Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Radiofrequency ablation, particularly pallidotomy, thalamotomy, and subthalamotomy, has been an effective surgical option for symptomatic relief of Parkinson disease for over 80 years.
Ablation is particularly useful in patients for whom implanted hardware is contraindicated (e.g., infection risk, limited access to follow-up) or in patients for whom cost or access to care is prohibitory.
Lesioning the posteroventral globus pallidus internus (GPi) or the dorsomedial subthalamic nucleus (STN) normalizes downstream pathologic oscillatory activity in related thalamic, cortical, and brainstem motor areas related to movement initiation. Lesioning the ventral intermediate (VIM) nucleus of the thalamus normalizes oscillatory activity underlying tremor in the cerebellothalamic to primary motor cortex circuit.
Radiofrequency ablation is guided by neurophysiologic mapping (e.g., microelectrode recording and/or macrostimulation mapping) and clinical responses in the awake patient. Electrodes are not considered MRI-compatible, therefore MR-guided thermometry is not a feasible approach.
Thalamotomy provides the most consistent tremor suppression, but pallidotomy also alleviates the other motor symptoms of Parkinson disease, including rigidity, bradykinesia, levodopa-induced dyskinesias, and dystonia. Subthalamotomy provides benefits similar to pallidotomy but has been additionally associated with decreased dopaminergic medication needs following the procedure.
Incisionless surgical approaches to thermocoagulative ablation, including stereotactic radiosurgery and the more recent high-intensity focused ultrasound and laser interstitial thermal therapy (all of which are leverage advances in MRI) are growing in use because of the appealing ease of surgery on patients, ever enriching the neurosurgical armamentarium for ablative therapy for Parkinson disease.
Patients with advanced Parkinson disease (PD) experience increasing motor and nonmotor symptoms and waning effectiveness and/or serious adverse effects of optimized medication management. For these patients, there are now several nonablative surgical options for symptomatic relief, including deep brain stimulation (DBS) and, in some centers, experimental neural transplantation or gene therapy. However, ablative procedures remain a viable and well-founded surgical option. For over 80 years, ablative surgeries of the basal ganglia and associated structures, most notably pallidotomy, thalamotomy, and subthalamotomy, have been used in the treatment of PD, and in fact were the primary treatment for decades before the advent of levodopa medical therapy. Particularly in the past 30 years, with great strides made in the understanding of basal ganglia physiology and PD pathophysiology coupled with significant advances in imaging technology and stereotactic equipment, these neurosurgical procedures have become safer, more effective, and more accurate, providing patients with years of improved quality of life.
Surgical interventions to address the symptoms of movement disorders—as striking and disabling as they are—have a long history, dating back nearly to the dawn of neurosurgery itself. The first published report, in 1890, described the treatment of choreoathetosis by resection of the premotor cortex. For the symptoms of PD, a variety of targets were tried in the 1930s and 1940s including lesions in the cerebral cortex and corticospinal system, in the pyramidal motor system (e.g., medullary pyramids and cerebral peduncles), in nonmotor targets (e.g., sympathectomy), and in extraneural targets (e.g., thyroid). Although these surgeries alleviated tremor, they often left other motor deficits. In the mid-1920s, Spatz discovered the extrapyramidal motor system, which included the modern basal ganglia nuclei and associated structures, through investigations into the caudate nucleus in Huntington disease. , Meyers was first to perform extrapyramidal lesions to address PD, initially ablating the caudate nucleus and then other regions of the basal ganglia circuitry in the 1940s, including the globus pallidus internus (GPi). , After sectioning of the pallidofugal fibers, parkinsonism was improved,particularly tremor,without creation of a pyramidal deficit. This laid the foundation for Spiegel, Wycis, and colleagues’ seminal 1947 paper on the use of a stereotactic apparatus in neurosurgical procedures, which was based on the original Horsley-Clarke stereoencephalotome for small animals. They began to perform pallidotomy for movement disorders in the late 1940s, providing a safer and more precise approach to the procedure. Soon other surgeons began to perform pallidotomies and develop their own instruments and protocols, including Bertrand, Leksell, Gillingham, , Guiot, Hassler along with colleagues Riechert and Mundinger, Narabayashi, , and Orthner and Roeder. , Cooper serendipitously happened upon pallidotomy when he damaged and then ligated the anterior choroidal artery during a pedunculotomy, only to find that his patient had marked tremor relief; he then went on to perform thousands of stereotactic pallidotomies.
