Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute tubular injury (ATI) characterizes damage to renal tubular epithelial cells and is the leading cause of acute kidney injury (AKI) in hospitalized patients. ATI is defined by a sudden decline in kidney function from ischemic or toxic insults and can occur at any epithelial segment along the nephron but is typically most profound in the proximal tubule. Packed with mitochondria and dependent on oxidative phosphorylation, the proximal tubule is particularly vulnerable to injury and ultimately cell death. The term ATI has largely replaced the term acute tubular necrosis (ATN) as we understand more about pathways of damage to and regeneration of the renal tubular epithelium. Damage can result in necrosis but is often limited to highly susceptible areas such as the outer medullary region largely due to its relative lack of oxygen supply. Damage can also result in cellular repair and apoptosis more broadly within the proximal tubule. This chapter will focus on the major ischemic and nephrotoxic mechanisms of acute tubular injury.
The renal tubular epithelium becomes susceptible to ischemic injury in pathologic states when autoregulation of blood flow and perfusion pressure to the renal microvasculature is disturbed. Pathologic microvascular ischemia manifests on a continuum between functional changes in glomerular filtration, often referred to as prerenal injury , to direct tubular cell injury, often referred to as intrarenal injury . The process is often patchy and does not occur in an all-or-none fashion due to normal variations in regional blood flow and differences in energy and oxygen consumption along the nephron. Modern classifications of tubular injury have attempted to move away from the pre-, intra-, and postrenal definitions, instead opting to classify cases based on changes in functional and damage biomarkers of kidney injury.
The point at which microvascular ischemia causes direct cellular injury rather than just functional change in glomerular filtration (as measured by serum creatinine or cystatin C) is incompletely understood. Generally, the more severe the perfusion defect, the greater the injury is at the cellular level. A number of comorbidities, including sepsis, chronic kidney disease (CKD), hypertension, and atherosclerosis, as well as a kidney’s reserve capacity to hyper-filter, will lower the threshold at which cellular injury begins to occur. There are numerous causes of renal microvascular ischemia that fall into two general categories. The first is hypotension-induced ischemia, resulting from decreased systemic arterial pressure and subsequent end-organ hypoperfusion. The second is localized obstruction or constriction within the renal vasculature that results in ischemia in downstream tissues. From the second category, this chapter will cover cholesterol atheroembolic kidney disease and kidney infarction.
Renal microvascular ischemia often results from systemic disorders that cause decreased arterial pressure and subsequent end-organ hypoperfusion. These include septic shock, cardiogenic shock, hypovolemic shock, autonomic dysfunction, and iatrogenic sources.
Septic shock creates a particularly pathologic environment for the kidney. Historically, well-recognized organ dysfunction from hypoperfusion and tissue hypoxia plays an important role. More recently, the role of inflammatory cytokines has been recognized as a critical additional mechanism of injury. The inflammatory milieu is thought to create variation in renal macro- and micro-circulation, diffusion limitation, disruption of the endothelial barrier and glycocalyx, and mitochondrial dysfunction, all of which contribute to and perpetuate ATI. While large animal models suggest all of this can occur in the absence of hypotension, clinical correlation in humans is under-investigation. Human sepsis seems to be associated with ischemic and inflammatory injury to the tubular epithelium.
Acute decompensated heart failure creates two cardinal issues that lead to renal microvascular ischemia and tubular injury: significant compromise to renal blood flow and venous congestion. In cardiogenic shock, there is underfilling of the systemic arterial vascular bed, including the kidneys, which leads to pathologic compensatory increases in sympathetic nervous system and renin-angiotensin-aldosterone system activity. This leads to maladaptive renal microvascular vasoconstriction. In states of overt volume overload in the setting of decreased forward flow rather than frank cardiogenic shock, it is venous congestion and related increases in intraabdominal pressure that drive hydraulic alterations in microvascular blood flow that result in ischemic injury.
Hypovolemic shock commonly occurs secondary to significant volume loss in the setting of diuresis, hemorrhage, vomiting, or diarrhea. Markedly reduced oncotic pressure from low albumin states such as cirrhosis, nephrotic syndrome, and protein-losing enteropathies can also result in severe intravascular volume depletion, despite an excess of total body fluid. Signs and symptoms of organ dysfunction do not typically manifest clinically until approximately 20% to 25% of effective arterial blood volume has been removed. Hypotension related to autonomic dysfunction is most commonly seen in the setting of diabetes mellitus; however, it is also associated with liver disease, Guillain-Barré syndrome, cerebral vascular accidents, dementia, and other processes.
