Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Abdominal aortic aneurysm (AAA) is a common disorder with an estimated incidence of 4%–7% in western countries. It the 13th-leading cause of death in the United States, with 15,000 deaths yearly. Ruptured AAA (RAAA) continues to carry an operative mortality of 40%–70% and overall mortality of 80%–90%, which have not improved much over the past four decades despite major advances in surgical care and the introduction of endovascular aortic aneurysm repair (EVAR). However, there appears to be a recent favorable trend in aneurysm-related mortality which may reflect increased eligibility to screening programs and sustained survival benefits from increasing use of an EVAR-first strategy in RAAA in patients with adequate imaging and suitable anatomy. , In contrast to RAAA, modern outcomes with open aortic repair (OAR) for intact AAA are excellent. Historically in the 5% to 8% range, early mortality of OAR in most population-based registries, carefully controlled trials, and high volume centers of excellence now approaches 3% ( Table 73.1 ). , , Early detection of AAA through screening and timely elective repair is the most reliable strategy for preventing aneurysm-related death with several randomized studies demonstrating 50% reduction in AAA rupture rate and overall AAA-related mortality.
| Series | Study Period | Number of Patients | Mortality | Data Source |
|---|---|---|---|---|
| Bradbury et al. | 1976–1996 | 842 | 7.5% | Royal Edinburgh Infirmary Database |
| Heller et al. | 1979–1999 | 358,521 | 5.6% | National (US) Hospital Discharge Survey |
| Johnston et al. | 1986 | 666 | 4.8% | Canadian Society for Vascular Surgery |
| Lawrence et al. | 1990–1994 | 32,389 | 8.4% | National (US) Hospital Discharge Survey |
| Galland | 1990–1995 | 2680 | 4.8% | British Joint Vascular Research Group |
| Dardik | 1990–1995 | 2335 | 3.5% | Maryland Heath Service Cost Review |
| Akkersdijk et al. | 1990 | 1289 | 6.8% | Dutch National Medical Registration |
| Kazmers et al. | 1991–1993 | 3687 | 4.9% | (US) Veterans Affairs Medical Centers |
| Bush et al. | 1991–2008 | 14,232 | 4.4% | US NSQIP-VA |
| Lee et al. | 2001 | 4607 | 3.8% | National (US) Inpatient Sample |
| Huber et al. | 1994–1996 | 16,450 | 4.2% | National (US) Inpatient Sample |
| Rigberg et al. | 1995–1999 | 9778 | 3.8% | California Statewide |
| Dimick et al. | 1996–1997 | 7980 | 3.8% | National (US) Inpatient Sample |
| Anderson et al. | 2000–2002 | 3064 | 3.9% | New York (US) Statewide |
| Schermerhorn et al. | 2001–2004 | 22,830 | 4.8% | Medicare (US) Beneficiaries |
| Schwarze et al. | 2001–2006 | 75,222 | 3.0% | National (US) Inpatient Sample |
| Davenport and Xenos | 2005–2009 | 3967 | 3.4% | US NSQIP |
| De la Motte et al. | 2007–2010 | 1176 | 3.3% | Danish nationwide cohort |
| Hicks et al. | 2007–2011 | 34,535 | 3.8% | Medicare (US) Beneficiaries |
| Grant et al. | 2008–2010 | 48,593 | 4.7% | UK National Vascular Database |
| Teixiera et al. | 2003–2014 | 3530 | 2.7% | Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) |
The landscape of AAA repair changed drastically in 1999 following approval by the FDA of two EVAR devices for commercial use. This minimally invasive therapy offered lower operative mortality compared to OAR with fast recovery and return to normal daily life and activities (see Ch. 75 , Aortoiliac Aneurysms: Endovascular Treatment). Within a decade, several high-quality randomized controlled trials had been conducted demonstrating better short-term and equivalent intermediate-term outcomes with EVAR compared to OAR. This helped propel EVAR to become the primary mode of therapy for the majority of patients with AAA, with OAR reserved for patients with increasingly complex anatomies or contraindications to EVAR. A study of Medicare recipients’ data from 2003 to 2013 illustrated the disruptive impact of EVAR on OAR, with a 76% decrease in OAR volume while EVAR volume nearly doubled. Continued growth of EVAR adoption also reflects introduction of newer generation devices and evolution of newer innovative complex EVAR techniques, notably fenestrated EVAR (FEVAR) and chimney EVAR (Ch-EVAR), enabling more minimally invasive treatment options for juxtarenal and pararenal AAAs. OAR is currently mostly offered to patients not amenable to EVAR, and as a result has become increasingly complex with requirement of suprarenal or supraceliac aortic cross-clamping in 30%–50% of cases, and a frequent need for additional reconstruction of concomitant aortoiliac occlusive disease. , , Despite the growing popularity of EVAR, OAR has maintained its status as time-tested standard of care in AAA treatment, owing to its versatility and established safety and efficacy. , , , A meta-analysis of case series comparing the outcomes of elective OAR (1575 patients) and FEVAR (751 patients) for juxtarenal AAA showed a similar perioperative mortality of 4.1%, although FEVAR was associated with higher rates of secondary reintervention and renal impairment during follow-up. A propensity-matched comparison of the outcomes of FEVAR and OAR in complex AAA of comparable anatomy showed higher morbidity and mortality with FEVAR, reaffirming the value of OAR as the gold standard and the need for further progress in endovascular approaches.
