Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The fifth edition of this chapter was written by Peter Lamb. We have retained much of the information, revising, and updating it as appropriate. We acknowledge the excellence of his work.
Acute disease of the small bowel, from which appendicitis is considered separately, contributes substantially to both the urgent and emergency workload of the abdominal surgeon. The pattern of acute small-bowel disease varies with the age of the patient, some being more common in young people, others in older patients. It primarily manifests itself in one of three ways: obstruction, inflammation (peritonitis) and haemorrhage. These are not mutually exclusive and may coexist in each clinical episode. Treatment may be operative or non-operative, and the timing of surgical intervention is often critical, particularly when bowel ischaemia/infarction is concerned.
Although there are many causes of small-bowel obstruction ( Box 15.1 ), the commonest in the developed world is adhesions secondary to previous surgery (approximately 60% of episodes) and malignancy. By comparison, in the developing world, the most common cause is hernia. A large retrospective study using Scottish National Health Service data estimated that 5.7% of all hospital admissions following abdominal and pelvic surgery over a 10-year period were directly related to adhesions. While an attempt should be made to diagnose the cause of the obstruction preoperatively (see Chapter 11 ) to eliminate conditions that might require special treatment, in practice, the cause of the obstruction is often made only at operation. A meta-analysis published in 2013 showed that the incidence of adhesive small-bowel obstruction (ASBO) requiring surgery was 2.5%. Adhesive obstruction requiring surgery has been found to be highest after paediatric surgery and surgery to the lower gastrointestinal (GI) tract. This review also showed significantly lower incidence of adhesions after laparoscopic (1%) versus open surgery (4%).
Gallstone
Food bolus
Bezoars
Parasites (e.g., Ascaris )
Enterolith
Foreign body
Primary
Small-bowel tumour
Carcinoma
Lymphoma
Sarcoma
Carcinoma of caecum
Secondary
Crohn’s disease
Radiation enteritis
Postoperative stricture
Potassium chloride stricture
Vascultides (e.g., scleroderma)
Congenital
Bands
Acquired
Postoperative
Inflammatory
Neoplastic
Chemical (e.g., starch, talc)
Pharmacological (e.g., practolol)
Intussusception
Primary
Congenital (e.g., diaphragmatic)
Acquired (e.g., inguinal, femoral, etc.)
Secondary
Incisional hernia
Internal postoperative hernia (e.g., lateral space, mesenteric defect)
The small bowel responds to obstruction by the onset of vigorous peristalsis. As the obstruction develops, the proximal intestine dilates and fills with fluid, producing systemic hypovolaemia. Further fluid is lost through vomiting, which occurs early if the obstruction is proximal. If the process continues and the blood supply is compromised, infarction and perforation will occur. If the blood supply remains intact and the bowel is decompressed by vomiting and nasogastric drainage, peristalsis will stop, leaving dilated, non-functioning bowel.
The typical clinical presentation of small-bowel obstruction is central abdominal colicky pain, vomiting (usually bile-stained), abdominal distension and a reduction or absence of flatus. If the blockage is in the distal ileum, early vomiting may be less of a feature and abdominal distension more obvious. The vomiting often becomes ‘faeculent’ as the stagnant small-bowel contents become degraded by bacterial colonisation. Bowel sounds increase and may be audible to the patient. Localised peritonitic pain and tenderness suggests ischaemia and incipient strangulation. In some patients there may be an obvious cause, such as an irreducible hernia or scars from previous surgery.
Although small-bowel obstruction can occur without the development of abdominal pain, the absence of this symptom should be viewed with caution. This is particularly the case in postoperative patients, where small-bowel obstruction and intestinal ileus can be difficult to differentiate.
The history and examination of the patient should be sufficiently thorough to identify small-bowel obstruction and its possible causes, as well as any suspicion of ischaemia or perforation aroused by tenderness and peritoneal irritation. Evaluation of the patient’s general state, particularly dehydration and its consequences, will ensure adequate resuscitation prior to any planned surgical treatment.
The aim of management is adequate resuscitation, confirmation of the diagnosis and the appropriate timing for surgery (when indicated).
Fluid resuscitation usually requires several litres of crystalloid fluid (ideally Hartmann’s solution/Ringer’s lactate) in the first few hours after admission. With proximal bowel obstruction, ongoing emesis leads to additional loss of fluid containing Na, K, H and Cl and metabolic alkalosis may occur. Continuous assessment of resuscitation using urinary output and lactate is essential. Monitoring of central venous pressure may be required in the elderly or patients with coexisting morbidity. Adequate fluid and electrolyte replacement should be given before any surgical intervention is planned.
