Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Early and late GI organ injury may occur following irradiation of thoracic, abdominal, and pelvic malignancies of GI and non-GI origin. As with all toxicities associated with radiation therapy (RT), GI side effects are categorized broadly into 2 types: early or acute reactions, such as diarrhea and nausea, which can occur during and soon after completion of a treatment course, and late or chronic effects, such as ulceration, stricture formation, and bowel obstruction, which can develop months to years later. Severe acute reactions can lead to treatment breaks and, in turn, a suboptimal treatment course, whereas the concern for chronic toxicity, particularly to the small bowel, is commonly a dose-limiting consideration in the creation of a treatment plan. The incidence and severity of radiation-induced GI morbidity depends on both total dose and fraction size, treatment volumes and techniques, the presence or absence of other treatment modalities such as systemic therapy and surgery, and underlying patient comorbidities. This chapter discusses the early and late toxicities of RT and combined chemoradiation therapy (CRT) regimens to the esophagus, stomach, small intestines, colon, rectum, anus, pancreas, and liver.
The sections to follow will focus on the various radiation toxicities that can occur in each of the individual organs of the GI tract, as well as the steps radiation oncologists take to reduce the likelihood and severity of said toxicities. Prior to that, we present a brief general discussion of the cellular mechanisms of radiation damage and introduce the concept of an organ that functions in series versus one that functions in parallel, which informs radiation dose constraints.
At the cellular level, both the therapeutic and injurious acute effects of RT are consequences of its ability to induce DNA double-strand breaks through the creation of free radicals. In some cell types, this damage will lead to programmed cell death (apoptosis), which proceeds through a well-defined signaling cascade that is commonly disrupted during the process of oncogenesis. In rapidly dividing cancer cells, generally lacking important components of the normal DNA-damage response system and the apoptotic cascade, RT-induced DNA double-strand breaks can result in lethal chromosomal aberrations that cause cell death when division is attempted, a phenomenon known as mitotic catastrophe.
The acute effects of radiation exposure to the normal GI tract have been studied in animal models, where a rapid increase in the rate of apoptosis of intestinal crypt/stem cells can be observed after exposure to low-dose irradiation (1 to 5 cGy). The rate of apoptosis is dose-dependent and reaches a plateau at 1 Gy. Radiation exposure increases expression of the TP53 gene product, p53, in GI epithelium, which induces expression of PUMA (p53 upregulated modulator of apoptosis, also known as BBC3 or Bcl-2-binding component 3), a proapoptotic protein that causes cell death via the intrinsic apoptotic pathway. Conversely, the rate of radiation-induced apoptosis in endothelial cells is significantly reduced in animals lacking the proapoptotic bcl-2 multidomain proteins, bax and bak. It is therefore postulated that p53 promotes apoptosis after irradiation whereas antiapoptotic members of the bcl-2 family protect the normal mucosa.
Beyond the acute loss of cells through apoptosis and mitotic catastrophe, radiation injury is the consequence of a complex set of interactions between cells involving multiple cytokines and molecular pathways that can acutely lead to mucosal edema, and chronically to fibrosis and organ dysfunction through excess deposition of extracellular matrix coincident with a reduction in the expression of remodeling enzymes such as matrix metalloproteinases. Radiation-induced fibrosis is, essentially, improper wound healing and, when combined with mucosal stem cell loss, underlies the chronic toxicities associated with RT such as dysmotility, stenosis, and fistula formation. This process typically begins several months to a year after the conclusion of a course of RT and can progress over the following years.
The initial step in this process is the recruitment of immune cells to the site of RT-induced injury, which in turn results in the increased local expression of a number of cytokines, including platelet-derived growth factor and transforming growth factor (TGF)-β. Platelet-derived growth factor promotes the migration of fibroblasts to the injured area, whereas TGF-β promotes both the proliferation of fibroblasts and their transdifferentiation into profibrotic myofibroblasts. Ongoing local expression of active TGF-β is thought to be the primary mediator of radiation-induced fibrosis. In addition to promoting the creation of myofibroblasts, TGF-β promotes the production of extracellular matrix proteins and reduces the expression of matrix metalloproteinases. Pathologic examination of bowel specimens from patients who underwent surgery for radiation enteropathy showed increased TGF-β in areas with vascular sclerosis and fibrotic areas of the serosa and muscularis propria as compared with patients who had surgery for other causes. In rat liver, TGF-β expression was found to be upregulated in a dose-dependent manner in hepatocytes up to 9 months after irradiation. Neutralizing antibodies to TGF-β and small molecule inhibitors have been shown to suppress or reverse fibrosis in preclinical models, but this has not been used in clinical practice to date.
The acute to subacute formation of fibrotic tissue and loss of epithelium can in turn lead to chronic and permanent fibrosis, the risk of which is also related to patient comorbidities such as smoking status, nutrition, diabetes, and certain inflammatory diseases. The overall picture is thought to be a product of the initial insult and damage response that leads to vascular changes with an end result of tissue ischemia and progressive fibrosis.
Through the loss of epithelial stem cells and the formation of scar tissue, RT can cause segments of organs of the GI tract to become less functional. Whether this manifests clinically is partly related to the functional arrangement of the organ, of which there are 2 types: serial and parallel.
Organs arranged in series are composed of segments that are reliant on the functionality of the preceding segment such that the loss of any individual segment will make the organ dysfunctional or even nonfunctional downstream, and possibly upstream, from the insult. The prototypical organ with this arrangement is the spinal cord, where significant damage at a single spinal level can cause loss-of-function at every level downstream of the injury. With respect to the GI system, much of the luminal GI tract has this type of organization.
Organs with parallel architecture, on the other hand, have some functional redundancy built in such that the loss of segments up to a point may not manifest clinically. The liver has this type of arrangement, although debate continues on exactly how much liver need be preserved to remain fully functional particularly in patients with underlying liver disease.
When creating RT plans, consideration of these arrangements informs the types of dose constraints that are used. Organs that function in series are subject to maximum dose constraints, because an ablative dose to a small area can manifest as dysfunction of the entire organ, such as a SBO. Organs with a parallel functional architecture are subject to constraints that allow for the protection of an adequate relative volume of functional tissue. As will be discussed in the case of the liver, it is important to not allow an absolute volume to receive more than a certain dose of radiation.
