Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Thoracic imaging plays a vital role in the management of intensive care unit (ICU) patients. The chest radiograph provides critical information regarding placement of life support devices, complications such as barotrauma, intravascular volume status, and a wide spectrum of underlying diseases. A systematic review of the life-support devices, lungs, pleura, mediastinum, bones, and extrathoracic soft tissues ensures that all important observations are made. To ensure radiographic interpretation is accurate, it is important to have as much clinical information as possible and to use the benefit of prior radiographs. Additional studies may be used to clarify a radiographic abnormality, including decubitus views, bedside ultrasonography, or computed tomography (CT).
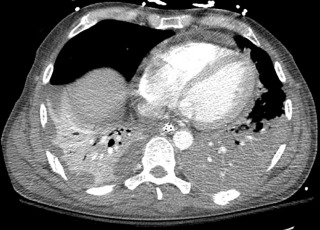
Atelectasis, a common finding in critically ill patients, represents areas of nonaerated lung that in the acute setting is often due to obstructive mucous plugging or nonobstructive relaxation atelectasis. The extent may vary from linear bands of subsegmental atelectasis to more extensive patchy opacification to lobar collapse. Radiographically, the appearance may be indistinguishable from pneumonia because both can demonstrate air bronchograms or manifest clinically with fever. However, atelectasis is usually basal and tends to have a left lower lobe predominance, particularly after cardiac surgery. Typically, atelectasis appears and resolves more rapidly than pneumonia and is associated with signs of volume loss, including fissural displacement, bronchovascular crowding, or hemidiaphragm elevation, among others. On contrast-enhanced CT, atelectasis may be differentiated from pneumonia as atelectasis manifests with intense enhancement owing to crowding of the vascular structures ( Fig. 10.1 ).
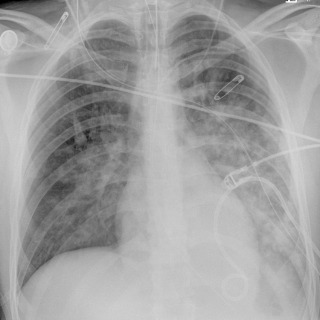
Patients with an altered level of consciousness are at risk for aspiration. The clinical and radiographic appearance is determined by the volume and nature of the aspirate and by the position of the patient. Aspiration occurs in the dependent regions of the lung; with the patient in a supine position, it involves the posterior segments of the upper lobes and the superior and posterior basal segments of the lower lobes ( Fig. 10.2 ). Importantly, aspiration is a common phenomenon in intubated patients, often occurring despite the presence of an inflated endotracheal balloon.
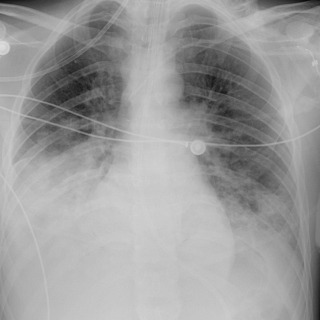
Three types of aspirates have been described with different radiologic outcomes. The aspiration of acidic gastric contents produces a chemical pneumonitis (Mendelson syndrome), resembling noncardiogenic pulmonary edema. The onset of symptoms occurs within minutes, and there may be associated bronchospasm, hypotension, or hypoxemia. Fever and leukocytosis are common. Clinical and radiographic resolution usually occurs within a couple of days, although some patients may progress to severe acute respiratory distress syndrome (ARDS). The aspiration of innocuous fluids such as blood or water is rarely clinically significant unless the fluid volume is large. The radiograph is typically normal, but the aspiration of food or oral pathogens may result in pneumonia with the typical appearance of persistent consolidation in the dependent regions of the lung.