Although mild adhesive obstruction may resolve without a nasogastric tube (NGT), its use in established small-bowel obstruction will reduce vomiting, decompress the bowel and reduce the risk of aspiration. Nasogastric losses should be replaced with additional electrolyte-rich intravenous crystalloid fluids and potassium supplementation if necessary. Adequate analgesia should be given early and will not mask signs of peritonitis, so there is no justification for withholding adequate analgesia while awaiting further clinical assessment. The analgesia requirement should be reviewed regularly, especially in the early stages, as a persistent requirement for increasing amounts may indicate developing strangulation. Anti-thromboembolic prophylaxis should be commenced early and continued until at least discharge from hospital.
These are aimed at:
Assessing the general state of the patient
Confirming the diagnosis of small-bowel obstruction and possible cause
Identifying those patients who need early surgery (those with a high risk of strangulation) and those in whom a non-operative approach is appropriate
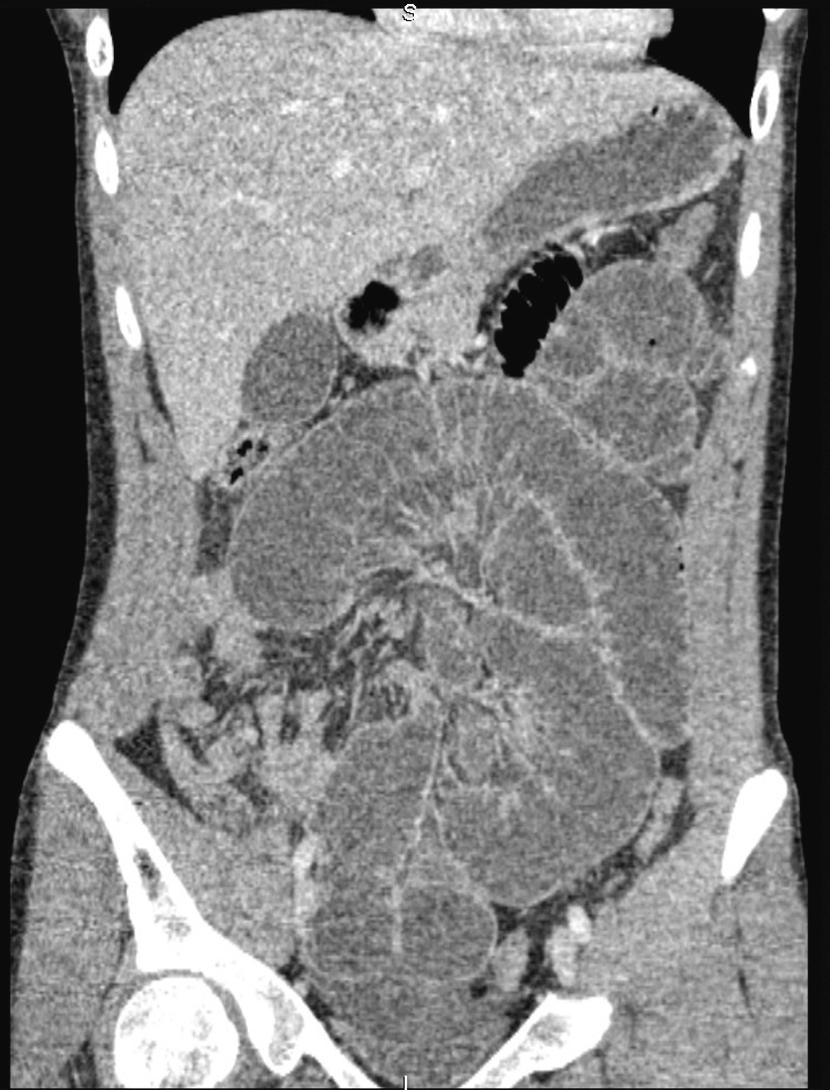
Contrast-enhanced computed tomography (CT) ( Fig. 15.1 ) is increasingly used in the early assessment of patients with small-bowel obstruction, both to identify the underlying cause (particularly malignancy) and to identify features of possible strangulation. CT features of intraperitoneal free fluid, mesenteric oedema and lack of the ‘small-bowel faeces sign’, in combination with a history of vomiting, have been reported to be highly predictive of requiring operative intervention. A subtle sign on contrast-enhanced CT scanning is hyperaemia of a segment of bowel, which indicates ischaemia. Identifying patients with possible strangulation remains difficult, and where concern persists, early surgery (laparoscopic or open) is advised. The small-bowel faeces sign refers to the presence of particulate faeculent material mingled with gas bubbles in the lumen of the small intestine (as seen in the colon on CT). It is believed to be the result of delayed intestinal transit and to be caused by incompletely digested food, bacterial overgrowth or increased water absorption of the distal small-bowel contents due to obstruction.