The first case of radiation injury of the small bowel, or radiation enteropathy, was described in 1897 in the context of irradiating the skin of the abdomen. RT can damage the small bowel during the treatment of virtually all GI and gynecologic cancers, while rarely being used as part of the primary treatment of cancers of the small bowel. Mitigating the risk and severity of radiation enteritis and chronic small bowel injury is commonly the dose-limiting factor in the radiotherapeutic management of most abdominal and pelvic malignancies.
The epithelium of the GI tract has a high proliferative rate, with turnover every 3 to 5 days, making it susceptible to radiation and chemotherapy-induced mucositis. Irradiation of intestinal mucosa primarily affects the clonogenic intestinal stem cells within the crypts of Lieberkühn (cells that provide, via self-replication and eventual maturation, replacement cells in the intestinal villi). Stem cell damage, as a result of direct radiation damage or radiation-induced microvascular damage, leads to a decrease in cellular reserves for the intestinal villi. This results in mucosal denudement, shortened villi, a decreased absorptive surface area, and associated intestinal inflammation and edema. Histologic changes are seen within hours of irradiation.
Within 2 to 4 weeks, an infiltration of leukocytes with crypt abscess (microabscess) formation can be seen, leading to ulcer formation ( Fig. 41.1 ). This acute injury can result in impaired absorption of fats, carbohydrates, proteins, bile salts, and vitamin B 12 , with loss of water, electrolytes, and proteins. Impaired ileal bile salt absorption increases loads of conjugated bile salts entering the colon, which are deconjugated by colonic bacteria, causing intraluminal salt and water accumulation and subsequent diarrhea. Furthermore, impaired digestion of lactose may occur following radiation, leading to increased bacterial fermentation with associated flatulence, distention, and diarrhea. There is also evidence of acutely altered gut motility following RT.
Patients with acute radiation enteritis experience diarrhea, abdominal cramping or pain, nausea and vomiting, anorexia, and malaise. Radiation-induced diarrhea often appears during the third week of a fractionated radiation course, with reported rates of 20% to 70%. Acute radiation enteropathy with diarrhea may be seen in some patients after delivery of doses of 18 to 22 Gy using conventional fractionation, which coincides with the start of the third week of therapy, and is seen in most patients by 40 Gy. The symptoms and pathologic findings typically subside 2 to 6 weeks following completion of RT, although evidence suggests that patients who develop acute small intestinal toxicity may be at higher risk for chronic effects.
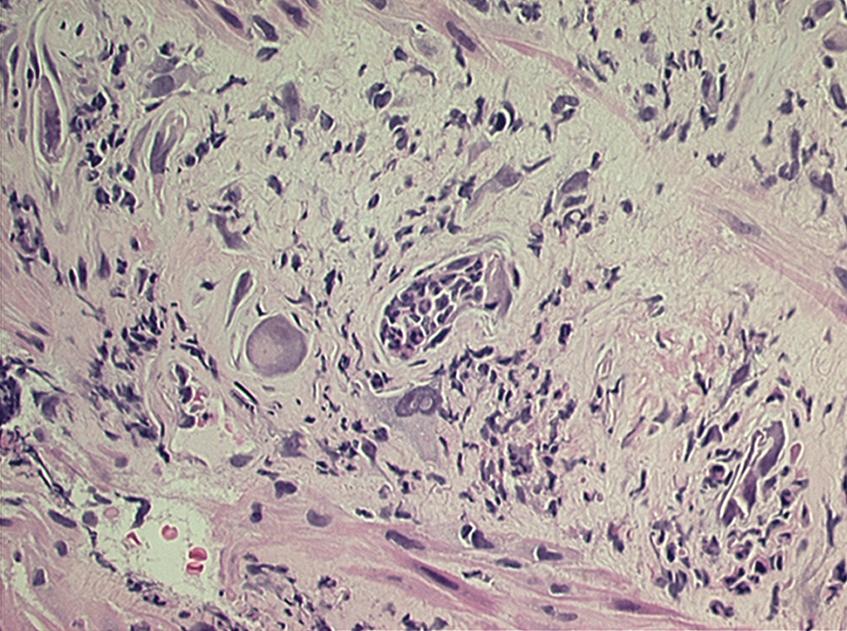
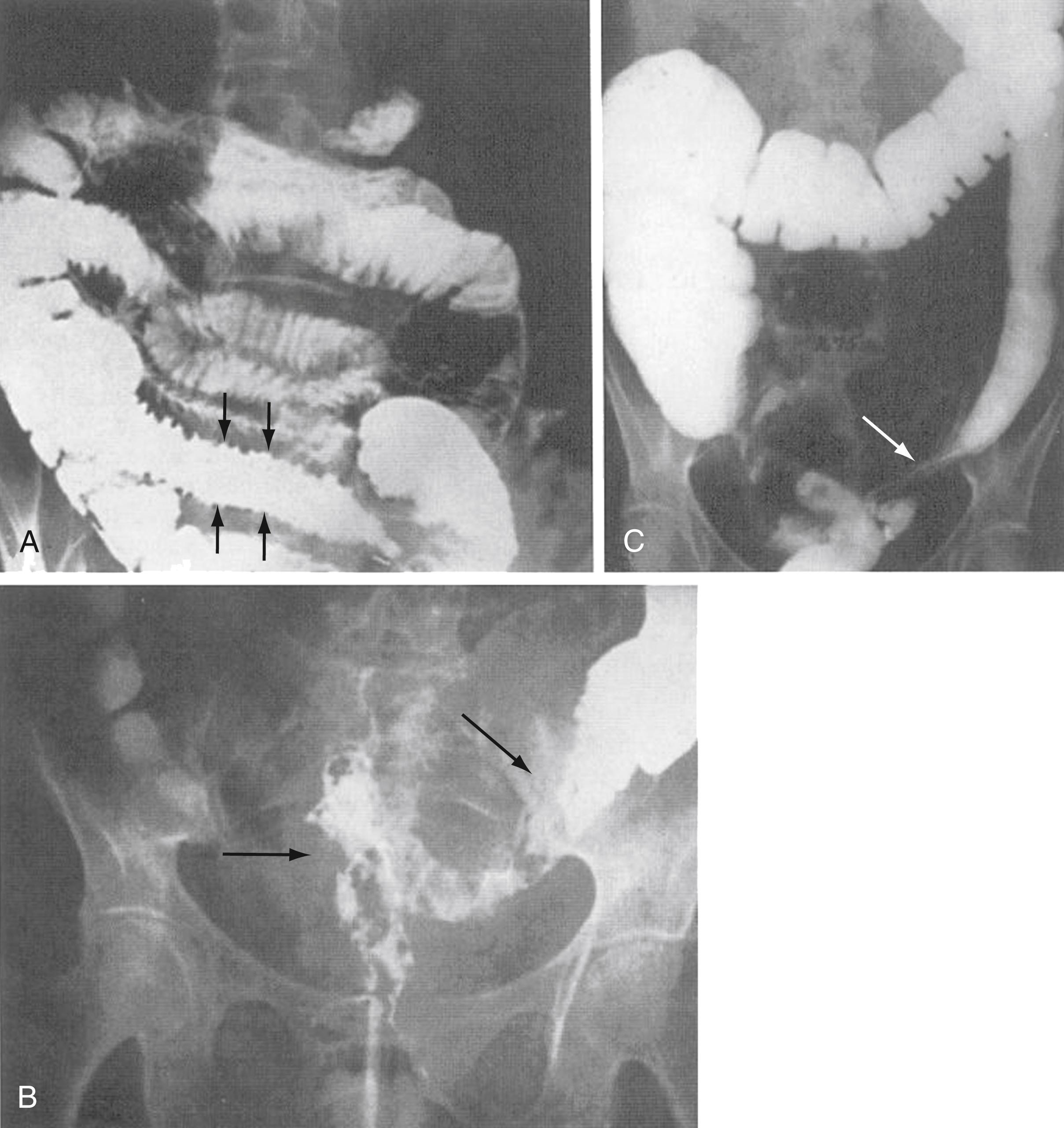
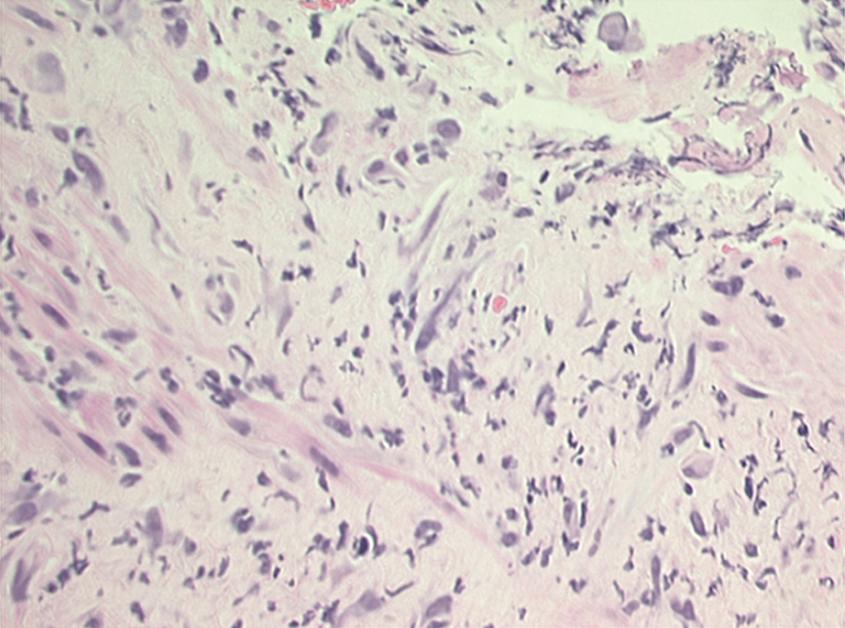