Despite growing complexity of OAR procedures, it is remarkable that the mortality rate of OAR has not increased accordingly. When examining contemporary series of juxtarenal and suprarenal AAAs and extent IV thoracoabdominal aortic aneurysms, the outcomes are quite comparable to those seen in infrarenal AAA ( Tables 73.2 and 73.3 ). A relatively recent analysis of Vascular Study Group of New England (VSGNE) registry data compared OAR outcomes in 443 complex AAA (suprarenal clamp in 340, supraceliac clamp in 103) and 1432 infrarenal AAA, demonstrating an early mortality rate of 3.6% for complex AAA repair compared to 1.2% for routine infrarenal AAA repairs.
| Publication Year | Patients | Early Mortality | Long-Term Survival | |
|---|---|---|---|---|
| Suprarenal Clamp | ||||
| Chong et al. , a | 2009 | 171 | 1.8% | 67.7% at 5 years |
| Landry et al. , a | 2009 | 82 | 6.1% | NR |
| Knott et al. , a | 2008 | 126 | 0.8% | 63.8% at 5 years |
| Chiesa et al. | 2006 | 85 | 3.5% | NR |
| Nathan et al. | 2011 | 97 | 3.4% | 69.1% at 5 years |
| Tsai et al. , a | 2012 | 199 | 2.5% | 74% at 5 years |
| Supraceliac Clamp | ||||
| Martin et al. | 2000 | 57 | 1.8% | NR |
| Martin et al. , b | 2000 | 53 | 11.0% | NR |
| Coselli et al. , b | 2007 | 329 | 3.60% | 65.3% at 5 years |
| Chiesa et al. | 2006 | 34 | 2.9% | NR |
| Kieffer et al. , b | 2008 | 171 | 13.4% | NR |
| Richards et al. , a , b | 2010 | 53 | 6.0% | 78% at 3 years |
| Nathan et al. | 2011 | 108 | 5.4% | 52.9% at 5 years |
| Nathan et al. , b | 2011 | 83 | 5.6% | 50% at 5 years |
| Patel et al. , b | 2011 | 179 | 2.8% | 62% at 5 years |
| Tshomba et al. , b | 2015 | 222 | 4.9% | NR |
| Trial | Recruitment Period | Publication Year | Follow-up (Years) | OAR Arm | EVAR Arm | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | 30-Day Mortality | Long-term All-Cause Mortality | N | 30-Day Mortality | Long-Term All-Cause Mortality | ||||
| DREAM , | 2000–2003 | 2004, 2010 | 6 | 178 | 4.6% | 70% | 173 | 1.2% | 69% |
| OVER , , | 2002–2008 | 2009, 2019 | 9 | 437 | 2.5% | 70% | 444 | 0.5% | 68% |
| EVAR-1 , | 1999–2004 | 2010, 2018 | 15 | 626 | 6.2% | 68% | 626 | 2.1% | 73% |
| ACE | 2005–2008 | 2011 | 3 | 149 | 1.3% | 54% | 150 | 0.7% | 53% |
A strong association has been repeatedly observed between hospital and operator volume of OAR procedures and perioperative adverse events and mortality. , Although outcomes with EVAR are less dependent of the surgeon’s case volume, worse EVAR outcomes are more likely to occur in low-volume facilities. However, the shift towards EVAR, along with the recent apparent decrease in AAA incidence in western countries, have lowered the exposure of vascular surgical trainees to OAR, raising concerns about maintenance of competency and highlighting a need for new training paradigms and assessment tools. ,
The argument for retaining open operative skills is important and will likely be addressed in the future by the creation of dedicated open aortic surgical training pathways and/or centralization of complex aortic care to higher volume centers. , , Sequestration of high-end operator skills and large operative volume into a smaller number of centers has been proposed as an option to provide ideal training opportunities in those facilities. , ,
Widespread adoption of EVAR has permanently changed the landscape of AAA treatment, with EVAR becoming the preferred treatment modality in most patients meeting the indications for repair. However, even in the current “EVAR era,” several scenarios remain in which OAR is preferable to EVAR and newer complex endovascular aortic interventions ( Box 73.1 ).
Unfavorable anatomy for EVAR or FEVAR (unmet IFU requirements for current approved devices)
Need to preserve patency of IMA (hypertrophied vessel, occluded SMA/CA or bilateral hypogastric arteries, or prior hemicolectomy)
Renovascular variants (horseshoe kidney, renal ectopia, multiple small renal arteries arising across the abdominal aorta, etc.)
Inability to preserve at least one hypogastric with EVAR
Symptomatic or ruptured juxtarenal AAA (inability to await construction of a custom-built FEVAR device)
Any prohibitive access issues such as excessive aortoiliac tortuosity and/or small access vessels and/or iliofemoral occlusive disease not amenable to staged optimization
Strong patient preference, with no prohibitive risk factors for OAR
Prolonged life expectancy (younger than 65 years)
Known connective tissue disorder or genetic vascular syndrome
Likely non-compliance with required follow-up requirements of EVAR, in the absence of prohibitive risk factors for OAR
Open conversion:
Infection of prior EVAR/FEVAR
Failure after prior EVAR, F/B-EVAR not amenable to endovascular rescue. Usually due to type 1 or 3 endoleak, device migration ∗
∗ Delayed open conversion with partial or total explantation.
Prior EVAR with type 2 endoleak and sac growth, refractory to endovascular treatment ∗∗
∗∗ Treatment of choice consists of oversewing of back-bleeding branches, obliterative endoaneurysmorrhaphy with stent graft preservation.
Failure after prior OAR, not amenable to endovascular rescue
Inflammatory AAA
Mycotic AAA
Infection or aortoenteric fistula ∗∗∗
∗∗∗ EVAR may be considered in those instances as a bridge option prior to definitive OAS after stabilization and/or infection source control.