Adhesive obstruction may initially be treated non-operatively, with a ‘drip and suck’ regimen, i.e. intravenous fluids and NGT aspiration. Spontaneous resolution can be expected in the majority of adhesive obstructions managed in this way. This treatment plan should be abandoned at the first suggestion of underlying strangulation. These patients are good candidates for a water-soluble contrast medium (WSCM), which has both diagnostic and therapeutic properties. The radiological appearance of contrast in the colon within 24 hours from administration predicts resolution in 99% of patients. , Although non-operative management (NOM) can be continued for several days in the absence of any suggestion of strangulation, surgical exploration is generally indicated if the obstruction fails to resolve after 48–72 hours, or the WSCM test fails. It might be worth waiting longer in some patients with known extensive adhesions from multiple previous explorations where surgery is likely to be complex, prolonged and high risk. In this setting, attention should be paid to intravenous nutritional support.
![]() Adhesive bowel obstruction with the absence of clinical or CT signs of strangulation (free fluid, mesenteric oedema, small-bowel faeces sign, devascularised bowel or hyperaemia), or for patients with partial obstruction, initial non-operative management is appropriate. These patients are good candidates for a trial of orally administered water-soluble contrast medium with both diagnostic and therapeutic purposes. The appearance of water-soluble contrast in the colon on X-ray within 24 hours from administration predicts resolution. The use of contrast is safe and reduces the need for surgery, time to resolution and hospital stay. In the absence of signs of strangulation or peritonitis, non-operative treatment can be prolonged up to 72 hours (Bologna Guidelines for Diagnosis and Management of Adhesive Small Bowel Obstruction).
Adhesive bowel obstruction with the absence of clinical or CT signs of strangulation (free fluid, mesenteric oedema, small-bowel faeces sign, devascularised bowel or hyperaemia), or for patients with partial obstruction, initial non-operative management is appropriate. These patients are good candidates for a trial of orally administered water-soluble contrast medium with both diagnostic and therapeutic purposes. The appearance of water-soluble contrast in the colon on X-ray within 24 hours from administration predicts resolution. The use of contrast is safe and reduces the need for surgery, time to resolution and hospital stay. In the absence of signs of strangulation or peritonitis, non-operative treatment can be prolonged up to 72 hours (Bologna Guidelines for Diagnosis and Management of Adhesive Small Bowel Obstruction).
The particular circumstances of any given patient determine the need for surgical intervention, but some of the commonest features in decision-making are listed in Box 15.2 .
Generalised peritonitis
Visceral perforation
Irreducible hernia
Localised peritonitis
Palpable mass lesion
‘Virgin’ abdomen
Failure to improve (continuing pain, high nasogastric aspirates)
Incomplete obstruction
Previous surgery
Advanced malignancy
Diagnostic doubt (possible ileus)
Adhesive small bowel obstruction is mainly caused by which two types of surgery?
If doing an anastomosis after resection of a small bowel radiation stricture, what is critical to know about the two ends of bowel being joined together?
What is the potential 5-year recurrence rate of appendicitis after successful antibiotic management?
Are interval appendicectomies indicated after successful conservative management of complicated appendicitis?
What is the best management of a low-grade mucinous neoplasm of the appendix?
Once a decision to operate has been made, patients should be resuscitated, their co-morbidity optimised, and the stomach emptied with an NGT. The wide range of possible surgical procedures should be explained to the patient, including the possibility of a stoma. Antibiotic and anti-thromboembolic prophylaxis should be administered.
A midline incision has the most utility when the diagnosis is unknown. Where there is a previous midline incision, this should be utilised and extended cranially or caudally so that the peritoneal cavity can be entered through a ‘virgin’ area. Loops of small bowel may be densely adherent to the back of the old scar and care taken to avoid an inadvertent enterotomy of the attenuated, dilated bowel.
In open surgery, having entered the abdominal cavity, the first step is to identify the point between dilated and collapsed bowel. It is important to demonstrate this transition as it confirms the diagnosis of mechanical obstruction and identifies the obstructing point. The presence of uniformly dilated small bowel, or no definite point of change in diameter of the bowel, suggests that the clinical diagnosis of mechanical obstruction may be incorrect.