Histologic changes of chronic toxicity to the small intestine include progressive occlusive vasculopathy with foam cell invasion of the intima and hyaline thickening of the arteriolar walls, with collagen deposition and fibrosis. The small bowel becomes thickened, with development of telangiectasias, whereas the vessel walls of small arterioles are obliterated, causing ischemia ( Fig. 41.2 ). As the vasculopathy progresses, mucosal ulceration, necrosis, and occasionally perforation of the intestinal wall can be seen, leading to fistula and abscess formation. Lymphatic damage contributes to mucosal edema and inflammation. Histologically, the mucosa atrophies, with atypical hyperplastic glands and intestinal wall fibrosis ( Fig. 41.3 ). As the ulcers heal, there can be fibrosis and narrowing of the intestinal lumen, with subsequent stricture formation and even obstruction with dilatation of the proximal bowel. Bacterial overgrowth may be an indirect complication arising from stasis in a dilated loop of bowel proximal to the stricture. Although the affected segments of intestine and serosa appear thickened with areas of telangiectasias, it should be noted that even if the gut appears normal, patients can still be at risk of spontaneous perforation.
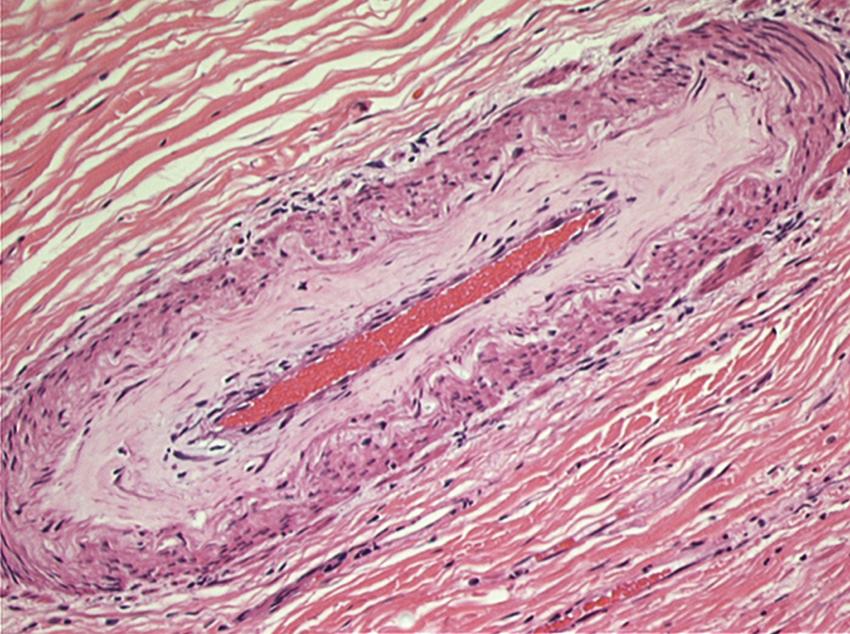
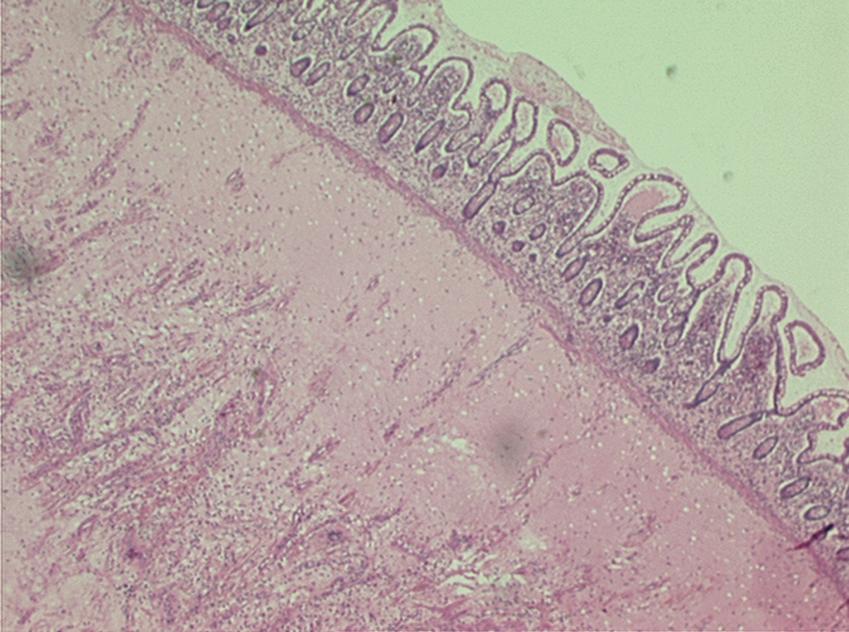
Chronic radiation enteritis can cause significant morbidity. This complication tends to be progressive, with an onset at least 6 months after radiotherapy. Late radiation injury to the small intestine occurs at a median of 8 to 12 months following RT, although it can appear years later. There are many clinical manifestations of the chronic phase of radiation enteritis ( Table 41.1 ). Fibrosis and vasculitis of the bowel may lead to dysmotility, stricture formation, and malabsorption. More rapid transit times can occur in the affected bowel, which can cause chronic malnutrition and resultant anemia and hypoalbuminemia. Malabsorption and other complications may require surgical intervention and parenteral alimentation. Patients with severe chronic radiation enteritis have a poor long-term prognosis and a mortality rate of approximately 10%.
| Complication | Lesion(s) | Clinical features |
|---|---|---|
| Obstruction | Stricture | Constipation, nausea, vomiting, postprandial abdominal pain |
| Infection | Abscess | Abdominal pain, fever, chills, sepsis, peritonitis |
| Fistulization | Fistula | Fecal, vaginal, or bladder discharge; pneumaturia |
| Bleeding | Ulceration | Rectal pain, tenesmus, rectal bleeding, anemia |
| Malabsorption | Small bowel damage | Diarrhea, steatorrhea, weight loss, malnutrition, cachexia |
The overall incidence of chronic radiation enteritis has not been precisely defined. Retrospective series suggest an incidence of 20%, but these studies often included a large number of patients who were lost to follow-up or died between the end of RT and the completion of the study. A review of randomized trials of adjuvant RT for rectal cancer shows severe long-term complications as low as 1.2% and as high as 15%, which is greatly improved in comparison with older trials, suggesting that technical advances have reduced chronic small bowel toxicity rates.
