Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The liver is the central organ responsible for carbohydrate metabolism. The liver stores carbohydrates in the form of glycogen and synthesizes glucose through glycogen breakdown and gluconeogenesis. Glucose is an essential nutrient for the function of both the central nervous system and muscle. Disorders of carbohydrate metabolism may be acquired or inborn. This chapter highlights the molecular basis, clinical presentation, diagnosis, and therapy of the most common errors in carbohydrate metabolism. Nonalcoholic fatty liver disease (NAFLD), galactosemia, hereditary fructose intolerance, fructose 1,6-bisphosphatase deficiency, and glycogen storage disease (GSD) are discussed.
NAFLD, the most common reason for chronically elevated aminotransferases among both adults and children in the United States, is a clinicopathologic condition characterized by abnormal lipid deposition in the absence of alcohol intake, genetic abnormalities, and medications. NAFLD represents a spectrum of diseases, ranging from simple steatosis to steatosis in association with necroinflammation and fibrosis (nonalcoholic steatohepatitis [NASH]) to cirrhosis. ,
The reported prevalence of NAFLD worldwide ranges from 6% to 33% of the general population, with variation based on the characteristics of the population assessed and diagnostic methods used to assess NAFLD. The exact prevalence of NAFLD in pediatrics is unknown. Elevated alanine aminotransferase (ALT) levels occur in 10% to 14% of adolescents in the United States, likely underrepresenting the true prevalence of NAFLD, which may occur despite normal aminotransferases. Estimates based on radiologic imaging suggest an 18% to 53% prevalence but also fail to identify all patients with NAFLD. A large pediatric autopsy study of NAFLD found a prevalence of 9.6% after adjusting for age, gender, and ethnicity, with a 38% prevalence in obese children. The natural history of pediatric NAFLD has been inadequately studied but suggests that children with simple steatosis may have little histologic progression, whereas those with NASH may show significant histologic progression and the development of cirrhosis. ,
Obesity, both as measured by overall body mass index (BMI) and visceral adiposity, is a significant risk factor for NAFLD. In addition, hypertriglyceridemia, type 2 diabetes mellitus, insulin resistance, and metabolic syndrome are common in patients with NAFLD. Although most children with NAFLD are between the ages of 11.5 and 13.5 years, likely corresponding to a peak in pubertal insulin resistance, an increasing number of young school-age children are also coming to medical attention. In pediatrics, males are more commonly affected (2:1) than females, suggesting that sex steroids may affect the development of NAFLD. Ethnic variations also exist, with a relative paucity of NAFLD in African Americans, compared with whites and Hispanics.
The etiopathogenesis of NAFLD and its progression to NASH are multifactorial. Children at increased risk for NAFLD may have a genetic predisposition, including mutations or single nucleotide polymorphisms (SNPs) in genes such as PNLA3, which encodes adiponutrin, a lipase that regulates triglyceride and retinoid metabolism, or TM6SF2, which also encodes a protein that regulates hepatocyte lipid content. , Epigenetic factors that cause heritable changes in gene expression without directly altering an individual’s DNA code itself may also impact the dysregulation of metabolic pathways that influence the development of NASH. Central to the development of fatty liver disease is abnormal lipid homeostasis. Insulin resistance suppresses glycogenesis, promotes gluconeogenesis and glycogenolysis, and increases the release of free fatty acids from adipose tissue. Uptake of circulating free fatty acids by hepatocytes is unregulated, resulting in increased triglyceride synthesis and impaired free fatty acid oxidation, producing excess hepatocyte lipid. , Hyperinsulinemia may also increase hepatic triglyceride synthesis by overstimulating sterol regulatory element–binding protein (SREBP)-1c. ,