Although great successes were observed with pallidotomy, some disappointing results led to the exploration of other ablative targets, seen in Hassler’s work in thalamotomy , and Cooper and Bravo’s work in chemothalamotomies. They discovered that what they intended to be a pallidotomy was sometimes found on postmortem analysis to be a thalamotomy. Hassler specifically targeted the ventrolateral (VL) nucleus of the thalamus, basing his procedure on a study of the anatomic connections between the pallidum and thalamus. VL thalamotomy was also perceived as more favorable for patients with severe bilateral symptomatology as it could be performed contralateral to a pallidotomy, thus having a bilateral effect while unilaterally preserving each structure, although some did choose to perform bilateral thalamotomies. Additionally, thalamic lesions were considered to carry less risk than pallidal lesions, with GPi ablation having a higher risk for collateral damage to the optic tract and internal capsule and a higher risk for hemorrhage. Hence beginning in the mid to late 1950s and reaching a peak in the 1960s, stereotactic neurosurgery, especially thalamotomy, became a prominent mode of therapy for PD. Through the use of microelectrode recording, first used in the 1960s, Guiot and Narabayashi , were able to define the ventral intermediate (VIM) nucleus of the thalamus and determine that it was the best target to control tremor in PD. The VIM target provided little relief of akinesia and rigidity, however it provided a rationale for combining pallidotomy with thalamotomy, where the second target would be lesioned only if necessary, depending on the response to the initial lesion. Subthalamotomy to relieve parkinsonian symptoms such as tremor and rigidity was also explored beginning in the 1960s and 1970s, such as with Andy and colleagues, Mundinger, and Velasco and colleagues, although the exact subthalamic region targeted was quite varied among surgeons.
Levodopa treatment for PD was introduced in 1961 and became widely available by 1968, , close to half a century after dopamine’s discovery in 1913 by Guggenheim. Its remarkable benefits and rapidly growing use led to a significant decline in surgical interventions for PD. However, within a few decades it became appreciated that long-term use of levodopa required an increasing cumulative dose to maintain effective symptom management and could result in medication-induced toxicity (e.g., worsening dyskinesia and “on-off” fluctuations), waning efficacy with disease progression, and significant end-dose deterioration. Given these observed effects with chronic use, there was a clear renewed need for supplementary treatment options. This drove a resurgence of stereotactic neurosurgical treatments with Kelly and coworkers reintroducing thalamotomy , and Laitinen and coworkers reintroducing Leksell’s pallidotomy, which coincided with advances in brain imaging (CT, MRI) and intraoperative electrophysiologic techniques. Moreover, physiologic and anatomic support for these targets was coincidentally being propelled by the work of DeLong and colleagues, Filion and Tremblay, and Albin and colleagues on the circuitry of the basal ganglia and the pathophysiology of PD, in large measure based on the advent of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) nonhuman primate model of PD. Thus the basal ganglia and associated structures were further solidified as the surgical target of choice, being associated with significant and sustained motor benefit contralateral to the lesion. , ,
Although through the 1990s pallidotomy and (to a lesser extent) thalamotomy were increasingly reevaluated for PD and other movement disorders, this resurgence was short-lived. In the late 1980s and early 1990s, Benabid and Siegfried and their colleagues introduced DBS of the basal ganglia (subthalamic nucleus [STN] and GPi) and thalamus as an alternative to lesioning, which rapidly became the standard surgical therapy for providing relief of symptoms in patients with advanced levodopa-responsive PD and/or tremor. DBS supplanted lesioning because of equivalent efficacy and improved safety profile, including in the setting of bilateral implantations. Nevertheless, in many areas of the world, especially the developing world, and where cost is a prohibiting factor, subthalamotomy and pallidotomy still remain the first-line surgical option. , , Moreover, because of its incisionless nature, lack of need for programming, and cost-benefit position, the advent of MR-guided focused ultrasound (MRgFUS) has driven rapid proliferation of both thalamotomy and more recently pallidotomy and even subthalamotomy in both more and less economically advanced regions.