Certain factors have been found to predispose patients to radiation toxicity to the small intestine. Women, older patients, and thin patients may have a larger amount of small bowel in the pelvic cul-de-sac, which can increase the likelihood of radiation injury in the treatment of pelvic malignancies. Patients with a history of pelvic inflammatory disease or endometriosis also appear to be at higher risk of radiation complications. Patients who have had previous abdominal surgery can develop adhesions that decrease the mobility of the small bowel, allowing it to be consistently exposed to fractionated RT. In addition, patients with prior pelvic surgery may have an increase in the amount of small bowel within the pelvis. In a series published by Eifel and associates, the risk of small bowel complications was significantly higher in women who had undergone a previous laparotomy.
Smokers, and patients with diabetes, hypertension, and cardiovascular disease, also have an increased risk of preexisting vascular damage or occlusion. These comorbid conditions are compounded by the pathologic changes of chronic radiation injury, which include vasculopathy and ischemia, predisposing the patients to radiation-related small bowel toxicity. Patients with collagen vascular and inflammatory bowel diseases have a higher risk of acute as well as chronic radiation-induced injury. Patients with these diseases may have pathologic changes that include transmural fibrosis, collagen deposition, and inflammatory infiltration of the mucosa. The late effects induced by RT to the small bowel are likely additive to these preexisting changes, and studies have shown that these patients have a lower GI tolerance to RT. Patients whose IBD or nonmalignant systemic disease is quiescent or well controlled appear to fare better than patients with active disease.
Studies have also addressed the effect of radiation dose on occurrence of small bowel toxicity. Volume of the treatment field, volume of irradiated small bowel, total dose, fraction size, treatment time, and treatment technique all influence small bowel tolerance. The TD5/5 (dose at which there is a 5% risk of toxicity at 5 years) for small volumes of small bowel has been estimated to be 50 Gy. Patients can generally receive 45 to 50 Gy in 1.8- to 2-Gy daily fractions to a pelvic field without a significant rate of toxicity. Retrospective analysis of patients with locally advanced pancreatic cancer treated with CRT found a maximum dose of 55 Gy to 1 mL of the duodenum to be an important metric for preventing long-term toxicity.
For postoperative patients, radiation to 45 to 50 Gy in 5 weeks is associated with an approximately 5% incidence of SBO requiring surgery, whereas at doses greater than 50 Gy the incidence rises to as high as 25% to 50%. Doses greater than 2 Gy per fraction in the postoperative setting also increase the risk of toxicity. At radiation doses of 70 Gy or greater, the incidence of toxicity rises precipitously.