The progression to NASH may also be caused, in part, by increased hepatocyte susceptibility to oxidative stress, through the generation of reactive oxygen species (ROS) formed by lipid peroxidation and peroxisomal β-oxidation. The upregulation of various cytochrome P450 (CYP) systems, particularly CYP2E1 and CYP4A, also supports the role of oxidative stress in the pathogenesis of NASH. CYP2E1 and CYP4A are key enzymes responsible for microsomal lipooxygenation. Peroxisome proliferator activated receptor α (PPAR-α), a transcription factor that regulates microsomal and peroxisomal lipid peroxidation, also contributes to the development of NASH through the formation of ROS. By-products of oxidative stress and lipid peroxidation are powerful chemoattractants of neutrophils and stimulate hepatic stellate cells responsible for fibrosis. Oxidative stress also stimulates the release of inflammatory cytokines, including leptin and tumor necrosis factor α (TNF-α). TNF-α, a proinflammatory and proapoptotic cytokine important in white blood cell recruitment, is increased in patients with NAFLD. , Leptin, a regulator of body weight and energy expenditure, is crucial in preventing lipid accumulation in nonadipose tissue such as myocardial and skeletal muscle and liver. Leptin likely has a role in the shunting of fat toward β-oxidation and away from triglyceride synthesis. In addition, adiponectin, an antiinflammatory cytokine that typically inhibits fatty acid uptake, stimulates fatty acid oxidation and lipid export, and enhances insulin sensitivity, is decreased in NAFLD. Recent studies suggest that prolonged disruptions in the circadian rhythms in mice with NAFLD may induce development of NASH. This may occur by means of dysregulation of the cross talk between two nuclear hormone receptors, farsenoid X-receptor (FXR) and constitutive androstane receptor (CAR), with suppression of FXR, hepatic bile acid accumulations, bile acid induction of CAR, and eventually liver injury and fibrosis. , , Although much has been learned about the pathophysiology of NAFLD and its progression to NASH and cirrhosis, many questions remain unanswered and are under active investigation.
Most children with NAFLD are asymptomatic. Elevated aminotransferases or increased hepatic fat on abdominal imaging is often discovered during testing performed for unrelated reasons. The typical patient is overweight or obese, although NAFLD may occur in lean individuals. Patients may have complaints of fatigue, constipation, or mild abdominal pain that is generalized or localized to the right upper quadrant. Physical examination findings may be normal or demonstrate obesity (particularly central obesity), mild-to-moderate hepatomegaly, and acanthosis nigricans (a sign of insulin resistance).
Laboratory evaluation reveals mild-to-moderate elevations of serum aminotransferase levels, typically less than 1.5 times normal, with an ALT-to–aspartate aminotransferase (AST) ratio greater than 1. Transaminase elevation cannot reliably confirm the diagnosis of NAFLD or predict the presence of fibrosis. Importantly, biopsy-proven NAFLD may occur with completely normal transaminase levels. Total and direct bilirubin levels are typically normal, although γ-glutamyl transpeptidase (GGTP) and alkaline phosphatase may be mildly elevated in less than 50% of cases. Patients affected by NAFLD may also have hyperglycemia and hyperlipidemia, particularly hypertriglyceridemia.
NAFLD, to some extent, is a diagnosis of exclusion. In patients with chronic hepatitis in whom NAFLD is suspected, a thorough evaluation and systematic exclusion of other etiologies of liver disease is necessary. These include Wilson disease (with a serum ceruloplasmin), α-1-antitrypsin deficiency (using an α-1-antitrypsin level and/or phenotype), viral hepatitis (using a hepatitis C antibody and hepatitis B surface antigen [HBsAg]), and autoimmune hepatitis (with an antinuclear antibody, antiactin antibody, and anti–liver kidney microsomal antibody). Low titers of elevated serum autoantibodies may occur in up to 3% of adults and 15% of children with NAFLD. Care providers should also consider the potential for regular alcohol use as a cause of liver disease, particularly in adolescents.
Abdominal imaging may help to confirm hepatic fatty infiltration consistent with NAFLD. Abdominal ultrasound, widely used because of ease of performance, noninvasive nature, and relative low expense, requires that at least 30% hepatic fat be present for detection, is not quantitative, and may be technically challenging to perform in patients with significant central obesity. Current guidelines do not recommend the routine use of abdominal ultrasound to diagnose NAFLD. Ultrasound may be used to evaluate for other anatomic abnormalities within the liver. An abdominal computed tomography (CT) scan may be also be used but has the additional disadvantage of radiation exposure. Magnetic resonance imaging (MRI), although more costly, is more sensitive than other imaging modalities in detecting lesser amounts of fat and allows for more definitive hepatic fat quantification when performed using the modified Dixon technique or with magnetic resonance spectroscopy (MRS). , However, none of the currently available imaging modalities allows differentiation of benign steatosis from NASH or has the ability to grade the severity of inflammation or definitively stage fibrosis. Emerging data show promising results using transient elastography for noninvasive prediction of fibrosis in pediatric NAFLD.