The fundamental deficiency in PD lies in the substantia nigra pars compacta (SNc). Loss of nigrostriatal dopaminergic neurons ultimately results in increased inhibitory γ-aminobutyric acid (GABA)-ergic output from the GPi and the substantia nigra pars reticulata (SNr), the latter being less important in primates than in rodents. , ( Fig. 110.1A – B ). GPi hyperactivity excessively inhibits the thalamus and its excitatory drive of the downstream cortical motor systems and brainstem motor areas. This dysregulation is exacerbated by the irregular output of GPi in PD, and thus also of the thalamocortical projections. More recently it has been appreciated that pathologic beta-range oscillations reverberate through the circuitry. , Ultimately the thalamus and brainstem are less able to regulate movement, especially the initiation of movement, leading to the classic clinical symptoms of disrupted and impoverished movements. Pallidotomy (specifically posteroventral pallidotomy) involves selective ablation of the sensorimotor regions of the GPi, decreasing its inhibitory output and thereby normalizing downstream thalamocortical activity and brainstem motor area function (see Fig. 110.1C ). The lesions are targeted to the sensorimotor portion of the GPi, which is defined by a population of neurons that respond to movement, whereas the limbic and associative territories of the GPi are avoided, as are the adjacent globus pallidus externus (GPe), optic tract, and internal capsule.
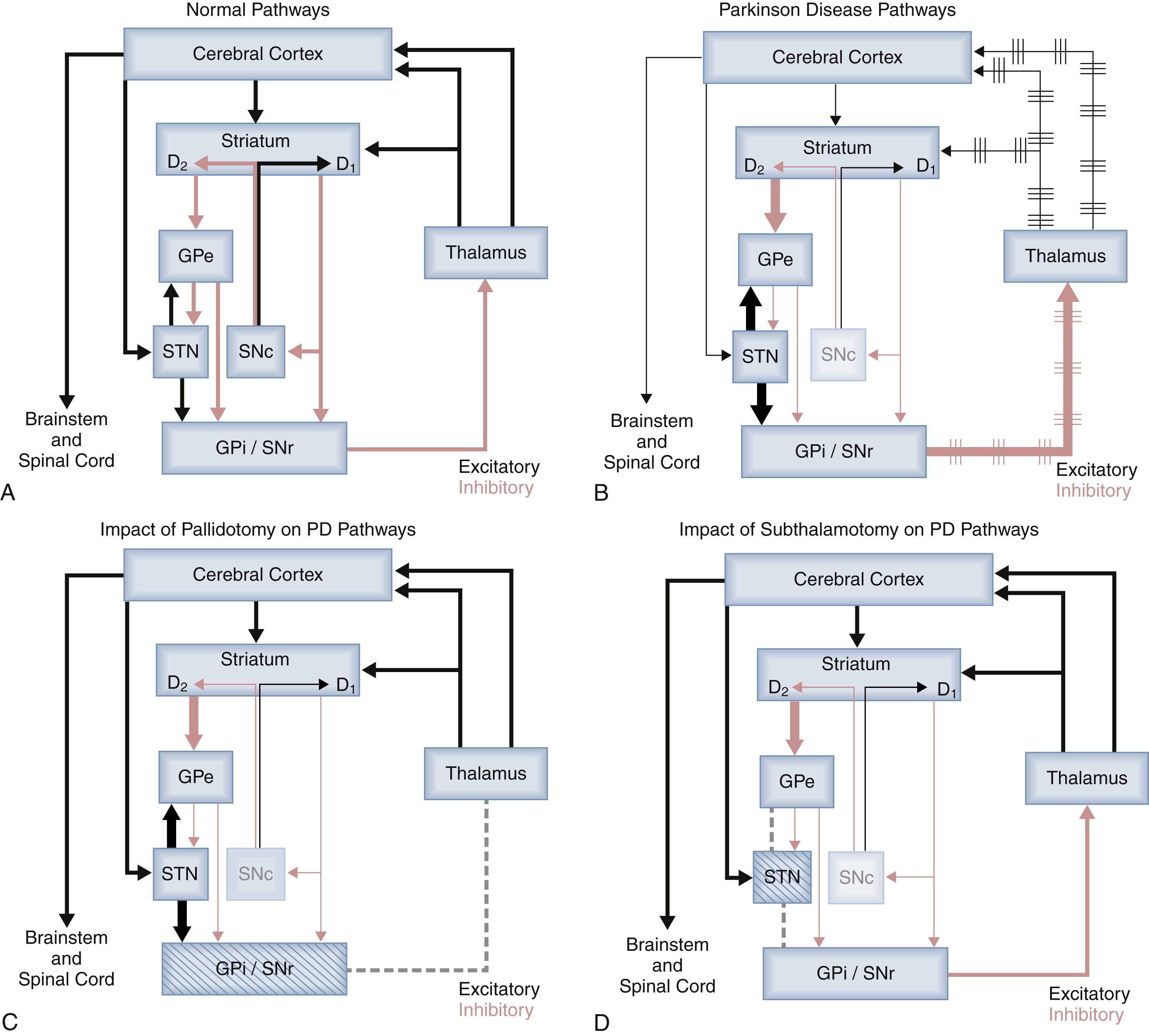
Historically, subthalamotomy involved lesions of the thalamic afferent fibers coursing below the thalamus rather than the STN itself, which was carefully avoided because of the specter of known association of hemiballismus with strokes involving the nucleus itself. In the 1990s during the resurgence of pallidotomy, subthalamotomy as previously performed did not receive renewed attention, perhaps because of lack of a known physiologic basis for this operation. However, the seminal research of DeLong and colleagues identified the central role of STN hyperactivity resulting from dysregulation of the indirect pathway caused by the dopaminergic deficit (see Fig. 110.1B ). Subsequently, they demonstrated in the nonhuman primate MPTP model that blocking this activity leads to amelioration of symptoms, thus providing a physiologic basis for STN ablation (subthalamic nucleotomy) in PD patients, as well as STN DBS , , , (see Fig. 110.1D ). Further physiologic evidence substantiating this target is that ablation of the STN leads to decreased GABAergic activity downstream in GPi outputs to the thalamus.