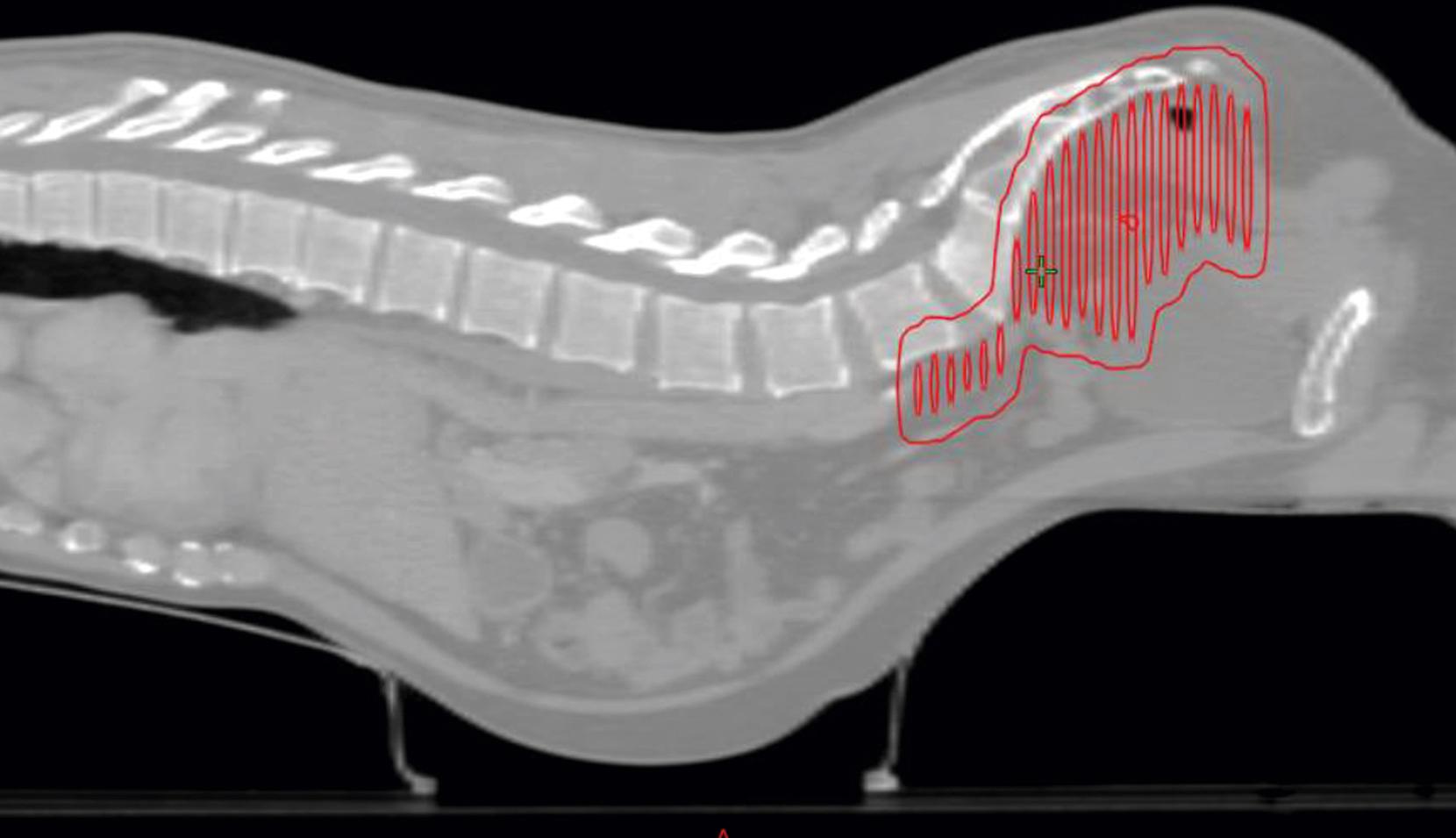
Studies that have analyzed dose-volume parameters associated with acute small bowel toxicity in patients undergoing treatment with 5-fluorouracil (5-FU)–based CRT therapy for rectal cancer found strong correlations between acute toxicity and the amount of small bowel irradiated at each dose level analyzed. A study of different treatment techniques to minimize the effect of pelvic radiation on the small bowel showed that irradiating smaller volumes of bowel yielded less toxicity. In addition, treating patients in the prone position with external compression and bladder distention decreased side effects, likely from exclusion of portions of the small bowel from the radiation field ( Fig. 41.4 ).
CRT, commonly with concurrent 5-FU or capecitabine, is used in the treatment of GI malignancies and is known to increase the risk of small bowel toxicity. In the French Federation Francophone de Cancerologie Digestive trial, which randomized patients to RT or CRT with 5-FU and leucovorin, the rate of acute toxicity was 2.7% with RT and 14.6% with the addition of chemotherapy to RT. A second trial conducted by the European Organization for Research and Treatment of Cancer randomized patients with advanced rectal cancer to preoperative RT or CRT, with or without adjuvant chemotherapy. The addition of chemotherapy resulted in higher grade 3 acute toxicity rates: 13.9% versus 7.4%. Rates of grade 2 diarrhea occurred more frequently in patients receiving concurrent chemotherapy: 37.6% versus 17.3%, with no differences in late toxicity.
There is ongoing investigation into the integration of novel chemotherapeutic and “targeted” agents with RT in neoadjuvant therapy for GI cancers. Promising results from early phase II studies incorporating oxaliplatin into the neoadjuvant regimen were shown later to be more toxic and to offer no disease benefit in subsequent randomized trials. Data from phase I and phase II trials using novel agents such as irinotecan, VEGF receptor and EGF receptor inhibitors suggest that the addition of these agents may significantly increase grades 3 and 4 GI toxicity rates relative to conventional neoadjuvant chemoradiotherapy (CRT) regimens, further emphasizing the importance of careful radiation planning to maximize normal tissue sparing in these patients.
Diagnosis of chronic (late) radiation enteropathy is made clinically ( Table 41.2 ). The cause of symptoms can be variable from patient to patient, and individualization of diagnostic and therapeutic approaches is indicated. Therapeutic options are displayed in Table 41.3 . Consultation with the treating radiation oncologist should be requested if the clinical presentation is consistent with radiation enteritis. Review of the patient’s previous radiation treatment record will reveal the total dose, fractionation, volume of treatment, and other radiation parameters. Analysis of the treatment plan may show areas of high dose, especially if the patient had an intracavitary implant or brachytherapy. Lesions encountered at endoscopy or imaging studies are usually localized in the area of high dose. Ulceration of the mucosa, thickening of jejunal folds, and thickening of the intestinal loops are radiologic signs that suggest radiation damage to the small bowel ( Fig. 41.5 ). Faster intestinal transit and reduced bile acid and lactose absorption can be observed in patients with chronic radiation enteritis. These effects may be improved after the administration of loperamide. Antibiotics are indicated if there is SIBO syndrome (see Chapter 105 ).