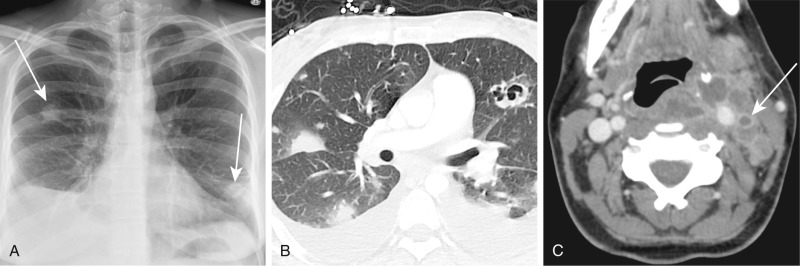
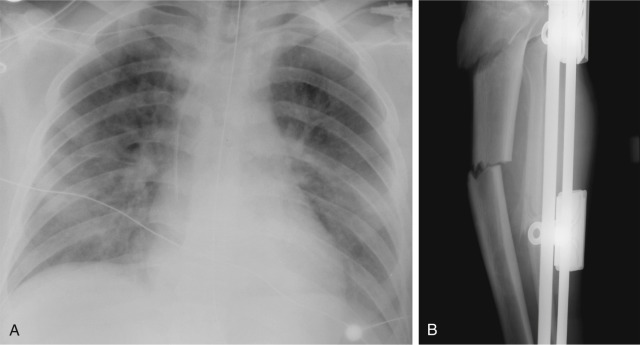
Pneumonia may be divided broadly into infection acquired in the community and infection acquired in hospital or nursing home patients, with the latter more often caused by multidrug-resistant bacteria. Health care–associated pneumonia (HCAP) is defined as pneumonia developing in a patient receiving health care services, including recent hospitalization, nursing home care, wound care, or dialysis. Hospital-acquired pneumonia (HAP) is defined as pneumonia manifesting 48 hours or later after hospital admission. HAP is typically caused by bacterial pathogens and is associated with high morbidity and mortality rates. It is the second leading cause of nosocomial infection, trailing only urinary tract infections. Ventilator-associated pneumonia (VAP) is defined as pneumonia manifesting at least 48 to 72 hours after endotracheal tube (ETT) intubation ( Fig. 10.3 ). VAP complicates the clinical course of up to 30% of ventilated patients, with a mortality rate as high as 80%.
The diagnosis of pneumonia is sometimes difficult in ICU patients but is defined by the presence of a new or progressive airspace opacity on the chest radiograph that is associated with purulent sputum, fever, or leukocytosis. However, fever and leukocytosis may be absent, or the white blood cell count may be elevated from a number of other causes. The radiographic appearance may be similar to that of atelectasis, pulmonary edema, pulmonary hemorrhage, or pulmonary infarct. Asymmetric pulmonary edema caused by underlying emphysema or asymmetric clearing of edema can also mimic pneumonia. In the presence of ARDS, the airspace opacification of diffuse alveolar damage may mask the appearance of pneumonia. The presence of positive sputum cultures may be misleading, reflecting colonization rather than infection. Colonization of the oropharyngeal tube and ETT by pathogenic bacteria occurs early, usually within 24 hours of intubation. The reliability of cultures from aspirated or expectorated tracheal sections is low, and protected brush catheter specimens obtained through bronchoscopy provide a more accurate diagnosis of a lower respiratory tract infection. Compared with radiography, the diagnosis of pneumonia is easier to make on contrast-enhanced CT, with pneumonia manifesting as areas of hypoattenuating and hypoenhancing lung parenchyma in contrast to densely enhancing lung in atelectasis (see Fig. 10.1 ). Other CT features helpful in the diagnosis of pneumonia include airspace nodules, tree-in-bud opacities, and nondependent consolidation. Complications of pneumonia include abscess and empyema and are more likely to occur with hospital or HCAP compared with community-acquired pneumonia.
Septic pulmonary emboli can originate from multiple extrapulmonary sources, including infective endocarditis, infected intravenous catheters, or thrombophlebitis. The classic appearance of septic emboli consists of peripheral, poorly marginated nodular or wedge-shaped opacities with or without cavitation, which may increase in number or change appearance from one day to the next. The nodules may manifest the feeding vessel sign . They may be seen on chest radiography but are best demonstrated on CT ( Fig. 10.4 ). When large, tricuspid or pulmonic valvular vegetations may be visible on CT.