The VIM nucleus of the thalamus, which predominantly receives contralateral cerebellar inputs, sends projections ipsilaterally to the primary motor, premotor, and supplementary motor cortical areas. In PD, it has been shown that many cells in the VIM nucleus have a discharge pattern synchronized with the patient’s tremor, which have been dubbed tremor cells . Given these findings, additional research was undertaken to further characterize the pathogenesis of PD as related to the tremor cells. However, a satisfactory understanding of the pathogenesis of tremor and tremor cells in PD remains elusive. As a justification for surgical treatment within the VIM for tremor (not just from PD, but tremor of essentially all origins such as essential tremor and multiple sclerosis), we still rely on the empirical observations made decades ago: intraoperative electrical stimulation of the tremor cells, ostensibly to inhibit them, produces tremor arrest, and VIM thalamotomy produces long-term tremor relief. , That VIM plays a peripheral role in the pathogenesis and pathophysiology of PD generally is reflected in the fact that thalamotomy has little or no impact on the other features of PD such as rigidity, bradykinesia, and gait disturbances.
Stereotactic ablative surgery is indicated for PD patients who respond suboptimally to dopaminergic therapy, experiencing significant impairments in quality of life in the form of “off” periods, dyskinesia, and/or tremor. The “off” state is characterized by the presence of bradykinesia, rigidity, tremor, and gait disturbances. Early in PD, patients have an adequate albeit decreased reserve of dopaminergic nerve terminals in the striatum, so dopaminergic therapy is effective, depending on uptake of the levodopa precursor of dopamine, conversion to dopamine, and its storage and physiologic release. For 5 to 10 years, typically, symptomatic treatment with dopaminergic agents is effective to the point that minimal symptoms are encountered throughout the day. However, with progression comes further nerve terminal loss; the loss of vesicular storage capacity shortens the response time to levodopa, the benefits of which become more and more dependent on the pharmacokinetics of delivery. This produces the “wearing off” phenomenon and motor fluctuations. Furthermore, the periods during which the synapse is devoid of dopamine lead to postsynaptic remodeling, with sensitization of receptors and the development of tolerance, and resultant drug-induced dyskinesia. , Wearing off, unpredictable motor fluctuations, and drug-induced dyskinesia compose the effects of disease progression and complications of therapy that determine the timing of surgical candidacy.