Currently, liver biopsy is the only reliable method for assessing the presence and extent of necroinflammation and fibrosis in NAFLD. Liver biopsy should be considered in patients who are a poor clinical fit for the previously described classic picture of NAFLD and for patients with chronic hepatitis (elevated aminotransferases for more than 6 months). Liver biopsy should also be performed prior to the start of any pharmacologic therapy for NASH. Liver biopsy is safe, even in obese children, with no greater risk for complications than in nonobese children. Evidence of steatosis in more than 5% of hepatocytes is necessary for the histologic diagnosis of NAFLD. In addition, patients with NASH have a mixed inflammatory infiltrate with scattered polymorphonuclear leukocytes and mononuclear cells and hepatocyte ballooning. , There are two prevalent histologic patterns of NASH. In type 1, or “adult type” NASH, the injury occurs in zone 3. In contrast, in “pediatric” type 2 NASH, inflammation and fibrosis are accentuated in the portal areas. Type 2 NASH occurs in 28% to 51% of pediatric patients, type 1 NASH in 2.4% to 17% of pediatric patients, and an overlap pattern in 16% to 52% of pediatric patients. Therefore a spectrum of disease patterns likely exists in pediatric NASH.
Currently, two main scoring systems are used to assess histologic severity of NAFLD. The Brunt scoring system, a semiquantitative three-tiered grading and staging system to evaluate steatosis, ballooning, and lobular and portal inflammation, is widely used clinically. , The other scoring system, developed for research purposes by the NASH Clinical Research Network (NASH CRN), generates an NAFLD Activity Score (NAS), which results from adding up individual scores for steatosis (0 to 3), lobular inflammation (0 to 2), and ballooning (0 to 2). Patients with a score of greater than 5 are classified as “definitive NASH,” patients with a score of 2 or less are classified as “not NASH,” and scores of 3 to 4 are “borderline NASH.”
The goal of treating NAFLD is regression of disease. Treatment of NAFLD focuses on slow, progressive weight loss through a combination of dietary modifications and exercise programs. Loss of 3% to 5% of body weight may improve steatosis, whereas up to 10% of body weight loss is necessary to improve necroinflammation. , Although the optimal diet for treating NAFLD is not well established, the importance of insulin resistance in the pathogenesis of NAFLD suggests that diets low in fructose and those with a low glycemic index may be beneficial. , , Bariatric surgery in adults, both gastric bypass and laparoscopic adjustable gastric banding, has shown early promise, although a lack of randomized controlled trials for NAFLD treatment precludes a definitive assessment of the risk/benefit profile. , , Bariatric surgery is not recommended as a specific therapy for pediatric NAFLD, because there is a lack of NAFLD-specific outcomes data in adolescents. However, it may be considered in adolescents with a BMI of 35 kg/m 2 or greater with noncirrhotic NAFLD and other accompanying significant comorbidities, such as type 2 diabetes, sleep apnea, and hypertension.
There are currently no available medications recommended to treat pediatric NAFLD, although great interest lies in potential pharmacologic therapies for NAFLD. Although orlistat (an enteric lipase inhibitor) has been used for weight loss, it does not improve liver histologic findings. Metformin does not improve either liver aminotransferases or liver histologic findings in children with NAFLD. Treatment with the thiazolidinediones such as pioglitazone may improve aminotransferases, in addition to demonstrating histologic improvement of NASH, as was seen in the Pioglitazone or Vitamin E for NASH (PIVENS) study, a large multicenter randomized controlled trial of pioglitazone, vitamin E, and placebo. However, a meta-analysis noted that, although pioglitazone improved both steatosis and inflammation, it did not affect fibrosis. Furthermore, there remains concern about the long-term safety of thiazolidinediones, including cardiovascular disease, congestive heart failure, bladder cancer, and bone loss. Clinical trials of antioxidants have occurred based on the role of oxidative stress in the pathogenesis of NAFLD. The PIVENS trial noted improvement in liver histology using 800 IU/day of vitamin E in adults. In a parallel randomized controlled trial of pediatric NAFLD subjects given either metformin, vitamin E, or placebo (TONIC), a sustained reduction of ALT level was not detected between groups, but those treated with vitamin E did exhibit significant histologic improvement compared to placebo. Therefore vitamin E may offer histologic benefits to children with biopsy-proven NASH, but further studies are needed to confirm these initial findings. Ursodeoxycholic acid, a cytoprotective bile acid, is not effective in improving histologic parameters in NASH. , Omega-3 fatty acids, which are commonly used to treat hypertriglyceridemia, show promising early results in preliminary studies.