The fluid within the bowel makes it heavy and if it is removed from the abdominal cavity, it should be handled and supported carefully, utilising surgical assistants to ensure the mesentery is not damaged or twisted. The large surface area of dilated loops results in considerable insensible fluid loss, and if it is anticipated that the viscera will lie outside the abdominal cavity for a significant length of time, it should be placed in a transparent ‘bowel’ bag or wrapped in moist swabs.
Having identified the point of obstruction, it should be released ( Fig. 15.2 ). Although it is not necessary or helpful to divide every adhesion within the abdomen (as these will inevitably re-form), enough should be divided to confirm that there remains no possible site of obstruction between the duodenojejunal flexure and the caecum. It is essential to recognise the patient in whom the clinical diagnosis of mechanical small-bowel obstruction is incorrect, as the presence of adhesions does not in itself confirm the diagnosis.
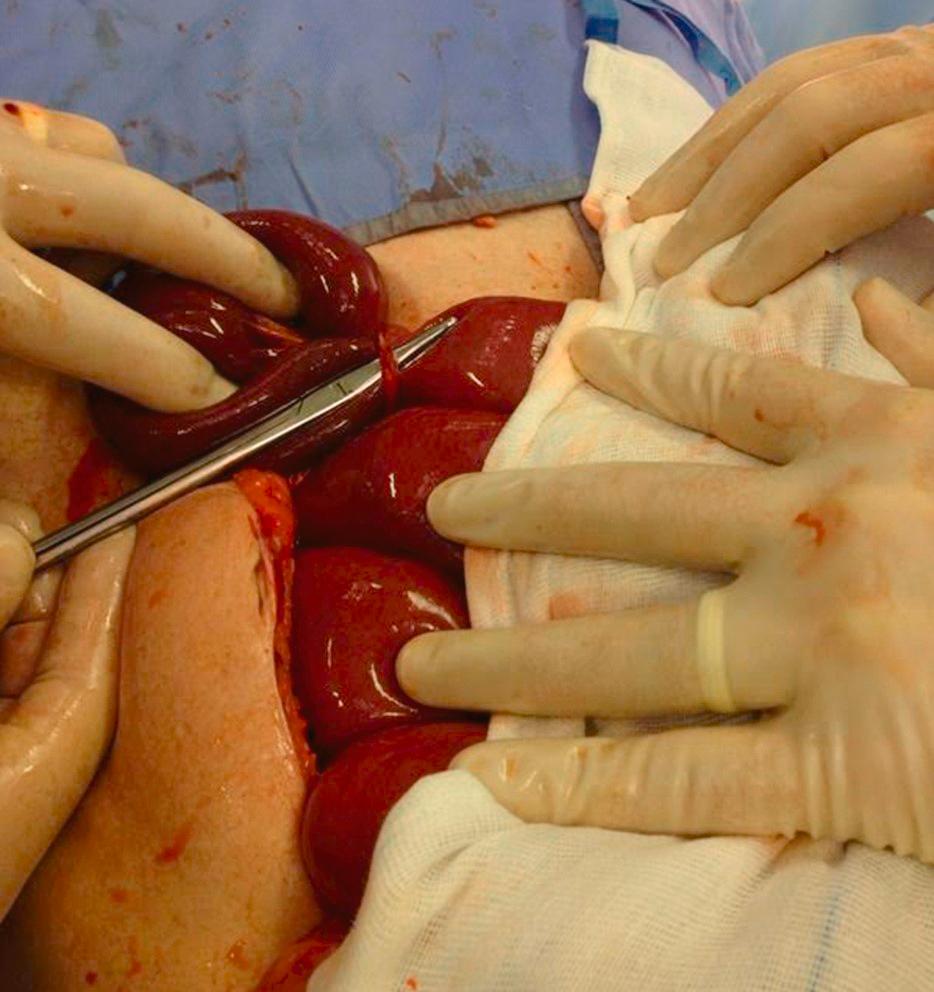
The small bowel should be resected if it is irreversibly ischaemic, or there is disease or fibrotic narrowing in the bowel at the point of obstruction and anastomosed if both ends of the bowel are healthy and the patient has no other contraindication from associated co-morbidity. If the viability of a segment of bowel is unclear, it should be wrapped in warm moist swabs for several minutes (approximately 15 minutes while you continue with other parts of the operation) and re-examined. If available, vascular status of the bowel may be assessed using near-infrared imaging with indocyanine green (ICG). Where viability remains in doubt, the segment should be resected, or a planned re-look laparotomy arranged 24–48 hours later. Even after removing the obstruction, exteriorisation of the bowel may be indicated if there is generalised disease of the bowel and when there is a high risk of anastomotic dehiscence. In patients who are septic and unwell, stapling off the ends of resected bowel with a planned re-look in 24–48 hours (such as is carried out in the trauma setting) is useful, with subsequent anastomosis performed in more favourable conditions. An ileostomy may be indicated in patients with Crohn’s disease as part of their long-term management, and this possibility should be considered and discussed with the patient beforehand.
