortisol, the major free circulating adrenocortical hormone, is a hydrophobic hormone and circulates in the bloodstream bound to protein. Cortisol-binding globulin (or transcortin)–protein complexes account for about 95% of circulating cortisol, but only the free form is biologically active with a plasma half-life of 60–120 minutes. Cortisol is metabolized by hydroxylation in the liver, and metabolites are excreted in the urine. Steroid hormones enter the cytoplasm of cells, where they combine with a receptor protein. Metabolic, immunologic, and hemodynamic responses to adrenocortical steroid hormones are regulated in a highly complex manner that includes transactivation, transcription, posttranscriptional/translational regulation, and nongenomic effects. The immediate nongenomic effects of steroid hormones are primarily attributed to mineralocorticoids (aldosterone), with rapid activation of the sodium-proton exchanger, increase in intracellular Ca ++ levels, and activation of second messenger pathways. , A randomized trial in patients during cardiac catheterization revealed that within minutes after aldosterone injection, cardiac index and arterial pressure increased significantly for 10 minutes and returned to baseline thereafter. Interestingly, the genomic effects of aldosterone seemed to be mediated by binding to GC receptors (GRs) and not to mineralocorticoid receptors. There is evidence that GC, like cortisol, also modulates immune functions by rapid, nongenomic effects via nonspecific interactions with cellular membranes and specifically binding to membrane-bound GRs. Nonspecific membrane effects have been demonstrated for inhibition of sodium and calcium cycling across plasma membranes by impairing Na + /K + -ATPase and Ca ++ -ATPase. Moreover, the rapid activation of lipocortin-1 and inhibition of arachidonic acid release after GC were independent of GR translocation. Finally, high-sensitivity immunofluorescence staining revealed membrane-bound GRs on circulating B lymphocytes and monocytes.
The multiple mechanisms by which GCs modulate cellular responses include mainly genomic pathways. Nongenomic effects are thought to account for immediate immune effects of high doses of GC, whereas membrane-bound receptors probably mediate low-dose GC effects. The classic model is that GCs bind to the cytoplasmic ligand-regulated GC receptor alpha (GRα), which is an inactive multiprotein complex consisting of two heat shock proteins (hsp90) acting as molecular chaperones, in addition to other proteins ( Fig. 136.1 ). Upon GC binding to GRα, a conformational change causes dissociation of hsp90, with subsequent nuclear translocation of GRα homodimers, binding of GRα to GC response elements (GREs) of DNA, and transcription of responsive genes (transactivation) such as lipocortin-1 and beta-2-adrenoreceptors. Alternatively, GRα may bind to negative GRE (nGRE) and repress transcription of genes (transrepression) such as pro-opiomelanocortin (POMC). More important, transrepression without direct binding of GRα to GRE by protein-protein interactions of GRα with transcription factors, nuclear factor kappa B (NF-κB), and AP-1 has been recognized as a key step by which GC suppresses inflammation. In turn, synthesis of TNF-α, IL-1β, IL-2, IL-6, IL-8, inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)-2, cell adhesion molecules, and growth factors is inhibited and apoptosis promoted. In addition, NF-κB repression may be mediated by GC-induced up-regulation of the cytoplasmatic NF-κB inhibitor, IκBα (see Fig. 136.1 ), which prevents translocation of NF-κB. Clinical investigations provide support for the presence of endogenous GC inadequacy in the control of inflammation and peripheral GC resistance. With GC treatment, the intracellular relations between the NF-κB and GRα signaling pathways change from an initial NF-κB–driven and GRα-resistant state to a GRα-sensitive one. However, data are conflicting and probably do not explain the early (<2 hours) suppressive effects of GC, but may account for the longer-term dampening effect of GC on inflammatory processes.