Iatrogenic causes of hypotension include medications, such as excessive exposure to antihypertensive agents, antiarrhythmics, narcotics, and sedatives. Medications that interfere with the autoregulation of renal blood flow can also contribute to or exacerbate tubular injury from other primary causes. Nonsteroidal antiinflammatory drugs (NSAIDs) can reduce blood flow through relative constriction of the afferent arteriole and can impair medullary blood flow in prostaglandin-dependent patients. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) can reduce GFR by altering intrarenal hemodynamics through efferent arteriolar dilation, and could thereby perpetuate ischemia from other causes. This potential mechanism is not fully understood and there is no consensus that discontinuation of ACE inhibitors and ARBs improves outcomes in acute tubular injury, although there is mounting evidence that restarting ACE inhibitors and ARBs following an episode of AKI may improve short- and long-term outcomes.
Along with history and clinical suspicion, urinalysis and urine microscopy remain the mainstay of diagnosis of ischemic acute tubular injury. Laboratory studies in ATI reflect dysfunction of the proximal tubule and include a blood urea nitrogen-to-creatinine ratio of less than 20, fractional excretion of sodium greater than 2%, and fractional excretion of urea more than 50%. However, the fractional excretion of sodium (FENa) may be found to be less than 1% in several circumstances where there is a mix of functional change in glomerular filtration and tubular cell injury (e.g., presence of diuretics, exposure to radio-contrast or calcineurin inhibitors). As such, the clinical utility of the FENa in the differential diagnosis of AKI remains unclear. Other common features on urinalysis include isosthenuria with a specific gravity of 1.010 and urine osmolality less than 350 mOsm/kg. This latter biomeasure reflects impairment in distal tubule and collecting duct ability to effectively concentrate or dilute urine.
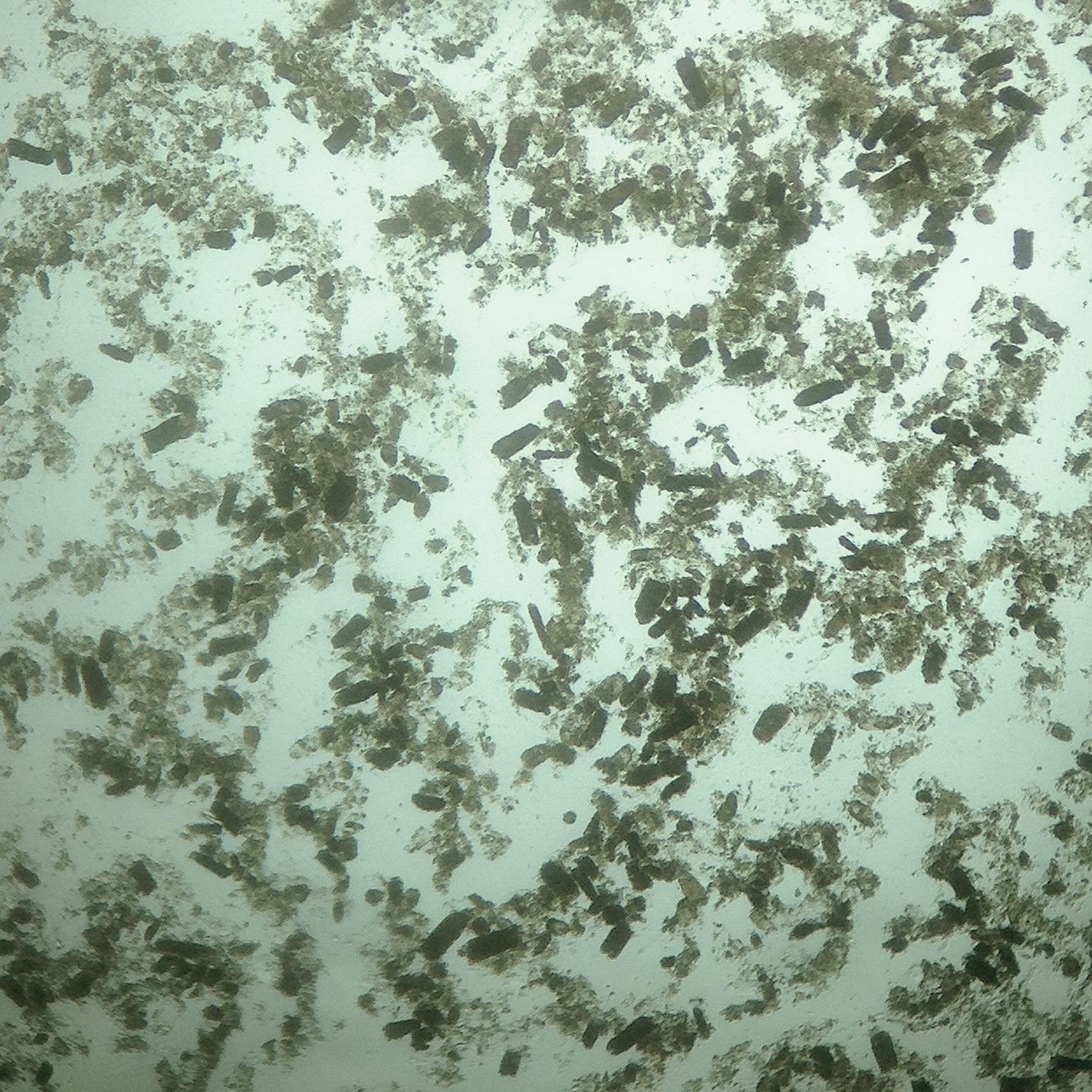
Manual urine microscopy provides crucial diagnostic and prognostic information. The classic finding of dense granular or “muddy brown” casts ( Fig. 32.1 ) is specific for the diagnosis of ATI. Fine granular casts, renal tubular epithelial cell casts, and renal tubular epithelial cells are also commonly found in ATI. While several urine sediment scoring systems have been published, there has been