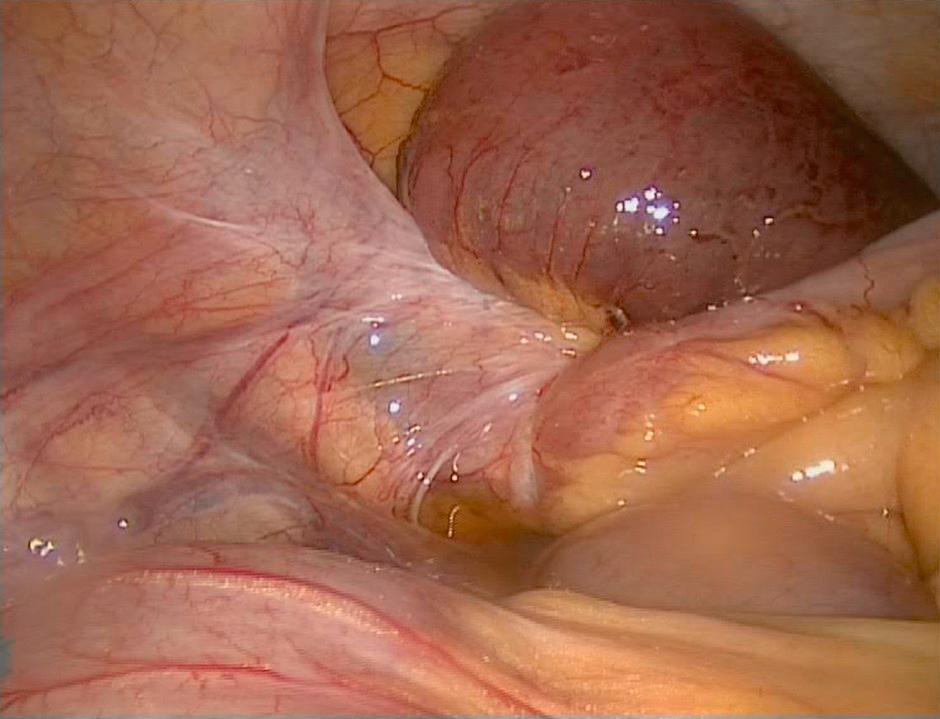
In suitable patients, laparoscopy can be attempted as an alternative to laparotomy using an open access technique. Laparoscopic adhesiolysis, with a low threshold for conversion, may be suitable during the first episode of small-bowel obstruction, particularly when there has been limited previous surgery and a single band is anticipated, for example in a patient with a virgin abdomen or who has had an appendicectomy, oophorectomy or hysterectomy ( Fig. 15.3 ). , If laparoscopic adhesiolysis is to be attempted, the obstructed bowel must be manipulated with great care to prevent perforation as it is usually thin-walled and distended.
There has been considerable research aimed at reducing the development of further adhesions after surgery. Anti-adhesion barriers are compounds that do not actively interfere with inflammation and wound healing. Rather, they act as a spacer that separates injured surfaces of the peritoneum, allowing these surfaces to heal without forming fibrinous attachments, which eventually lead to adhesions. To accomplish this task, such barriers should ideally be inert to the human immune system and be slowly degradable. Anti-adhesion barriers have been demonstrated to reduce adhesion formation and the incidence of subsequent complications. There is moderate evidence that a hyaluronate carboxymethylcellulose adhesion barrier can reduce the incidence of reoperations for ASBO in colorectal surgery. In three trials involving 1132 patients undergoing colorectal surgery, hyaluronate carboxymethylcellulose reduced the incidence of reoperations for ASBO (RR 0.49, 95% CI 0.28–0.88). In surgery for adhesive bowel obstruction, there is evidence that the intraperitoneal administration of icodextrin 4% solution at the end of surgery reduces intra-abdominal adhesion formation and the risk of re-obstruction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here