Galactosemia, an inborn error of galactose metabolism, is an autosomal recessive condition affecting one in 50,000 live births. It is caused by a cellular deficiency in one of three enzymes in the pathway of glucose-to-galactose conversion. The classic form of galactosemia, presenting with malnutrition, growth failure, and progressive liver disease, results from a deficiency in galactose-1-phosphate uridyl transferase (GALT). GALT is a 43-kDa protein encoded by a 4-kb gene on chromosome 9p13. A majority of patients with galactosemia have missense mutations, of which more than 150 have been identified. , The Q188R mutation, affecting 60% to 70% of whites, where arginine is substituted for glutamine, results in no enzymatic activity. , The milder Duarte variant ( N314D mutation) involves a change from asparagine to aspartate, resulting in decreased enzymatic activity. A rarer deficiency in uridine diphosphate galactose-4-epimerase results in a clinical presentation similar to that of GALT deficiency, whereas a galactokinase deficiency results primarily in cataract formation. , Galactose is a monosaccharide derived from the hydrolysis of the milk sugar lactose. Lactose is converted to glucose and galactose by the enterocyte brush border enzyme lactase. Galactose is then transported across the enterocyte by a sodium-glucose/galactose transporter. Galactose is metabolized to glucose by a series of reactions that begin with the phosphorylation of galactose to galactose-1-phosphate ( Fig. 73.1 ). Galactose 1-phosphate is further converted to glucose 1-phosphate by GALT. In the absence of GALT, alternative pathways overproduce galactitol and galactonate, potentially toxic metabolites.
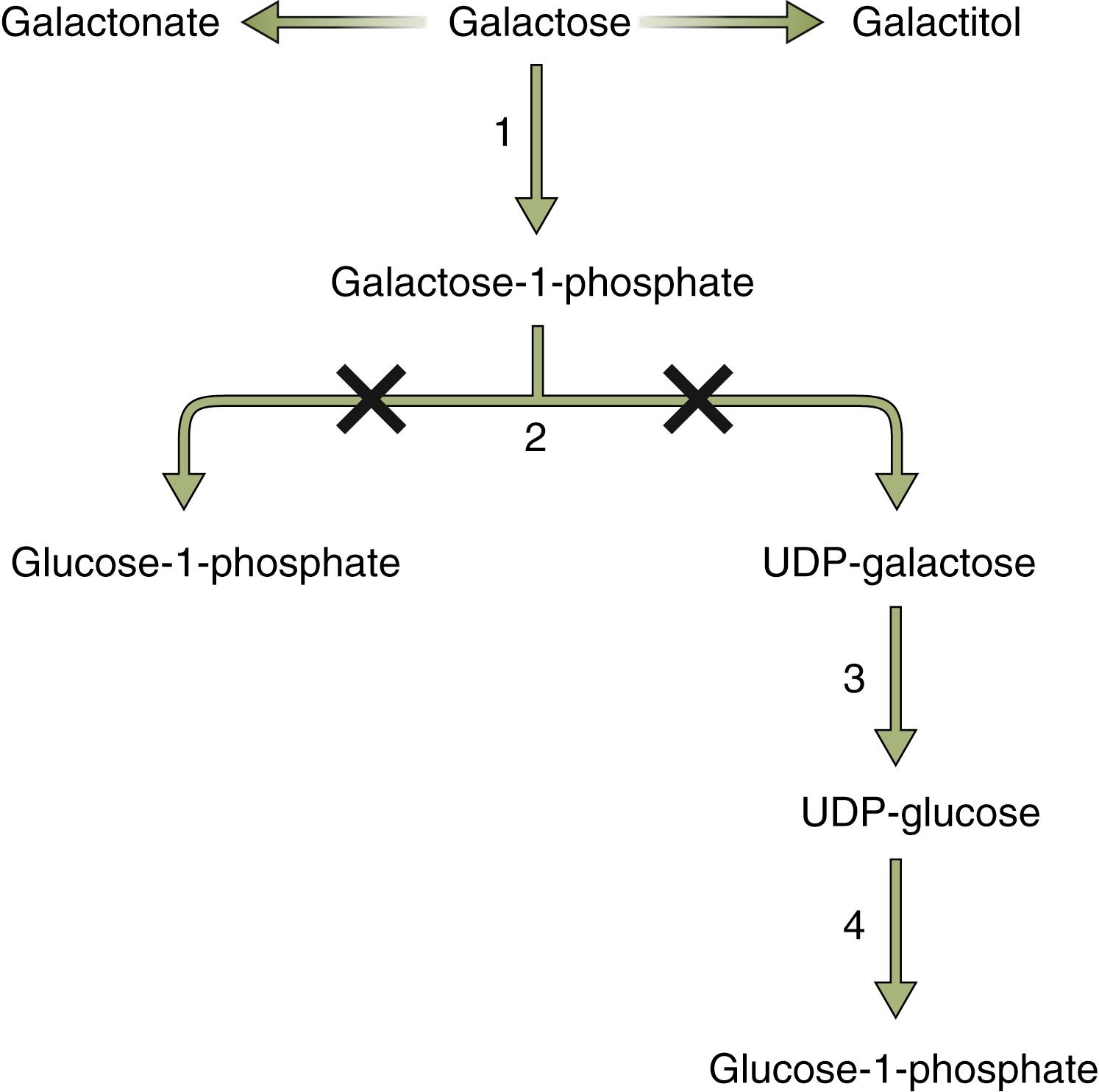