Critically ill patients are likely to have risk factors for venous thromboembolism. In a study of medical intensive care patients, 33% developed deep venous thrombosis (DVT) despite prophylaxis, and most cases occurred within the first week. Approximately 10% of patients with DVT will develop pulmonary emboli, and 10% of these are fatal. Many thromboembolic episodes, however, are unrecognized. Autopsy studies of hospitalized patients report a prevalence ranging from 9% to 28%.
Pulmonary CT angiography has proved invaluable in the assessment of the dyspneic intensive care patient. CT angiography provides an accurate, noninvasive assessment of the pulmonary arteries by demonstrating even small peripheral emboli and allows identification of nonembolic causes of respiratory distress. Studies have shown that up to two thirds of patients with an initial suspicion of pulmonary embolism have an alternate diagnosis. The radiographic, scintigraphic, CT, and conventional pulmonary angiographic features of pulmonary thromboembolism are discussed in Chapter 11 .
Fat embolism is a rare complication of long bone fracture. Most patients are asymptomatic and may have normal radiographs, but a percentage go on to develop fat embolism syndrome (FES). FES is a symptom triad of lung, skin, and cerebral involvement that develops within 1 to 3 days of injury. The mechanism of injury is believed to be primarily due to an endothelial inflammatory reaction incited by the production of free fatty acids rather than mechanical vessel obstruction. Clinically, FES can be diagnosed by identifying fat in the urine. CT rarely demonstrates endoluminal fat filling defects but instead resembles diffuse alveolar damage of ARDS, including focal or diffuse consolidation, ground-glass opacity, and small (<10 mm) pulmonary nodules ( Fig. 10.5 ). The lag in appearance of radiographic abnormalities helps differentiate FES from pulmonary contusion, with the latter diagnosis typically manifesting as geographic airspace consolidation or ground-glass opacities within 24 hours of the inciting trauma. Unlike cardiogenic pulmonary edema, pleural effusions are often absent in FES.
Amniotic fluid embolism (AFE) is a rare pregnancy complication with a reported maternal mortality rate of 80%. Most cases begin during early labor but can develop up to 48 hours after delivery and result from uterine contractions forcing amniotic fluid into the bloodstream through small uterine vein tears. Other cases may occur during surgical or traumatic placenta disruption, including cesarean delivery, curettage abortion, and amniocentesis. Similar to FES, the pathophysiology of AFE is thought to be due to an inflammatory and anaphylactic reaction rather than mechanical vessel obstruction. Radiographic findings consist of diffuse bilateral heterogeneous opacities resembling pulmonary edema or diffuse alveolar damage.
Air embolism can be classified into two types: venous air embolism and arterial air embolism. Venous air embolism occurs when air is introduced into systemic veins and travels to the right ventricle and pulmonary arteries. Arterial air embolism occurs when air enters the pulmonary veins, left ventricle, and systemic arterial circulation. Air embolism is discussed in more detail in Chapter 11 .