Patients are therefore screened and evaluated on and off medication to determine their medication responsiveness, including evaluations of timed motor tasks, and complications from therapy. It has become standard practice in many institutions to evaluate patients in the off-medication and on-medication states with the Unified Parkinson’s Disease Rating Scale (UPDRS) and the Hoehn and Yahr staging scale. Patients who do not manifest a significant reduction with dopaminergic medications, with the exception of tremor, are typically not good candidates for surgical treatment, and a high index of suspicion should be maintained for Parkinson plus syndromes.
Whereas nonablative DBS surgery can in many instances be performed in patients under general anesthesia, lesion surgery—being irreversible—relies almost exclusively on patients being awake and able to be examined during the ablation. A variety of medical conditions can contraindicate the surgery; therefore patients must be healthy enough to undergo an awake procedure.
Neuropsychological assessment is critical to screen out patients with cognitive decline, a risk factor for further decline with surgery. Assessment is performed using tools such as the Folstein Mini Mental State Examination or the Mattis Dementia Rating Scale. Furthermore, the gains of an improved motor status may be dampened in patients with a preexisting cognitive impairment, such that they may not derive significant improvement in quality of life. All patients who are considered “at risk” after screening should undergo further, more detailed, neuropsychological evaluation, both before and after surgery. ,
Because the main complication of the surgery is intracranial hemorrhage, cardiovascular health and stability must be ensured. This includes but is not limited to control of bleeding tendency, coagulopathy, and hypertension, the latter of which has been strongly correlated with an increased risk for intracranial hemorrhage during ablation ; discontinuation of all antiplatelet regimens for the appropriate time to reverse their actions before surgery; and clearance with cardiac stress tests for patients with a history of cardiovascular disease.
All patients are screened with MRI or, if not possible because of a cardiac pacemaker, CT. The presence of significant cerebral atrophy may predispose the patient to increased risk for subdural hematoma from traction injury to bridging veins and is therefore a relative contraindication. The presence of linear high signal in the posterolateral putamen (on T2 or proton MRI scans), sometimes combined with evidence of low signal on T2, is indica-tive of iron deposition in the lenticular nucleus and strongly suggests a diagnosis of multiple system atrophy (MSA).
An ablative procedure may be preferred over DBS for a variety reasons, including its wider availability, lower cost, decreased need for follow-up, the absence of implantation-related complications (e.g., infection, hardware damage, and malfunction), and its immediate benefit. , , However, ablation is irreversible and not modifiable. Accordingly, there may be a case in which having this more permanent treatment may preclude the patient from treatment with future therapeutic modalities. Ablative procedures may be performed contralaterally to a DBS system in patients who require bilateral treatment. However, bilateral treatment often is more prone to complications and side effects. Caution should be used when considering the combined use of ablation and DBS when mixing nuclei, as several researchers have experienced difficulty with managing the varied side effects of both the different surgical approaches and targets, particularly with respect to balancing medication and stimulation parameters. In patients who have undergone DBS but in whom hardware removal is necessary (e.g., because of infection, malfunction, or erosion), ablative surgery may be considered in lieu of reimplanting the DBS hardware. Indeed, ablative surgery can be an approach to preventing DBS withdrawal syndrome in patients who are predisposed to it (e.g., those with long-standing STN DBS). ,
Pallidotomy is indicated for the following PD features: levodopa-induced dyskinesias; severe “wearing off” or “on-off” fluctuations characterized by rigidity, bradykinesia, and tremor; “off”-period dystonia; and gait disturbances present only during “off” periods. Patients who respond well to levodopa are likely to respond well to pallidotomy, whereas those who do not respond to dopaminergic therapy are unlikely to receive much benefit with pallidotomy. The exception is tremor, which, although it may not be levodopa-responsive, will likely still improve with pallidotomy. Such patients may be better served with thalamotomy. Midline symptoms that persist during “on” periods, such as swallowing difficulty, hypophonic speech, postural instability, and freezing, are not responsive to pallidotomy and may even worsen.
The other causes of parkinsonism such as MSA, progressive supranuclear palsy, diffuse Lewy body disease, and parkinsonism secondary to multifocal ischemic white matter disease must be ruled out because patients with these disorders are unlikely to derive benefit from pallidotomy and may actually worsen. Other secondary forms of parkinsonism (e.g., pugilistic, vascular) also contraindicate surgery. Fluorodeoxyglucose–positron emission tomography (FDG-PET) can be used to distinguish a Parkinson plus syndrome from PD because Parkinson plus syndrome patients will likely show lentiform hypometabolism, whereas PD patients will likely show lentiform hyper-metabolism. In addition, PD patients will show reduced striatal 18 F-dopa uptake, reflecting loss of dopamine transporters, particularly in the posterior putamen. Furthermore, preoperative FDG-PET measurements of lentiform glu-cose metabolism have been shown to positively correlate with pallidotomy clinical outcomes.