The age and general health of a patient plays an important role in procedure choice. Reports on suboptimal long-term results of EVAR suggest that younger patients with long life expectancy and low perioperative risk may benefit more from open repair. Recent evidence from randomized trials and population-based studies suggests significant increase in cancer deaths in patients who underwent EVAR, raising concern for the impact of radiation exposure from lengthy complex endovascular procedures and reinterventions as well as numerous CTA studies. , This, in addition to personal preference, could impact procedure choice in favor of OAR, especially in patients with prolonged life expectancy.
Although classic cardiovascular risk factors are the leading cause of AAA, diagnosis of AAA in patients younger than 60 years should prompt evaluation for underlying genetic and/or connective tissue disorders, especially in the presence of a family history. There is a list of more than 30 inheritable conditions that can potentially manifest with aortic aneurysm. Although these are commonly associated with thoracic aortic aneurysms, they can also affect the abdominal aorta, albeit to a lesser extent. These include Marfan syndrome (MFS), vascular Ehlers–Danlos syndrome (VED), Loeys–Dietz syndrome (LDS), arterial tortuosity syndrome (ATS), and aneurysm osteoarthritis syndrome (AOS) (see Ch. 141 , Aneurysms Caused by Connective Tissue Abnormalities). Mutations in genes encoding for extracellular matrix components are associated with increased risk of abdominal aortic pathology and aneurysm formation. However, considerable variability exists in clinical presentations. Clinical decision making is quite complex in those disorders. For example, the risk of rupture in LDS and AOS is higher at smaller aortic diameters than in MFS. Also, OAR is more challenging in VED than in MFS owing to increased arterial wall fragility. Although small series have been published on the selective use of EVAR in certain connective tissue disorders such as MFS, there is generally profound lack of knowledge on which of these syndromes, if any, would be amenable to EVAR.
OAR is the procedure of choice with these syndromes. The operative technique differs in that an attempt should be made at excluding all ectatic segments because of the propensity of future deterioration. Reinforcement of suture line with felt, and secondary reinforcement with tissue glue should be done routinely. Reimplantation of visceral side branches to the main aortic graft using in situ inclusion or Carrel patch technique should be avoided because of the risk of progressive aneurysmal degeneration or suture line disruption. Instead, visceral branch reconstruction is focused on the use of multi-branched synthetic graft (Coselli Thoracoabdominal Graft, Terumo Aortic, Sunrise, FL), with branch limb anastomoses performed endoaortically onto the ostia of corresponding visceral branches.
Careful patient selection and preparation is critical for optimal outcomes. Because of the physiologic derangements that occur as a result of the hemodynamic stress of aortic cross-clamping, a detailed understanding of the patient’s cardiac, pulmonary, and renal function is necessary to determine who is a candidate for OAR. Preoperative assessment of frailty cannot be overstated in open aortic surgery, given its known association with postoperative mortality and adverse outcomes. Frailty is a multidimensional syndrome of loss of reserves (energy, physical ability, cognition, health) that gives rise to vulnerability to adverse events. Preoperative identification of high-risk patients may help mitigate procedural and long-term outcomes and improve shared decision-making regarding AAA repair. Several preoperative frailty and nutritional parameters such as nutritional status and psoas muscle sarcopenia have been suggested to help identify high-risk patients for OAR so that endovascular aneurysm repair or no intervention can be recommended. The modified frailty index (mFI) score is a commonly used measure derived from comorbidity and preoperative functional status data that can be used as a predictive tool to aid in surgical planning of patients undergoing elective AAA repair. Similarly, in individuals with diminished physiologic reserve or compromised baseline functional status, it must be determined whether the patient’s current and anticipated postoperative quality of life are sufficient. This is especially pertinent in the elderly, where postoperative complications may condemn the patient to long-term skilled nursing care and permanent loss of independent functional status.
American College of Radiology appropriateness criteria suggests CTA as the optimal choice for detailed characterization of AAA, with MR angiography (MRA) to be considered if CT cannot be performed (see Ch. 29 , Computed Tomography). CTA data processing on modern workstation enables comprehensive assessment of vascular anatomy including geometric measures, angulation, tortuosity, underlying occlusive disease, extent of wall calcification, mural thrombus and shaggy aorta, which are crucial for planning the best approach including preoperative choice of clamp site (and endovascular treatment) ( Fig. 73.1 ). This can yield valuable preoperative planning information and has become a standard of care that most vascular surgeons are well versed in. CTA can also accurately assess for involvement of the visceral aortic segment and branches with the aneurysmal process or occlusive disease. , Preoperative information derived from CTA is crucial for determining if suprarenal or supraceliac clamping is anticipated and potential clamp placement sites can be examined for heavy or circumferential calcification or thrombus. Concomitant occlusive disease of the visceral vessels and aortoiliac segment is also readily apparent on CTA, allowing decisions regarding the need for endarterectomy or complex reconstruction to be made preoperatively. CTA also provides valuable information about variant anatomy that might alter the operative plan, such as retro-aortic or circumferential renal vein, multiple renal arteries or a horseshoe kidney. Concomitant distal stenotic, occlusive, or aneurysmal disease will influence the site of distal reconstruction, as well as the need for additional procedures. Numerous advances have occurred in CTA technology such as lower radiation dose, time-resolved dynamic imaging, and ability to perform comprehensive aortic imaging using a much smaller amount of iodinated contrast using dual energy beam CT technology.