Pulmonary edema is an abnormal accumulation of fluid in the extravascular compartments of the lung and is broadly divided into cardiogenic and noncardiogenic edema. Cardiogenic edema is caused by increased pulmonary capillary hydrostatic pressure, typically caused by left heart failure or fluid overload. In theory, when the pulmonary capillary wedge pressure (PCWP) rises to 12 to 18 mm Hg, pulmonary venous hypertension develops and results in pulmonary vascular engorgement. In chronic pulmonary venous hypertension, vascular redistribution may be seen on true upright chest radiographs where the upper lung zone pulmonary veins become larger than the lower lung zone pulmonary veins. As the PCWP increases to 18 to 24 mm Hg, interstitial edema develops and on radiography is characterized by vascular indistinctness or perihilar haze, peribronchial cuffing ( Fig. 10.6 ), subpleural edema, and septal thickening. Accumulation of fluid within the interlobular septa is seen as fine linear horizontal opacities extending to the pleural surface (Kerley B lines) ( Fig. 10.7 ) or as perihilar linear opacities that are longer and central (Kerley A lines). Further increases in the PCWP result in alveolar edema and may appear as the bat-wing pattern with bilateral perihilar opacities ( Fig. 10.8 ) or as diffuse consolidation. Although in theory a patient should progress from pulmonary venous hypertension to interstitial edema and eventually alveolar edema, the reality is that this sequence is often unpredictable. Pulmonary edema may also be asymmetric because of patient positioning or preferentially affect the right upper lung zone because of acute mitral insufficiency from papillary muscle rupture or severe mitral regurgitation of any cause ( Fig. 10.9 ). An atypical pattern and distribution of pulmonary edema may also be seen in emphysema or pulmonary fibrosis in which the pulmonary edema manifests in areas not destroyed by underlying lung disease ( Fig. 10.10 ). Associated findings in cardiogenic pulmonary edema include cardiomegaly, pleural effusions, and a widened vascular pedicle.
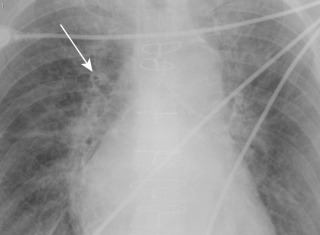
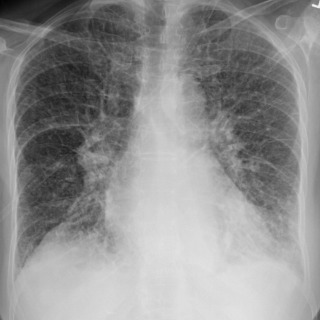
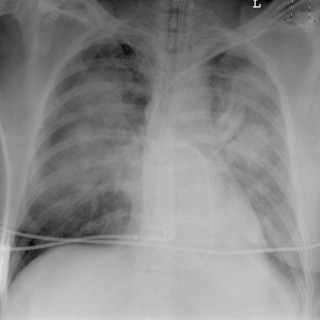
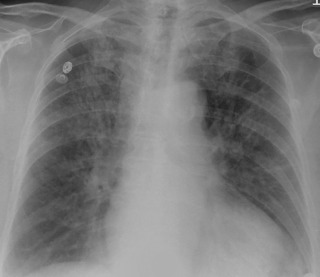
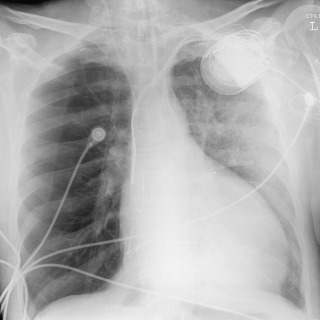
The vascular pedicle is the width of the superior mediastinum as measured from the right lateral border of the superior vena cava at the point at which it crosses the right main bronchus to the left lateral margin of the left subclavian artery as it arises from the aortic arch. A serial change in the width of the vascular pedicle reflects circulating blood volume and provides a useful assessment of the patient's intravascular volume status. In 95% of normal individuals, the vascular pedicle is between 38 and 58 mm wide. Because of the wide normal range, comparison of serial radiographs for an individual patient is more useful than an absolute measurement. However, similar radiographic positioning is required for accurate comparison, and portable supine radiographs are rarely directly comparable. With fluid overload, the vascular pedicle typically widens ( Fig. 10.11 ), but in cases of pulmonary edema from left heart failure, only half of patients have a widened vascular pedicle. Patients with permeability pulmonary edema have a normal vascular pedicle.