| Pathophysiologic Feature | Clinical Manifestations |
|---|---|
| Mucosal dysfunction | Lactose intolerance Vitamin B 12 deficiency Steatorrhea |
| Stricture or blind loop syndrome with SIBO | Diarrhea |
| Intestinal dysmotility | Bloating Constipation Diarrhea |
| Abnormal bile acid recirculation | Cholerheic diarrhea |
| Pathophysiologic Feature | Therapeutic Options |
|---|---|
| Nutritional deficits | Correction of specific deficits Low-fat diet Lactose-free diet Elemental diet TPN |
| Intestinal dysmotility (increased or decreased) | Loperamide Octreotide Prokinetic agent |
| Bile acid malabsorption | Bile-salt binding agent |
| SIBO | Antibiotics |
The management of acute radiation small bowel toxicity should be based on the severity of symptoms. Most cases of acute radiation enteritis are self-limited, requiring only supportive treatment. Diarrhea, nausea, vomiting, and abdominal cramping are treated symptomatically. Antidiarrheal medications such as loperamide, diphenoxylate atropine, or opiates can be used. Antiemetic agents may also be effective. A low-fat, lactose-free diet may improve symptoms. A study of oral sucralfate in patients receiving pelvic irradiation showed a decrease in frequency and an improvement in consistency of bowel movements at both early and late time points. Cholestyramine to treat diarrhea from bile acid malabsorption has also shown some benefit, and treatment with aspirin has been effective. Intractable diarrhea during combined-modality treatment (CRT) may require hospital admission for administration of parenteral fluids and electrolyte repletion. Patients who are refractory to conventional antidiarrheal medications may benefit from administration of a synthetic somatostatin analog such as octreotide.
The management of chronic radiation enteritis remains a major challenge, given the progressive evolution of the pathophysiology, including obstructive endarteritis and fibrosis. The treatment should be conservative, given the diffuse nature of the process and the high morbidity associated with surgery; however, surgical intervention is indicated in intestinal obstruction, perforation, fistulas, and severe bleeding.
Chronic effects of diarrhea are managed symptomatically with a low-residue diet. Fiber supplementation (e.g., Metamucil, Citrucel) has shown benefit in some cases. In the rare setting of malnutrition related to chronic radiation injury, TPN can improve clinical outcome, and methylprednisolone may add to the effects of TPN. Despite these interventions, the 5-year survival rate for patients undergoing TPN ranges from 36% to 54%. It has been estimated that overall mortality rate associated with chronic radiation enteropathy is approximately 10%.
Endoscopic techniques are sometimes required for diagnosis of bleeding intestinal ulcers. Double-balloon enteroscopy and capsule endoscopy may help facilitate this diagnosis. The double-balloon enteroscope method may allow therapeutic intervention in certain situations, including coagulation of small bowel telangiectasias. Significant bleeding refractory to endoscopic intervention may be managed surgically.
SBO is generally managed conservatively with bowel rest and tube decompression. In rare situations, the obstruction is severe or chronic enough that bowel resection or lysis of adhesions may be required. It is difficult to perform surgery for chronic radiation enteritis because of the diffuse fibrosis and alterations in the intestine and mesentery, resulting in high rates of surgical morbidity and reoperation. The risk of anastomotic leak is high if the anastomosis is performed using irradiated bowel. The risk of leak can be lowered if at least 1 limb of the anastomosis is previously unirradiated bowel. However, it may be difficult to distinguish between normal and irradiated tissues at time of surgery and even on pathologic evaluation. Another method the surgeon can use to circumvent this technical difficulty is to create the anastomosis with unirradiated colon. The accuracy in localizing injured bowel may be improved by intraoperative endoscopic examination, which can detect radiation-induced mucosal injury.
Limited resection of the diseased intestine is the goal, but if the lesion is too diffuse, a bypass procedure may be attempted. If feasible, resection of the affected bowel results in a better outcome than an enteric bypass procedure. However, extensive surgical resection of the diseased intestines may lead to short bowel syndrome (see Chapter 106 ) and the need for TPN. In selected patients who underwent extensive surgical intestinal resection, 5-year survival was approximately 65%, with two thirds of the patients weaned off of parenteral nutrition. Given the progressive evolution of fibrosis, the patient may require additional surgery if extensive surgical resection is not performed. Surgical bypass of the injured bowel may be associated with a blind loop syndrome, and the patient still may be at risk for perforation, bleeding, abscess, and fistulas due to the persistence of the affected bowel. Bypass procedures should be performed when resection is not possible or as temporary management before resection at a later date. Surgery should be performed by an experienced team familiar with the management of radiation enteritis. Perforations and fistulae are best managed surgically. It should be noted that many patients with chronic small bowel radiation toxicity are nutritionally depleted and more susceptible to anastomotic leakage and dehiscence after surgery. The postoperative mortality of these patients may be significant and must be taken into consideration before a decision to proceed with surgery is made.
Hyperbaric oxygen has been used in the treatment of chronic radiation enteritis, the rationale being that the creation of an oxygen gradient in hypoxic tissue will stimulate neoangiogensis. In a retrospective study of 36 patients with severe radiation enteritis refractory to medical management, improvement of clinical symptoms was reported in two thirds of the patients treated with hyperbaric oxygen. Hyperbaric oxygen may be helpful in management of bleeding due to chronic radiation enteritis in patients who are not controlled with conservative measures such as formalin and laser therapy (discussed later). A clinical series of 65 consecutive patients with chronic radiation enteritis (small bowel and rectum), primarily manifested as chronic bleeding, were treated with hyperbaric oxygen. Response rates for rectal and more proximal sites were 65% and 73%, respectively. The response rate for bleeding was 70% and for other symptoms (pain, diarrhea, weight loss, fistula, obstruction) was 58%. The authors concluded that hyperbaric oxygen therapy resulted in clinically significant improvement in two thirds of patients with chronic radiation enteritis.
Other agents to reduce the incidence of chronic enteritis have been investigated. There is some suggestion that pentoxifylline may abrogate radiation-associated fibrosis through antioxidant effects and inhibition of TGF-β1. In a small study, patients with radiation enteropathy were treated with pentoxifylline and vitamin E, with response assessment by subjective, objective, management, analytic scales. Regression of symptoms by subjective, objective, management, and analytic scales was seen in 40% of patients by 6 months and 80% of patients at 18 months.