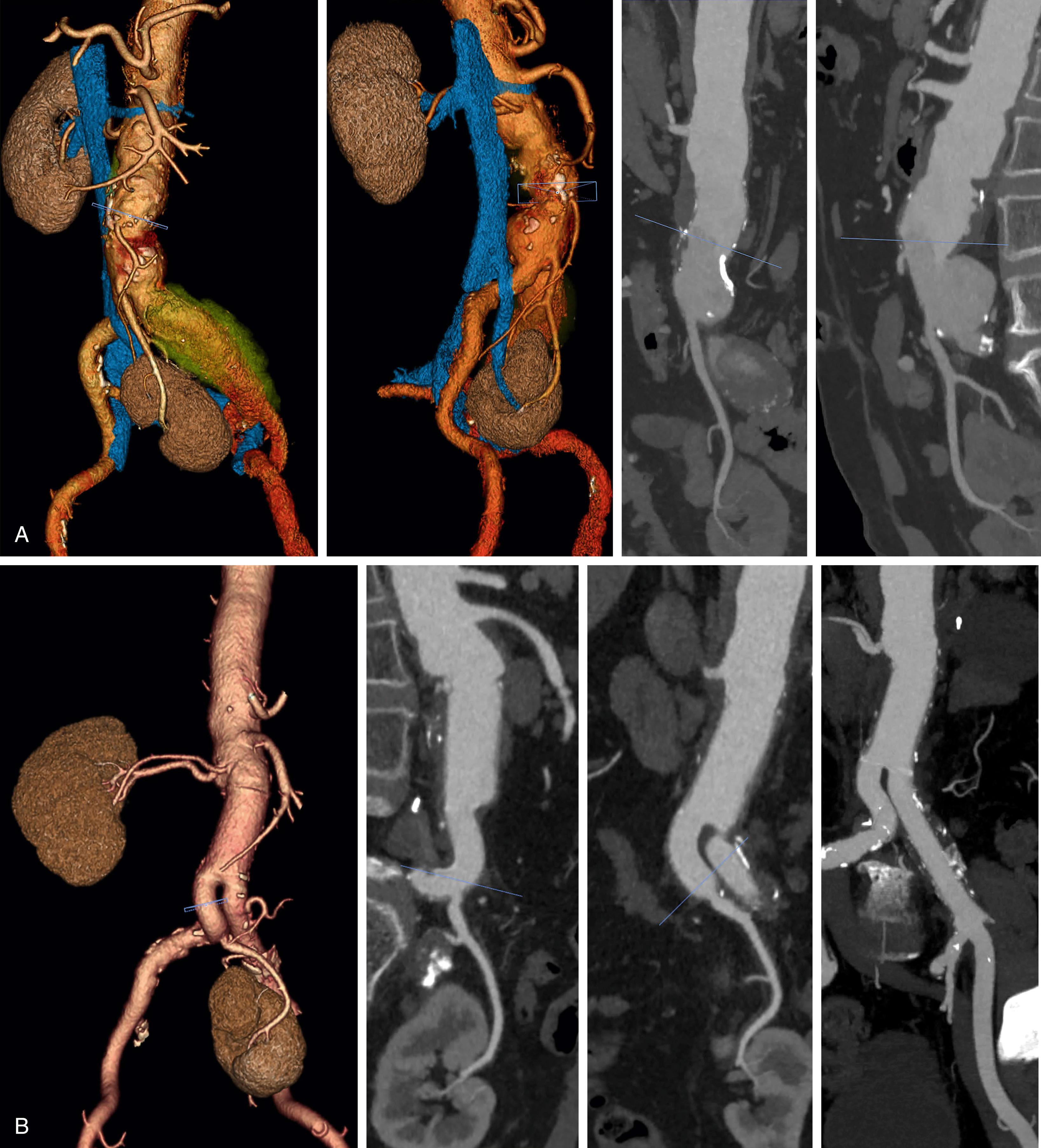
Magnetic resonance angiography (MRA) can be used for assessment of aneurysm extent, size and for preoperative planning (see Ch. 30 , Magnetic Resonance Imaging and Arteriography). , It can replace CTA for planning in patients for whom iodinated contrast is contraindicated. One major drawback is poor visualization of calcification and propensity to artifacts that can degrade diagnostic quality. However, MRA can better delineate the pathologic process in inflammatory AAA, or extent of phlegmon in mycotic aneurysms especially those related to direct spread from an adjacent source such as infectious spondylitis. Noncontrast MRA sequences for comprehensive evaluation of AAA in patients who cannot receive gadolinium are also becoming increasingly available. ,
There are two widely practiced approaches to open aneurysm repair: transperitoneal and retroperitoneal, dependent on surgeon’s preference and patient-specific anatomic requirements or comorbidities (see Ch. 56 , Abdominal Vascular Exposures). The transperitoneal approach offers rapid access to the infrarenal aorta with straightforward positioning and allows higher flexibility in terms of accessing the entire aortoiliac segments including the hypogastric arteries and all aortic visceral branches, albeit with additional expanded exposures which can be technically demanding. It is ideal for use in emergencies. While the retroperitoneal approach requires a more involved positioning process, it allows excellent exposure of the visceral aorta that can be easily extended into the distal thoracic aorta. In addition, the retroperitoneal approach is invaluable in patients with a hostile abdomen due to prior abdominal surgery, obese patients, and arguably in patients with poor cardiopulmonary reserve. , New SVS guidelines also favor a retroperitoneal approach for open repair of IAAA. Main drawbacks of the retroperitoneal approach include limited exposure of the distal right iliac artery and its branches. It can also be difficult to obtain adequate visualization of right renal artery beyond its proximal-most segment. Advocates of the retroperitoneal approach cite decreased postoperative fluid requirements, pulmonary edema, pneumonia, respiratory failure, postoperative ileus, shorter length of intensive care unit and overall hospital stays, shorter time to full recovery, and less cost, , , , although other studies disputed those advantages. , A recent study compared the two approaches in 1282 patients from the VQI national database, showing the transabdominal approach to be associated with higher rates of late reintervention and readmission.