Most contemporary cases of galactosemia are detected through the newborn screening process. However, patients may present with failure to thrive, weight loss, and emesis after the initiation of dietary lactose, either via breast milk or a lactose-containing formula. Vomiting and diarrhea are almost universally present. Patients may present with hypoglycemia and encephalopathy in the first few days of life, or they may present several days later with jaundice, ascites, hepatomegaly, splenomegaly, and liver failure. Hemolytic anemia may also be observed. Cataracts, formed because of increased oncotic pressure exerted by the accumulation of galactitol in the lens, may be present at birth if the mother ingested large amounts of dairy late in pregnancy, or may develop postnatally, but they may be difficult to detect without a slit lamp examination. A strong correlation between galactosemia and neonatal Escherichia coli sepsis exists. Thus the presence of E. coli sepsis in the neonate should prompt evaluation of galactosemia. Renal tubular dysfunction with albuminuria, aminoaciduria, and galactosuria may also occur. Furthermore, patients may manifest increased blood and urinary galactose levels and hyperchloremic acidosis.
Long-term prognosis is good for patients with early diagnosis and intervention. Acute symptoms and biochemical changes regress rapidly upon withdrawal of lactose products. Long-term follow-up data of those treated exhibit some variability. Growth and liver function revert to normal. Mental retardation is the most devastating result of toxicity. Intelligence, as measured by IQ, appears highly correlated with adequate dietary control. Some patients manifest residual defects in mental functioning despite dietary restrictions and normal IQ. These include speech and language delays, spatial and mathematic learning disabilities, short attention spans, abnormal visual perceptions, tremor, and ataxia. In addition, there is a high incidence of postnatal hypergonadotropic hypogonadic ovarian failure, and pregnancy in affected female patients is rare. , Osteoporosis also commonly occurs in affected female patients, who may benefit from appropriate nutritional supplementation. These long-term complications may be the result of endogenous galactose synthesis.