The optimum age for pallidotomy is questionable. Studies have shown more benefit in the young, more benefit with age, and no relationship with age. Thus age is a less important selection criterion. However, pallidotomy is not offered to end-stage, wheelchair-bound, or bedridden patients because, not unlike the aforementioned cognitively impaired patients, they may not derive optimal benefit from the procedure such that their activities of daily living (ADLs) and quality of life are improved. Given the disabling nature of these conditions, these patients are often unresponsive to medication or surgery.
Although bilateral pallidotomy has been shown to produce both short- and long-term benefit to tremor, rigidity, bradykinesia, ADLs, and dyskinesia, , bilateral surgery has not been extensively evaluated in controlled trails, and the risk for impairment to speech, swallowing, cognition, and gait after bilateral pallidotomy is generally considered to be too great. , The benefits of pallidotomy are predominantly unilateral, so it is typically performed contralateral to the patient’s worse presenting side. Hence, unilateral tremor is a positive predictive factor for favorable surgical outcome. In the case of symmetrical presentation, the pallidotomy is performed in the patient’s dominant hemisphere. However, it is important to note that unilateral surgery has shown some sustained benefit to gait and postural stability in both “on” and “off” states, , , but after bilateral surgery, gait and balance issues have actually worsened. Hence when treating axial symptoms, wherein one might believe bilateral surgery is necessary, a unilateral approach can be considered. For patients with severe bilateral symptomatology, unilateral pallidotomy and contralateral GPi DBS, bilateral GPi DBS, or bilateral STN DBS , can be considered.
Although pallidotomy used to be routinely performed and now, given DBS, has more limited indications, subthalamotomy is essentially no longer used as originally performed (i.e., lesion of the fibers below the STN), and the present manner in which it is performed (i.e., actual ablation of the STN) has not been as extensively used or studied. Thus the indications for subthalamotomy (i.e., subthalamic nucleotomy as currently performed) are relatively unclear. Nevertheless, based on physiology, preclinical studies, and limited clinical studies, the following tentative indications can be put forth.
Subthalamotomy is indicated for the same patient population as pallidotomy. However, the slight variations in outcomes can be used to provide guidance as to which is the more appropriate lesion. Subthalamotomy, like pallidotomy, significantly reduces tremor, bradykinesia, and rigidity contralateral to the lesion. However, there is a greater reduction in dopaminergic medication needs after subthalamotomy than after pallidotomy because of the decreased need for dopaminergic medication and/or the tendency for subthalamotomy to lower the threshold for drug-induced dyskinesia or even to induce it independent of medication. Thus, although subthalamotomy does reduce “on”-state dyskinesia, it does not seem to reduce it as well as pallidotomy because of the risk for inducing medication-related dyskinesias, which may even necessitate subsequent pallidotomy. , , , , In addition, subthalamotomies carry a risk for hemiballismus, although the extent of this risk is debated. , , Although the number of studies is too small to draw a reliable conclusion, subthalamotomy may be safer to perform bilaterally than pallidotomy.
VIM thalamotomy is indicated in PD patients with severe asymmetrical tremor that is unresponsive to dopaminergic therapy. It should provide significant tremor relief but will not improve the other PD symptoms of bradykinesia, rigidity, micrographia, speech disturbances, and gait disturbances. There is some evidence to suggest that lesioning the ventralis oralis posterior (VOP) nucleus, a pallidal receiving area situated anterior to the VIM nucleus that also contains tremor cells, may provide improvement with rigidity and dyskinesia. , It can also be used as a treatment for patients who do not experience significant tremor relief from a prior unilateral pallidotomy. , Given that the benefit of VIM thalamotomy is confined to tremor relief, there has been a progressive decline in the number performed. It is performed unilaterally because bilateral thalamotomies have a high incidence of adverse speech and balance effects.
The basic stereotactic lesioning procedures for pallidotomy, subthalamotomy, and thalamotomy are quite similar, so they are described here together. , ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here