The transperitoneal approach can be performed through a variety of incisions (midline, transverse, and bilateral subcostal with many other variants) depending on the surgeon’s preference and patient habitus. Surface anatomy and body habitus of the patient play an important role in planning the type of incision.
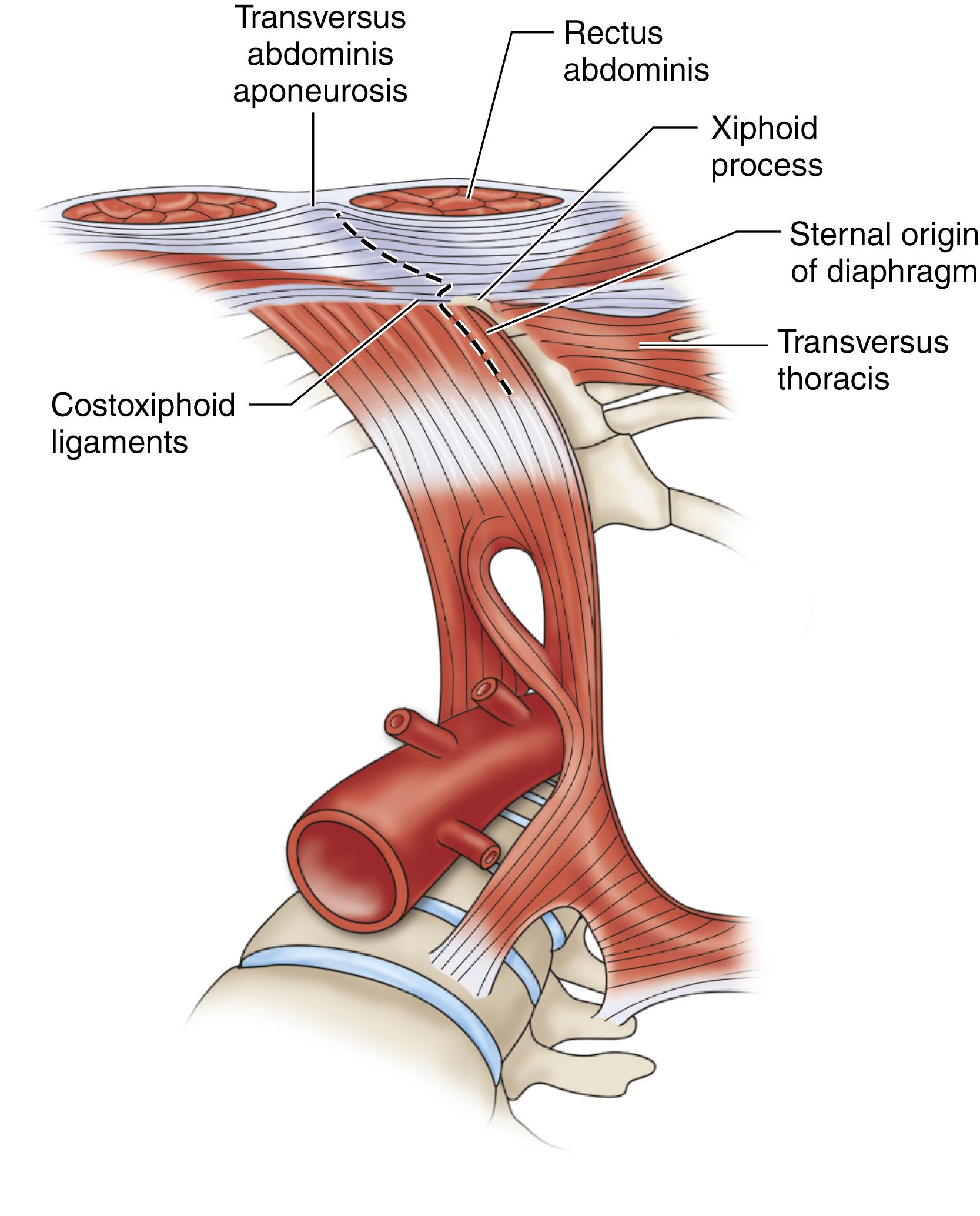
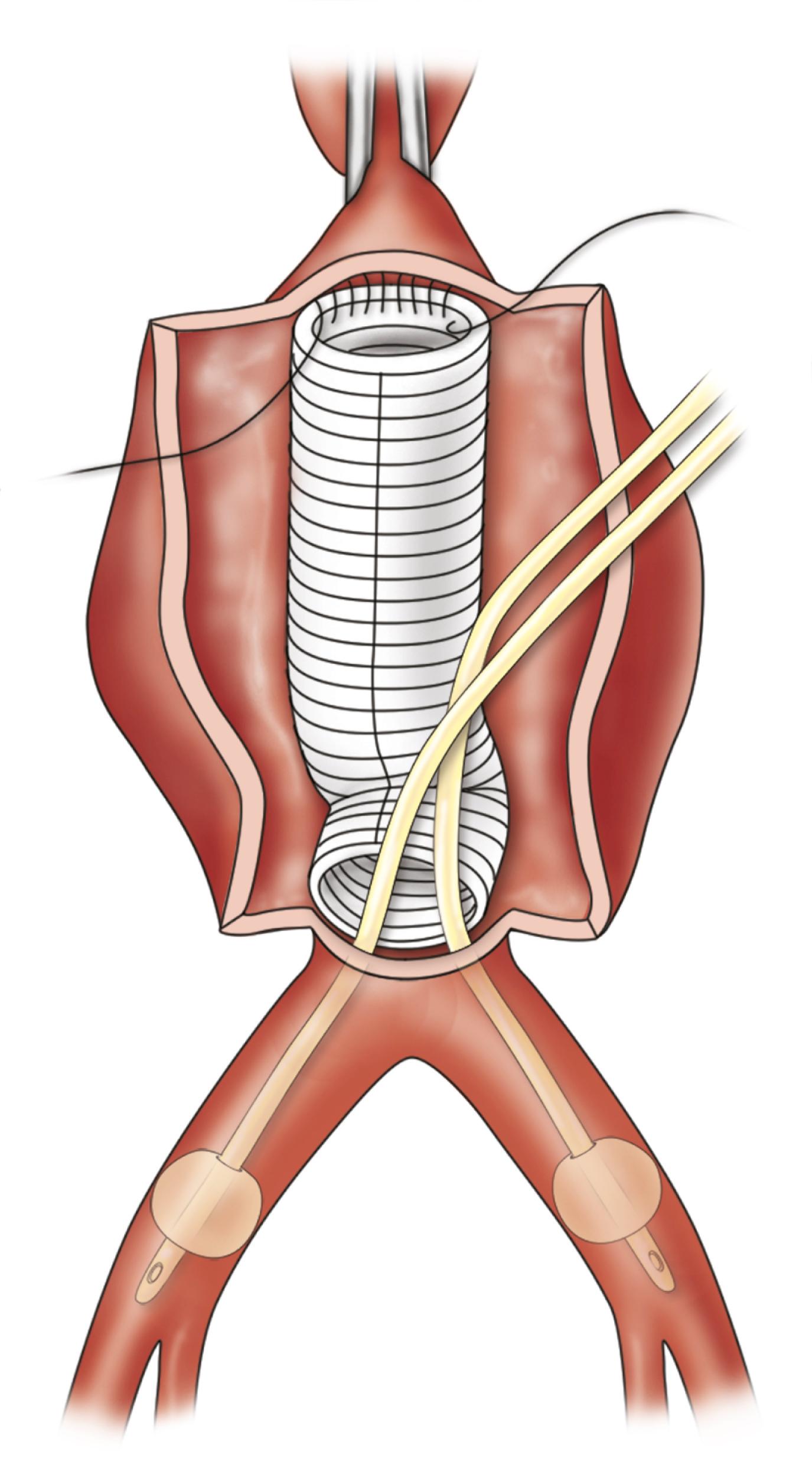
When a midline incision is used in a patient where visceral aortic exposure is required, an important detail is to extend its proximal extent alongside the xiphoid process. This simple maneuver allows the release of several attachments, ligaments and aponeuroses, appropriately referred to as the “linchpin of proximal aortic exposure.” By enhancing the mobility of the lower costal margin this extension allows wider retraction and greatly facilitates exposure of the paravisceral and celiac aorta ( Fig. 73.2 ). The omentum and transverse colon are retracted cephalad, and the small bowel is packed in the right hemiabdomen. A self-retaining retractor, such as the Omni (Omni-Tract, St. Paul, MN) or Thompson (Thompson Surgical Instruments, Traverse City, MI), greatly facilitates exposure and should be set up at this point. The ligament of Treitz is divided, the third and fourth portions of the duodenum are reflected to the patient’s right, and the periaortic lymphatic and connective tissue is ligated and divided ( Fig. 73.3 ). The inferior mesenteric vein can be divided to facilitate exposure. The incision in the posterior peritoneum is then continued caudally in this plane to expose the entire infrarenal aorta. Although starting to the left of the aorta at the ligament of Treitz, the incision in the posterior peritoneum should course to the right of the aortic midline to prevent injury to the IMA, sigmoid mesentery, and autonomic nervous plexus at the bifurcation ( Fig. 73.4 ). In cases where tube graft reconstruction is planned with distal anastomosis to the aorta itself, the dissection does not need to proceed beyond what is necessary to obtain distal control at the level of the bilateral proximal common iliac arteries. In certain cases where external clamping is to be avoided as when the common iliac arteries are heavily calcified, endoluminal clamping can be used with a Foley catheter or large Fogarty balloon ( Fig. 73.5 ).