limited external validation of these scores. A urine sediment scoring system developed by Patel and colleagues from prospective clinical work had positive predictive value of 100% and negative predictive value of 91% for the diagnosis of ATI. Perazella and colleagues showed scores had prognostic significance by associating higher urine sediment severity scores with dose-dependent, increased relative risk for worsening acute kidney injury (higher AKI stage, dialysis requirement, or death).
Owing to the imprecision of many of the aforementioned ATI diagnostic tools, innovation in the detection and management of ATI is a large focus in current clinical research. Tissue inhibitor of metalloproteinases (TIMP2) and insulin-like growth-factor binding protein 7 (IGFBP7) are two biomarkers that have been combined in use commercially (NephroCheck, Biomerieux, France) in both the United States and Europe for detection and risk stratification of severe AKI. Prediction of moderate to severe post-CABG AKI (KDIGO stage 2 or higher) with this test has been extremely good with an area under the curve (AUC) of 0.80 to 0.90. In high-risk groups (postcardiac surgery, intensive care unit), detection of ATI using these biomarkers combined with deploying AKI care bundles has been shown in several investigations to improve patient outcomes (less severe AKI, shorter ICU stays, decreased cost of care). Internationally, other biomarkers have been approved for clinical use and several others remain under investigation, including neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and proenkelphalin (Penkid). Several of these markers have been shown to correlate with adverse patient outcomes (dialysis requirement, prolonged length of stay, inpatient mortality) even in the absence of changes in serum creatinine or urine output, leading to the concept of “subclinical ATI.” While the data supporting this concept are emerging, they have yet to be validated with prospective trials and human biopsy samples. In general, adoption of biomarkers has been somewhat slow, perhaps related to a sense that there is no targeted “therapeutic” for ATI. However, the positive findings from deployment of AKI care bundles (kidney-focused care aimed to optimize kidney perfusion, prevent volume overload, and avoid additional kidney stressors) as well as recent inclusion of biomarkers in perioperative cardiac surgery guidelines are expected to spur increased adoption in the near future.