A diagnosis of galactosemia should be suspected in patients with any of the constellation of symptoms described previously. The presence of urinary reducing substances in the absence of glucosuria is suggestive of galactosemia but is neither sensitive nor specific. False negatives may occur with poor lactose intake or intermittent excretion of galactose. False-positives may occur with severe liver disease and some medications. A diagnosis of galactosemia should be confirmed by quantitative measurement of GALT activity in red blood cells, which will be decreased. If an infant affected by galactosemia has received a red blood cell transfusion, the red cell enzyme level may be falsely elevated and the diagnosis missed. Prenatal diagnosis is possible by measuring enzymatic activity of tissue or cells obtained by chorionic villus sampling or amniocentesis. In the United States, most patients are detected through newborn screening programs.
Treatment of galactosemia is based on elimination of dietary galactose. In infants, this is achieved by feeding soy or protein hydrolysate formulas. In older children and adults, dietary avoidance of dairy products, with special attention to food additives, is needed. Some concern exists regarding galactose toxicity from certain grains, fruits, and vegetables. Controversy exists regarding safe or acceptable levels of galactose. In addition to dietary manipulation, close monitoring of neurodevelopment in children and yearly ophthalmologic examinations to evaluate for cataracts are also recommended.
Hereditary fructose intolerance is an autosomal recessive metabolic disorder (OMIM 229600). Occurring in approximately 1 in 20,000 live births, it is caused by a deficiency of fructose-1,6-bisphosphate aldolase (aldolase B), one of a set of enzymes that converts fructose to intermediate constituents of the glycolytic-gluconeogenic pathway. These intermediary products are then further metabolized to glucose and glycogen ( Fig. 73.2 ). Aldolase B is the product of a 14.5-kb gene on chromosome 9q22.3 that encodes a 364-amino-acid polypeptide. It is expressed in the liver, kidney, and small intestine. More than 20 different mutations and five polymorphisms have been described. , Among people of northern European descent, the A149P allele is most common. The A174D allele is predominant in central and southern Europeans, whereas the N334K allele is predominant among those of central and eastern European descent.
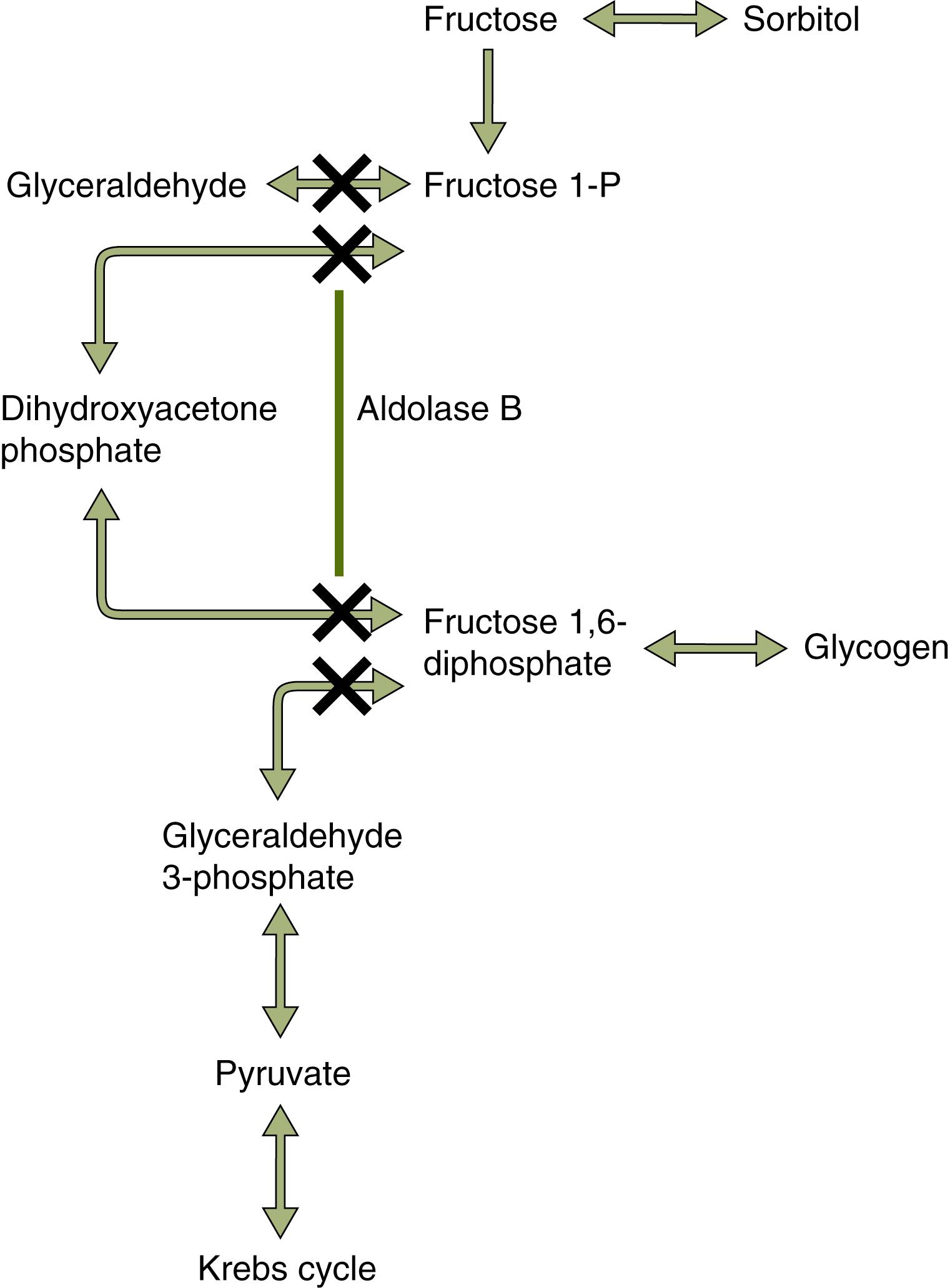