Given that chronic radiation enteritis is complex and rarely curable, prevention is key, and measures to decrease its incidence are imperative. Pancreatic enzymes can exacerbate acute intestinal radiation toxicity, and reducing pancreatic secretion with a synthetic somatostatin receptor analog such as octreotide may reduce early and delayed radiation enteritis in animal studies. One of the major risk factors for injury is previous abdominopelvic surgery, which leads to the prolapse of the small intestines into the pelvis and exposure to radiation. Anticipation for the need of radiation and chemotherapy before or after surgery requires close collaboration among surgical, radiation, and medical oncologists. If gross residual tumor is found unexpectedly at surgery, outlining the tumor bed with surgical clips to facilitate postoperative treatment planning and surgical techniques to keep the small intestine outside the pelvis (e.g., omentoplasty or polyglycolic mesh) may significantly decrease the rate of complications.
Postoperative bowel adhesions may increase the volume of bowel irradiated compared with normal small intestine, which is usually mobile. If RT is anticipated after surgery, attempts should be made at the time of surgery to displace the bowel outside the radiation field. One simple technique is the surgical placement of a polyglycolic, biodegradable mesh that moves the intestines out of the pelvis. This procedure has minimal morbidity and does not significantly increase operating time. It also does not require a second operation to remove the mesh, because it is absorbed 3 to 4 months after surgery. MRI can be used after surgery to verify the position of the mesh, the small bowel, and eventual disappearance of the mesh. A reduction of 50% of the volume of the small bowel exposed to the radiation has been demonstrated with placement of a mesh during surgery, allowing a higher dose of radiation to be given postoperatively where indicated. Other techniques such as pelvic reconstruction, omentoplasty, and transposition of the colon may also significantly decrease the volume of bowel exposed to RT.
RT technique is critical in reducing the rate of complications. The use of only anterior and posterior fields for pelvic radiation should be avoided if possible because of the high dose and large volume of bowel irradiated. The toxicity of RT correlates with the volume of small bowel irradiated. In many patients, treatment in the prone position with a “belly board” allows the displacement of the small intestines out of the radiation field. Patients should be instructed to maintain a full bladder during the radiation session, which further displaces the intestines out of the pelvis. Three-dimensional (3D) treatment planning optimizes the treatment technique by facilitating more accurate dose distributions. A 3D treatment algorithm ensures the sparing of excessive radiation dose to normal tissues by the judicious use of multiple fields to the target volume from multiple geometries. In addition, more modern techniques such as intensity-modulated radiotherapy (IMRT) use sophisticated planning techniques to avoid critical structures.
Treatment of radiation enteritis is often only partially successful. Management is patient specific and should be as conservative as possible because of the relentless progression of the disease, which can be exacerbated by further injury to the area. A better understanding of the mechanism of fibrosis and the interaction of the molecular events controlling apoptosis and fibrosis may assist in the identification of the patient at risk for radiation complications and in the development of new therapeutic approaches.
Early and late effects of the esophagus often occur following irradiation of thoracic and upper abdominal malignancies (e.g., esophageal/esophagogastric junctional carcinomas, lung carcinomas). Normal esophageal mucosa undergoes continuous renewal. Acute esophageal injury is believed to be primarily related to radiation damage to the basal epithelial layer, manifested histologically by vacuolization, resulting in epithelial thinning followed by denudation ( Fig. 41.6 ). These changes manifest clinically as dysphagia, odynophagia, and substernal discomfort, usually occurring within 2 to 3 weeks following initiation of RT. Patients may describe a sudden, sharp, severe chest pain radiating to the back. As treatment progresses, pain may become constant and may not necessarily be related to swallowing. The symptoms may be confused with Candida esophagitis, which may occur in conjunction with radiation esophagitis. Concurrent chemotherapy exacerbates these toxic effects. Endoscopically, mucositis and ulceration may be observed. Perforation and bleeding are rare in the acute phase. Shortly after treatment completion, basal proliferation returns and regeneration occurs. Chemotherapy administered concurrently with RT, as it commonly is in both lung and esophageal cancer, increases the rates of grade 3 or greater acute esophagitis approximately 5-fold.
Following recovery from acute injury, late effects such as benign stricture leading to persistent dysphagia, ulceration, and fistula formation may occur months to years following treatment. These effects are believed primarily due to inflammation and scar formation within the esophageal muscle. The connective tissues surrounding the esophagus may also exhibit severe fibrosis over time, and small vessel telangiectasias may be seen endoscopically. Histologic studies of the esophagus in previously irradiated patients have demonstrated epithelial thickening, chronic inflammation, and fibrosis of the submucosa and muscularis propria but rarely chronic ulceration. Complete epithelial recovery from radiation effects may take 3 to 24 months. Late effects often manifest as dysphagia due to stricture, as well as altered motility due to fibrosis or muscular damage, possibly with accompanying nerve injury. Fistula formation is unusual and radiation dose dependent. Barium swallow examination may show strictures and disruption of peristalsis at the level of the irradiated esophagus, with repetitive and nonperistaltic waves above and below the irradiated region. Abnormal peristalsis has been reported at 1 to 3 months following treatment completion, whereas most strictures occur 4 to 8 months following treatment completion. Late effects are usually not seen until 3 months following completion of RT, with a median time to onset of 6 months in some series.
Development of radiation-related late complications is dose related. Much of the randomized data regarding the dependence of acute radiation esophagitis on different dose-fractionation schemes are from lung cancer trials. The Intergroup 0096 trial of patients with limited stage small cell lung cancer compared CRT regimens of 1.5-Gy fractions delivered twice daily over 3 weeks against 1.8-Gy fractions delivered daily over 5 weeks to the same total dose of 45 Gy. Grade 3 esophagitis was nearly 3 times as likely in the group receiving treatment twice daily. In the Radiation Therapy Oncology Group (RTOG) 0617 trial, comparing total doses of 60 Gy versus 74 Gy in the treatment of locally advanced non–small cell lung cancer, a 3-fold increase in grade 3 or greater esophagitis was also noted. Historically the TD5/5 (i.e., dose at which 5% of patients will develop complications at 5 years) has been estimated to be 60 Gy when one third of the length of the esophagus is irradiated. Cumulatively, it is recommended that the mean esophageal dose be kept less than 34 Gy, while limiting portions of the esophagus treated to no more than 60 Gy.
Few randomized trials in esophageal cancer have reported late esophageal toxicities. In the RTOG study 0113, which used doses of 50.4 Gy with chemotherapy, the rate of severe late esophageal toxicity was 12% (3% grade 5 toxicity, which is death). In RTOG 85-01, a randomized trial comparing definitive radiotherapy to 64 Gy and CRT to 50 Gy, nearly 20% of patients in each arm experienced severe late esophageal toxicity. More recent analyses of patients treated with modern planning techniques have found significant reduction in the long-term esophageal sequelae.