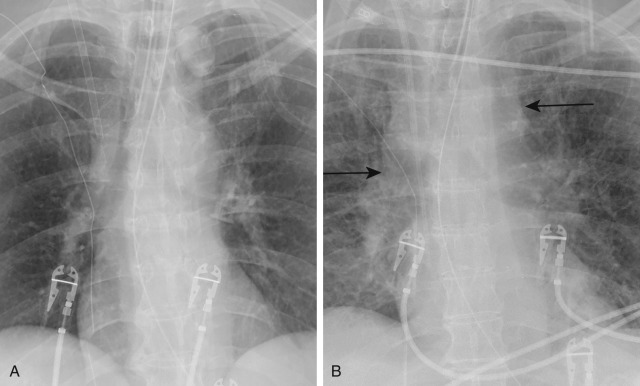
The posttherapeutic lag phase describes the discrepancy between the improving PCWP and the lack of radiographic resolution of edema. Even after the wedge measurements have returned to normal and the patient's symptoms have improved, it may still take hours or days for the reabsorption of large amounts of extracellular fluid and therefore clearing of the radiographic abnormality. This phenomenon is frequently seen in patients with left-sided heart failure.
Noncardiogenic edema is caused by increased capillary permeability or decreased plasma oncotic pressure and can be seen in multiple settings, including central nervous system injury, near drowning, high-altitude pulmonary edema, renal impairment, drug use, lung reexpansion, blood transfusion, postpneumonectomy, and diffuse alveolar damage. The radiographic appearance may be indistinguishable from cardiogenic edema, although cardiomegaly and pleural effusions are less common in noncardiogenic causes of edema. Diffuse alveolar damage (DAD) results from injury to the alveolar capillaries and epithelium. DAD is absent in some cases of noncardiogenic edema caused by drugs (e.g., heroin) but may be severe in other cases. The degree of DAD influences the time course of the radiographic abnormality: When DAD is absent, the radiographic findings are likely to be transient, but severe DAD may result in permanent lung damage.
Acute respiratory distress syndrome is a clinical diagnosis of acute respiratory failure characterized by profound hypoxia associated with a chest radiograph demonstrating widespread pulmonary opacification with air bronchograms ( Fig. 10.12 ). The risk factors for developing ARDS include multiple trauma, fat emboli, sepsis, severe pneumonia, aspiration of gastric contents, and multiple transfusions. Patients often have more than one risk factor. The initiating event may occur hours or days before, as with sepsis or fat emboli, or ARDS may develop acutely, as after gastric aspiration. The resultant DAD causes a generalized permeability defect that produces noncardiogenic pulmonary edema. Acute lung injury (ALI) is on the same spectrum as ARDS. Both involve acute onset, bilateral radiographic abnormalities, and absence of left atrial hypertension, but they differ in the degree of hypoxemia, with ARDS being the most severe form of disease. Although ALI has a ratio of arterial oxygen to the fraction of inspired oxygen (PaO 2 /FiO 2 ) of 300 mm Hg or less, ARDS is defined by a ratio of 200 mm Hg or less.