Cholesterol atheroembolic kidney disease describes the result of disruption of atheromatous plaques within walls of arterial beds, which release cholesterol crystals that flow downstream and lodge in various blood vessels and organs including the kidney. This phenomenon leads to ischemic kidney injury due to vessel obstruction from cholesterol crystals and the subsequently provoked inflammatory response. Plaque disruption is generally iatrogenic from vessel cannulation for cardiac catheterization (aortic plaques) and various vascular procedures (including both open and closed vascular surgery). The incidence of this syndrome appears to have decreased substantially over time. Retrospective analyses and case series from the 1970s and 1980s report incidence rates as high as 25%-30%, but more recent retrospective and prospective analyses from the 1990s and early 2000s show incidence rates consistently less than 2%. Study heterogeneity complicates assessment, but it is felt that widespread statin use and less invasive modes of vessel cannulation and manipulation (radial artery approaches, advances in technology, and surgical technique including distal clot-catching devices) have had a significant effect in disease reduction.
The disease can present in one of three ways. The first is an acute process with fulminant AKI occurring within one week of the triggering event. This scenario is often the result of a large burden of cholesterol emboli with multi-organ involvement. Severity can range from catastrophic tissue necrosis characterized by severe (stage 3) AKI, GI bleeding, necrotic skin ulcerations, spinal cord infarction, and rapid death, to less severe presentations with abdominal pain, livedo reticularis, and acalculous cholecystitis. A second presentation is a subacute decline in kidney function, with AKI appearing several weeks after the initial insult. Kidney dysfunction occurs in a stepwise fashion, representing ongoing cholesterol crystal embolization. The third presentation is with a chronic or delayed course, in which significant kidney impairment may not be noted until up to 6 months after the trigger. These cases are likely under-recognized and typically attributed to other causes of CKD, such as hypertensive nephrosclerosis. Kidney biopsy, however, demonstrates the classic needle-shaped clefts within the intrarenal vasculature.
Laboratory test findings are often non-specific and can include anemia, leukocytosis, thrombocytopenia, and elevated inflammatory markers such as ESR and CRP. Eosinophilia and hypocomplementemia are more specific laboratory findings that have been described, but the negative predictive value of these markers is quite low. Urinalysis typically reveals a bland sediment with minimal proteinuria. Kidney biopsy is regarded as the definitive method for diagnosis. However, the benefit of biopsy may be limited as there are no definitive therapeutic options, with anticoagulation, steroids, or other specific treatments not having shown consistent benefit in improving patient outcomes (see therapy section below).
After entering the renal artery, cholesterol crystals typically settle within the arcuate and interlobular arterioles of the kidney, but they can reach the afferent arteriole and glomerular capillary as well. An inflammatory reaction ensues that is characterized initially by granulocyte infiltration and then mononuclear cell infiltration and giant cell formation. Endothelial proliferation occurs, which leads to intimal thickening and concentric fibrosis. Ultimately this process results in arteriole obstruction and ischemic infarction of downstream tissues, including the glomeruli, tubules, and interstitium. Histologically, cholesterol crystal emboli are identified in the lumen of arcuate and interlobular arteries as biconvex, needle-shaped, and empty clefts, referred to as ghost cells because they dissolve during specimen processing. The tissue damage caused by these crystal emboli is patchy. Glomerular and interstitial changes are mainly ischemic, with a varying extent of glomerular obsolescence and interstitial fibrosis. In the early phases, areas of acute tubular necrosis can be identified.
Primary prevention remains the most effective target at this time, with data extrapolated from the cardiac disease prevention literature as there are no formal clinical trials specifically looking at atheroembolic kidney disease. This includes aggressive management of underlying predisposing conditions (e.g., hypertension, hyperlipidemia, and tobacco use) and careful consideration of the risks and benefits of initiation or continuation of anticoagulant or antithrombotic therapy (as these may increase risk for plaque disruption) and of proceeding with intervention at all. Once atheroembolic disease develops, there are no rigorously studied therapies that have shown consistent improvement in kidney outcomes. Steroids have been used in severe cases with acute multi-organ involvement with limited success but are not currently thought to be beneficial more generally. Other experimental therapies such as low-density lipoprotein (LDL) apheresis have been used but have not been evaluated in controlled settings.
Few outcome studies have been done in patients with atheroembolic kidney disease. The data available suggest kidney prognosis is poor with as many as 40%–60% of patients requiring dialysis at the time of initial presentation, and 25% of patients remaining dialysis-dependent in the two studies with more long-term follow-up. Statin use has been associated with improved outcomes in retrospective analyses. Preexisting CKD, longstanding hypertension, and presence of eosinophilia have all been identified as poor prognostic markers.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here