Brachytherapy (the temporary insertion of a radioactive source into or adjacent to a tumor) has also been used as a technique for radiation dose escalation in esophageal cancer. Although some institutions have reported low rates of fistula associated with brachytherapy, Gaspar and colleagues reported the results of a phase I/II study examining the role of brachytherapy in addition to external beam RT in the treatment of esophageal cancer. The 1-year actuarial fistula formation rate was 18%, and the authors recommended caution in the use of this approach, particularly in conjunction with concurrent chemotherapy. A more contemporary series of 62 patients treated with external beam and brachytherapy resulted in a 16% rate of severe toxicities including ulceration, stricture, esophageal perforation, fistula, and acute esophageal bleeding.
The intensity of cancer treatment, such as use of concurrent chemotherapy with RT, increases the rate of acute esophagitis. Maguire and colleagues evaluated 91 patients treated with RT for non–small cell lung cancer and found that the percentage esophageal volume and surface area treated to greater than 50 Gy predicted late esophageal toxicity. Patients who had preexisting GERD and esophageal erosions secondary to tumor were at increased risk for late toxicity. Hyperfractionation (multiple daily radiation treatments) was also associated with increased acute toxicity. Singh and associates studied patients with non–small cell lung cancer who received conformal daily RT with or without concurrent chemotherapy. They found that a maximal esophageal “point” dose of 69 Gy (RT alone) and 58 Gy (with concurrent chemotherapy) predicted significant toxicity. Twenty-six percent of patients receiving concurrent CRT developed grade 3 or higher esophageal toxicity, whereas only 1.3% of patients who received RT alone experienced this degree of toxicity.
Ahn and colleagues found that the most powerful predictor of late esophageal toxicity in 254 patients treated for non–small cell lung cancer was the severity of acute esophageal toxicity. Severe acute toxicity was predicted by the use of twice-daily radiation, older age, increasing nodal stage, and a variety of dosimetric parameters. The overall incidence of late toxicity was 7%, with a median and maximal time to onset of 5 and 40 months, respectively. Wei and coworkers, evaluating 215 patients who received concurrent chemotherapy, found that the relative esophageal volume receiving greater than 20 Gy predicted for grade 3 or greater acute toxicity, and a second series found that when greater than 30% of the esophageal volume received greater than 50 Gy (V50), this resulted in grade 1 or higher acute toxicity. Based on these and other data, it is clear that the addition of concurrent chemotherapy to RT increases the incidence of both acute and chronic esophageal toxicity.
The treatment and prevention of radiation-induced esophagitis have come under increased attention with the use of aggressive combination chemotherapy and RT regimens. Treatment interruptions may ease the symptoms of acute esophagitis but may also compromise treatment efficacy and is generally reserved for severe cases. The management of acute esophagitis usually includes symptomatic management such as topical anesthetics (including viscous lidocaine-based regimens), oral analgesics (including anti-inflammatory agents and narcotics), gastric antisecretory drugs (histamine blockers, PPIs), promotility agents (e.g., metoclopramide), and treatment of superimposed infection (candidiasis). Dietary modification, including bland foods, pureed or soft foods, and soups, can help patients maintain oral intake. Other modifications include avoidance of smoking, alcohol, coffee, spicy or acidic foods, chips, crackers, and fatty foods. A study of dietary modifications and pharmacologic prophylaxis for radiation-induced esophagitis reported decreased toxicity and fewer treatment interruptions. It was recommended to drink between meals and to eat 6 smaller meals per day, consisting of semisolid food, soup, high-calorie supplements, purees, puddings, milk, and soft breads. In addition, ingestion of hot or cold foods should be avoided if possible; instead, foods and liquids should be at room temperature. In severe cases, feeding tube placement may be required.
Radioprotective chemical agents have been investigated as a means of mitigating radiation-induced normal tissue toxicity. The best-studied radioprotector, amifostine, is an organic thiophosphate. This agent is a scavenger of free radicals and serves as an alternative target to nucleic acids for alkylating or platinum agents. Trials have had conflicting results and are limited by small patient numbers. In the largest randomized trial, patients treated with chemotherapy and RT for non–small cell lung cancer were randomized to receive amifostine or no drug. Although amifostine did not significantly reduce grade 3 or higher esophagitis, patient self-assessments suggested a significantly lower incidence of acute esophagitis in those who received amifostine. Patients receiving amifostine, however, experienced significantly higher rates of nausea, vomiting, infection, febrile neutropenia, and cardiac events. Given this, amifostine is not routinely recommended in the prevention of radiation esophagitis.
A second radioprotector, glutamine, has generated clinical interest. In hypercatabolic states, such as cancer, glutamine deficiency can develop. A retrospective study in 41 patients with lung cancer demonstrated that glutamine was well tolerated, with supplemented patients experiencing a lower incidence of grade 2 to 3 esophagitis, typically resulting in weight gain during treatment. A second analysis from the same institution evaluated 104 patients, 56 of whom received glutamine. Glutamine was associated with less grade 3 esophagitis, treatment breaks, and weight loss, and administration was not associated with differences in time to event end points. A pilot study of 75 patients corroborated retrospective data demonstrating no glutamine intolerance or toxicity. Most patients (73%) were treated with sequential chemoradiation, and 49% of those treated with concomitant chemoradiation did not develop esophagitis. A recent retrospective analysis of 122 patients with advanced lung cancer noted that patients treated prophylactically with glutamine had significant less acute esophagitis and, consequently, significantly less weight loss. Although glutamine is associated with little toxicity, further evaluation of efficacy is needed before its broad incorporation into clinical practice.
The management of late esophageal radiation stricture consists of serial endoscopic dilatation for symptomatic improvement. Dilations in advanced stricture can result in esophageal rupture and therefore should be approached cautiously. Long-term use of gastric antisecretory drugs, as well as prokinetic agents such as metoclopramide, has been recommended to decrease gastroesophageal (GE) reflux effects. Uncommonly, tube feedings may be required for patients with significant weight loss who are unable to maintain weight or for those only able to take in liquids. Surgical intervention may be required for patients who develop perforation or fistula. Finally, it is important to note that the clinical symptoms associated with late radiation injury are often difficult to distinguish from those caused by recurrent or new primary malignancies. Patients with strictures or ulcerations should also be evaluated to differentiate chronic radiation changes from cancer recurrence.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here