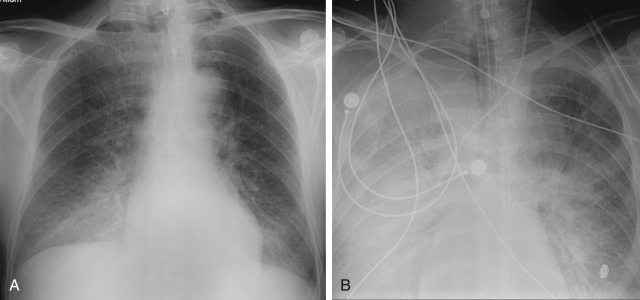
Acute respiratory distress syndrome is traditionally divided into three phases: exudative, proliferative, and fibrotic. The exudative phase involves the leaking of circulating fluid into the interstitium and alveolar space. Initial imaging may be normal, with eventual development of interstitial and subsequent alveolar edema. Typically, in acute exudative-stage ARDS, the supine radiograph demonstrates extensive airspace opacification, which appears to symmetrically involve both lungs, preferentially involving the lung periphery ( Fig. 10.13, A ). CT reveals the lung involvement as heterogeneous, with a mixture of ground-glass opacity and normal aerated lung anteriorly and areas of dense consolidation dependently. In addition to the ventral–dorsal gradient, the abnormal density of the lung increases in a cephalocaudal direction ( Fig. 10.13, B ). The parenchymal pattern represents the generalized capillary leak (i.e., ground-glass opacification), the local lung injury (i.e., consolidation), and the dependent atelectasis from a prolonged supine position. Dense, asymmetric consolidation predominates in patients with direct injury to the lung, such as pneumonia or aspiration (see Fig. 10.12 ), and ground-glass opacification (i.e., permeability edema) is the predominant finding in patients with alveolar damage from an extrapulmonary insult such as sepsis or hypotension. The “crazy paving” pattern of ground-glass opacities and interlobular septal thickening may also be seen on CT, although this is not specific for ARDS. In contrast to cardiogenic and hypervolemic edema, the vascular pedicle is not widened, the heart is not enlarged, pleural effusions are small or absent, and there is no upper lobe vascular redistribution. When visualized, the upper lobe vessels are constricted rather than dilated. The lung volumes are reduced, but because of mechanical ventilation, this may not be apparent.
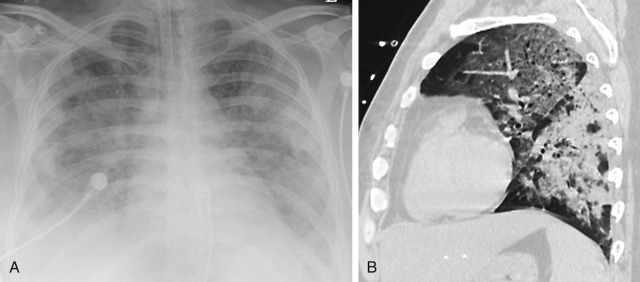
The second, proliferative, phase of ARDS occurs on days 3 to 10 after the initial insult and involves the migration of fibroblasts and type II pneumocytes, which secrete proteins such as collagen. The continuation of this process eventually leads to more collagen production with fibrosis and destruction of alveolar spaces, the so-called fibrotic stage ( Fig. 10.14, A ). In patients who survive ARDS, some have no long-term sequelae, but others have a residual pulmonary function deficit and variable amounts of lung fibrosis. Chronic CT findings that may be seen include cysts of varying sizes that occur in both dependent and nondependent lung ( Fig. 10.14, A ). Ground-glass and reticular opacities may also be seen preferentially involving the anterior nondependent lung. This nondependent distribution suggests that the major contributor to lung fibrosis may be ventilator-induced lung injury rather than alveolar damage because the dependent consolidated lung is protected from the ventilator and oxygen therapy.
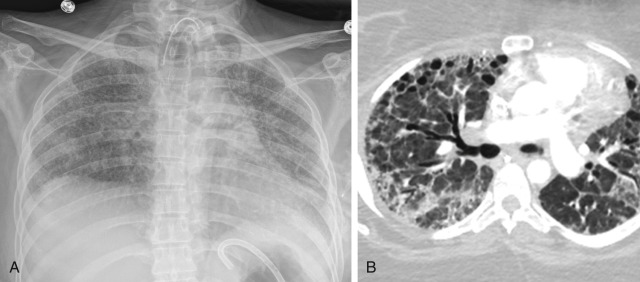
Bacterial pneumonia is a common and frequently missed complication of ARDS. The diffuse airspace opacification of ARDS often obscures the radiographic findings of pneumonia. However, the CT finding of dense, nondependent consolidation should raise the suspicion of coexistent infection.
Most patients with ARDS require mechanical ventilation for survival, often for prolonged periods and with high peak end-expiratory pressure. Barotrauma is a well-recognized complication associated with a high mortality rate. The most common manifestation is the development of extraalveolar air collections, particularly pneumothorax and pneumatocele formation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here