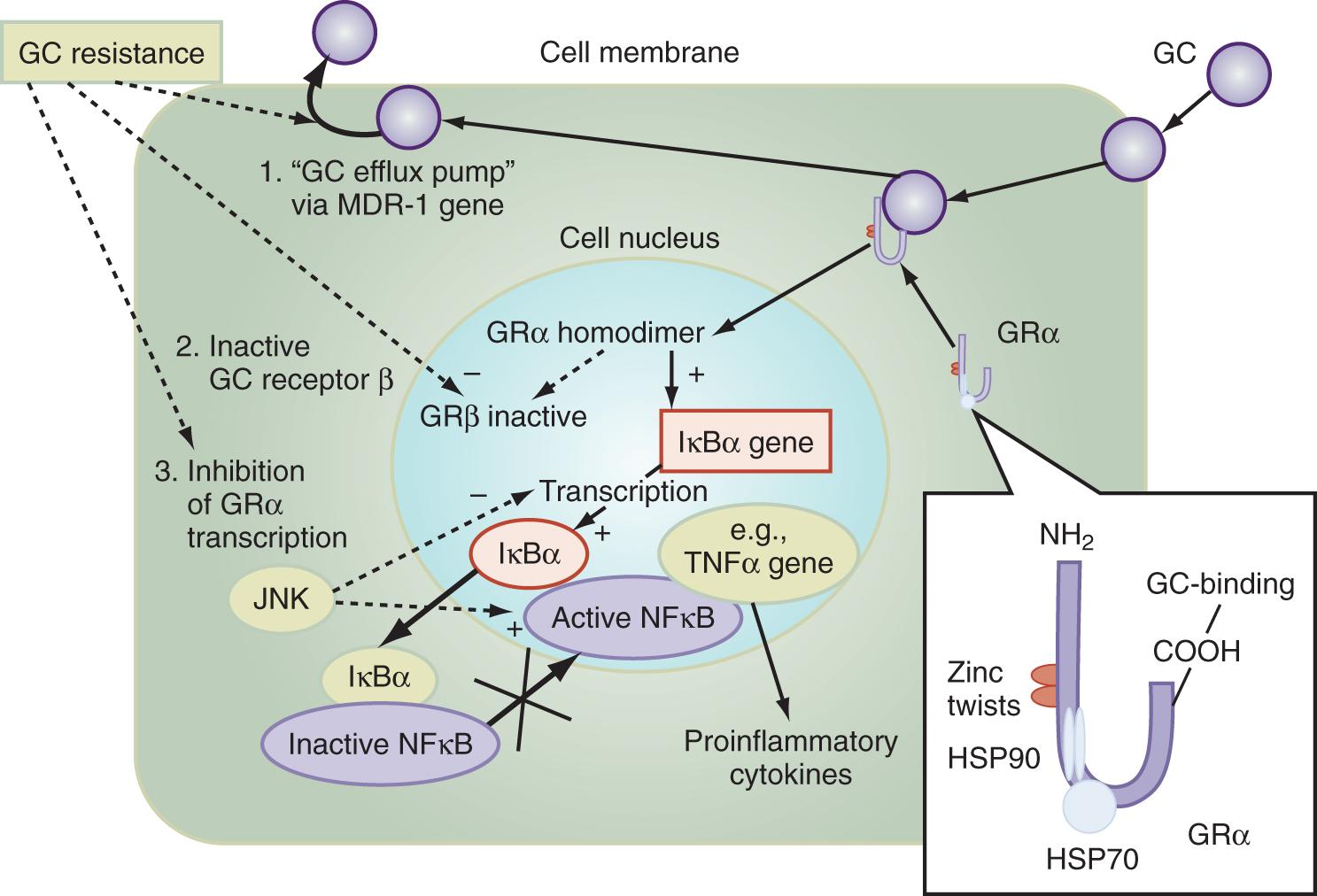
Besides transcriptional regulation, posttranscriptional, translational, or posttranslational processes have been described for GC-induced modulation of COX-2, TNF-α, GM-CSF, IL-1β, IL-6, IL-8, and interferon gamma (IFN-γ). Furthermore, GCs act at multiple levels to regulate iNOS expression via (1) decreased iNOS gene transcription and messenger RNA (mRNA) stability; (2) reduced translation and increased degradation of the iNOS protein by the cysteine protease, calpain ; (3) limitation of the availability of the NOS cofactor, tetrahydrobiopterin; (4) reduced transmembranous transport and de novo synthesis of the NOS substrate, l -arginine; and (5) lipocortin-1–induced inhibition of iNOS. , Together, these complex mechanisms result in the ability of GC to inhibit inflammation and to stabilize hemodynamics. Finally, GRs have been found in nearly every nucleated cell in the body, and because each cell type has specific responses to GC, it follows that GCs have many effects in the body, equally true of endogenously produced GC hormones or exogenously administered GC medications. Both increase hepatic production of glucose and glycogen and decrease peripheral use of glucose. Steroids also affect fat and protein metabolism. They increase lipolysis both directly and indirectly by elevating free fatty acid levels in the plasma and enhancing any tendency toward ketosis. GCs further stimulate peripheral protein metabolism, using the amino acid products as gluconeogenic precursors.
The adrenal glands may stop functioning when the HPA axis fails to produce sufficient amounts of the appropriate hormones. Primary adrenal insufficiency is defined by the inability of the adrenal gland to produce steroid hormones even when the stimulus by the pituitary gland via corticotropin is adequate or increased. Primary adrenal insufficiency affects 4–6 out of 100,000 people. The disease can strike at any age, with a peak between 30 and 50 years, and affects males and females about equally. In 70% of cases, the cause is a primary destruction of the adrenal glands by an autoimmune reaction (“classical” Addison disease or autoimmune adrenalitis), with about 40% of patients having a history of associated endocrinopathies. Most adult patients have antibodies against the steroidogenic enzyme, 21-hydroxylase, but their role in the pathogenesis of autoimmune adrenalitis is uncertain. In the other 30%, the adrenal glands are destroyed by cancer, amyloidosis, antiphospholipid syndrome, adrenomyeloneuropathy, acquired immunodeficiency syndrome (AIDS), infections (e.g., tuberculosis, cytomegaly, fungi), or other identifiable diseases ( Box 136.1 ). In these cases, the typical morphologic changes of the adrenal cortex are atrophy, inflammation, and/or necrosis. In primary adrenal insufficiency, the whole adrenal cortex is involved, resulting in a deficiency of GCs, mineralocorticoids, and adrenal androgens. ,
Autoimmune adrenalitis (Morbus Addison), often with concomitant endocrinopathies
Hemorrhage (trauma, anticoagulants)
Infarction, thrombosis
Tumors
Infections (tuberculosis, cytomegaly, fungi, AIDS)
Amyloidosis, hemochromatosis, sarcoidosis
Congenital hyperplasias or hypoplasias
Congenital ACTH resistance
Adrenomyeloneuropathy
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here