Fructose is transported into hepatocytes and intestinal cells via glucose transporter type 5 (GLUT5), a sodium-independent transporter. It is then phosphorylated into fructose-1-phosphate by fructokinase. Fructose-1-phosphate is cleaved by aldolase B into d -glyceraldehyde phosphate and dihydroxyacetone phosphate. These intermediates may then directly enter the glycolytic pathway or the gluconeogenesis pathway or become synthesized into glycogen (see Fig. 73.2 ). Accumulation of fructose-1-phosphate results in hypoglycemia secondary to impaired glycogenolysis as a result of concurrent inhibition of glycogen phosphorylase. Gluconeogenesis is also impaired because glyceraldehyde-3-phosphate and dihydroxyacetone phosphate cannot be converted. In addition, the formation and sequestration of large quantities of fructose-1-phosphate result in adenosine triphosphate (ATP) and glutamine triphosphate (GTP) depletion with impaired protein synthesis.
Patients affected by this disorder remain completely asymptomatic unless they consume fructose-containing foods. Newborns fed breast milk do not manifest symptoms because breast milk contains lactose (made of glucose and galactose). Symptoms occur as infants are weaned and either receive sucrose-containing formulas or begin baby foods, with accumulation of fructose-1-phosphate in tissues where aldolase B is usually expressed. The most common symptoms are poor feeding with failure to thrive, vomiting, and irritability. In addition, diarrhea, abdominal pain, and severe hypoglycemia with sweating, trembling, pallor, and metabolic acidosis can occur. Chronic exposure to fructose leads to failure to thrive, signs of liver disease (hepatomegaly, splenomegaly, ascites, and rarely liver failure), and proximal renal tubular dysfunction (renal tubular acidosis, hypophosphatemia, and rickets). , Occasionally, affected patients remain undiagnosed into adolescence or adulthood because of self-imposed dietary restrictions. Laboratory abnormalities are consistent with the affected organ systems. Patients may have elevations in serum liver transaminases, prothrombin time, uric acid, and magnesium and decreased serum levels of protein, potassium, and phosphate. Urine studies reveal increased reducing substances, proteinuria, aminoaciduria, organic aciduria, and fructosuria. Patients may also have anemia and thrombocytopenia. Histopathologic evaluation of liver biopsies shows steatosis with scattered hepatocyte necrosis and interlobular or periportal fibrosis. Electron microscopy reveals intracellular deposits of fructose-1-phosphate with polymorphous, electron-dense cytoplasmic inclusions in concentric membranous arrays.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here