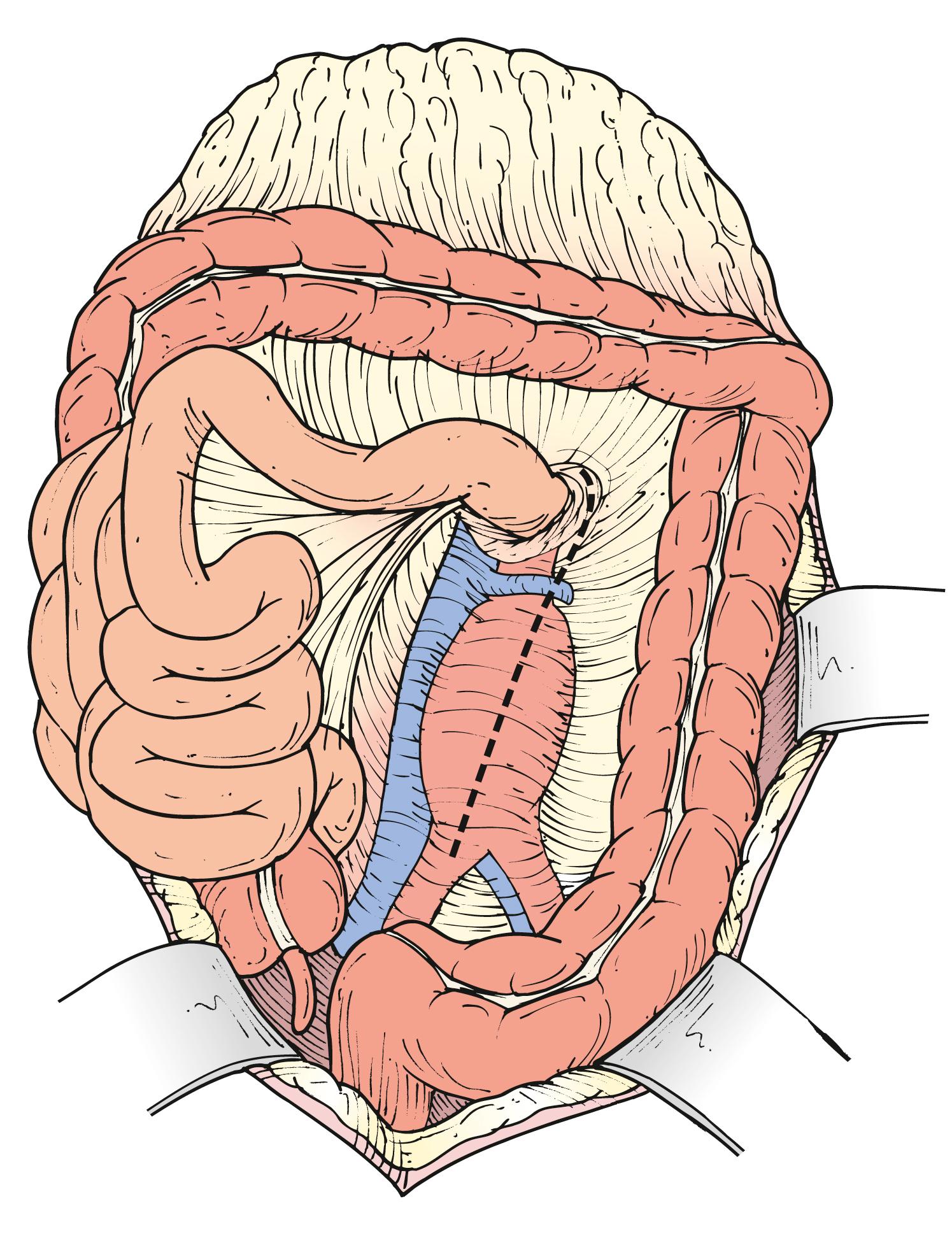
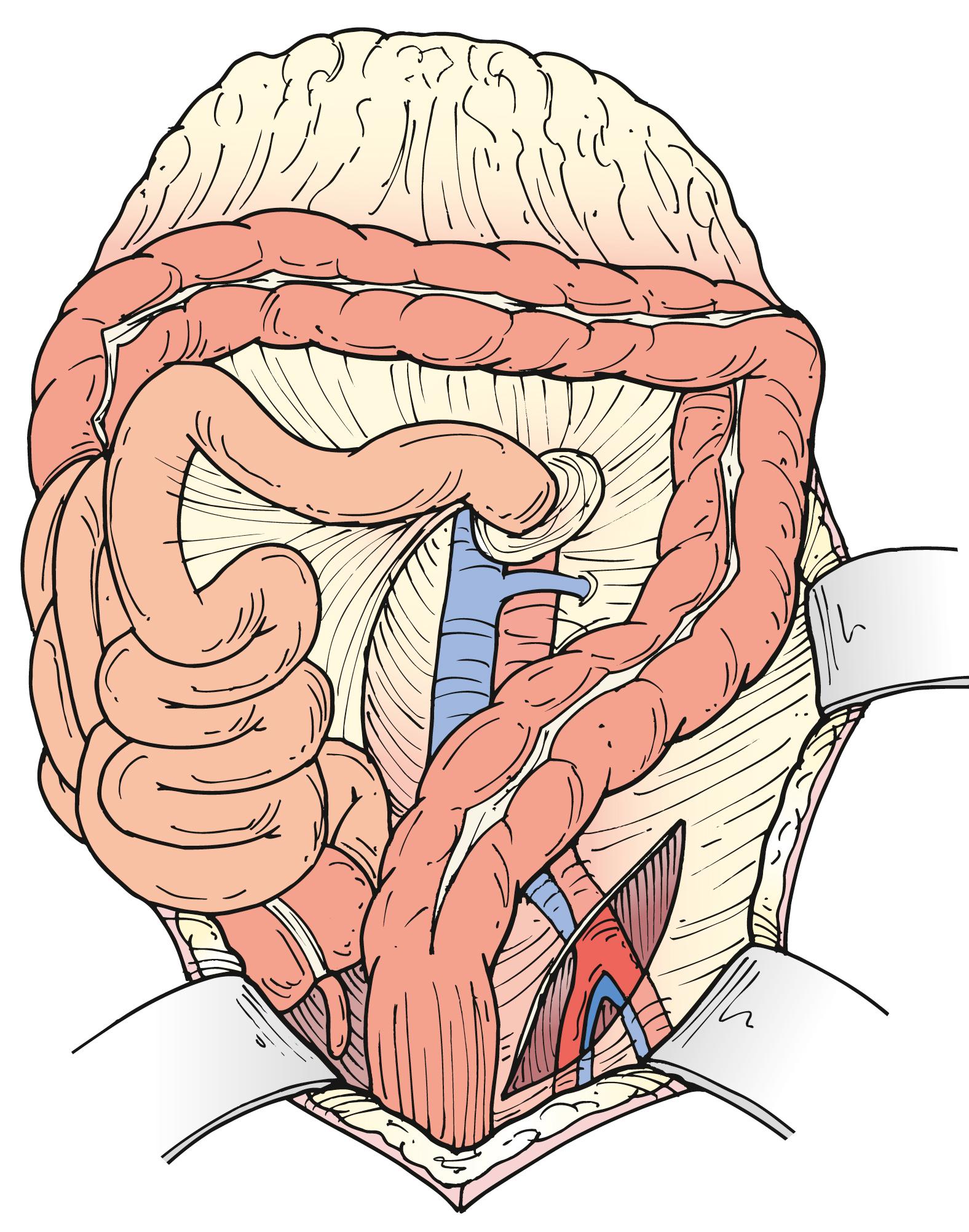
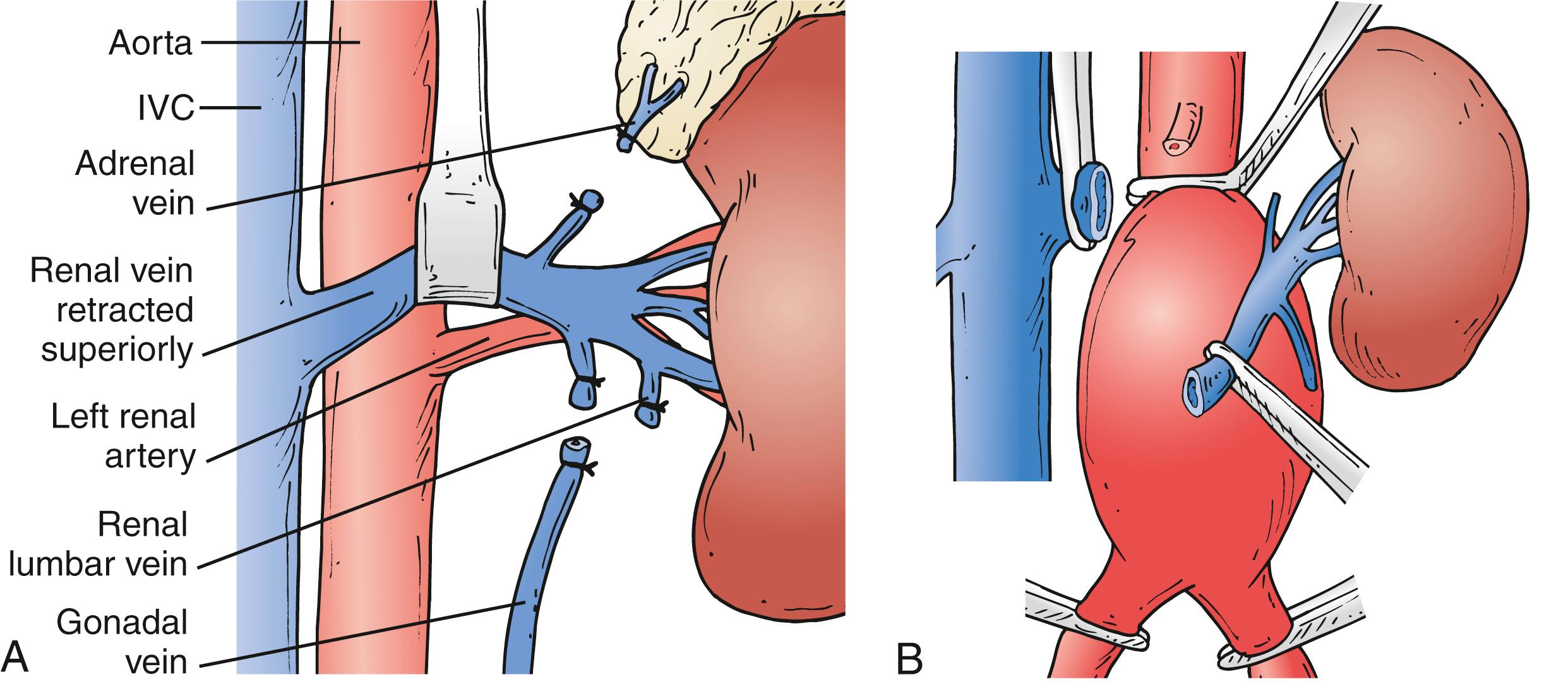
A growing proportion of patients requiring OAR have aneurysms with short or no infrarenal neck. Juxtarenal aneurysms often require suprarenal clamping, which necessitates additional proximal aortic exposure. To do so, the posterior peritoneum is opened cephalad to the level of the left renal vein. Location of the left renal vein should always be ascertained on the preoperative CT scan. It can be mobilized to gain more proximal aortic exposure by dividing some of the tethering branches (gonadal, adrenal, and lumbar branches) ( Fig. 73.6A ). Alternatively, the left renal vein can be divided safely, albeit an increased risk of transient azotemia has been reported. In order to preserve its drainage through side branches, the division point should be as close to the inferior vena cava as possible ( Fig. 73.6B ). The decision to divide the left renal vein needs to be made before proceeding with aggressive mobilization, because adequate renal venous drainage once it is divided requires patency of most side branches. If there is concern for compromised renal venous drainage after renal vein division, an end-to-end reanastomosis can be readily performed after completion of the aortic reconstruction. If a retro-aortic or circumferential left renal vein is present, care must be taken to avoid injuring the vein.
The supraceliac aorta is exposed separately (non-continuous with the primary inframesocolic exposure) by dividing the lesser omentum or gastrohepatic ligament. The aorta is identified by palpation, and the overlying fibers of the right crus of the diaphragm are divided. Mobilization of the left lobe of the liver by dividing the left triangular and coronary ligaments, as well as the falciform ligament, can assist with exposure but is often not necessary. A nasogastric tube is helpful for identifying and protecting the esophagus, which is retracted to the left. The aorta can be dissected anteriorly down to the level of the celiac axis, although care must be taken to avoid injury to the pancreas as one proceeds caudally.
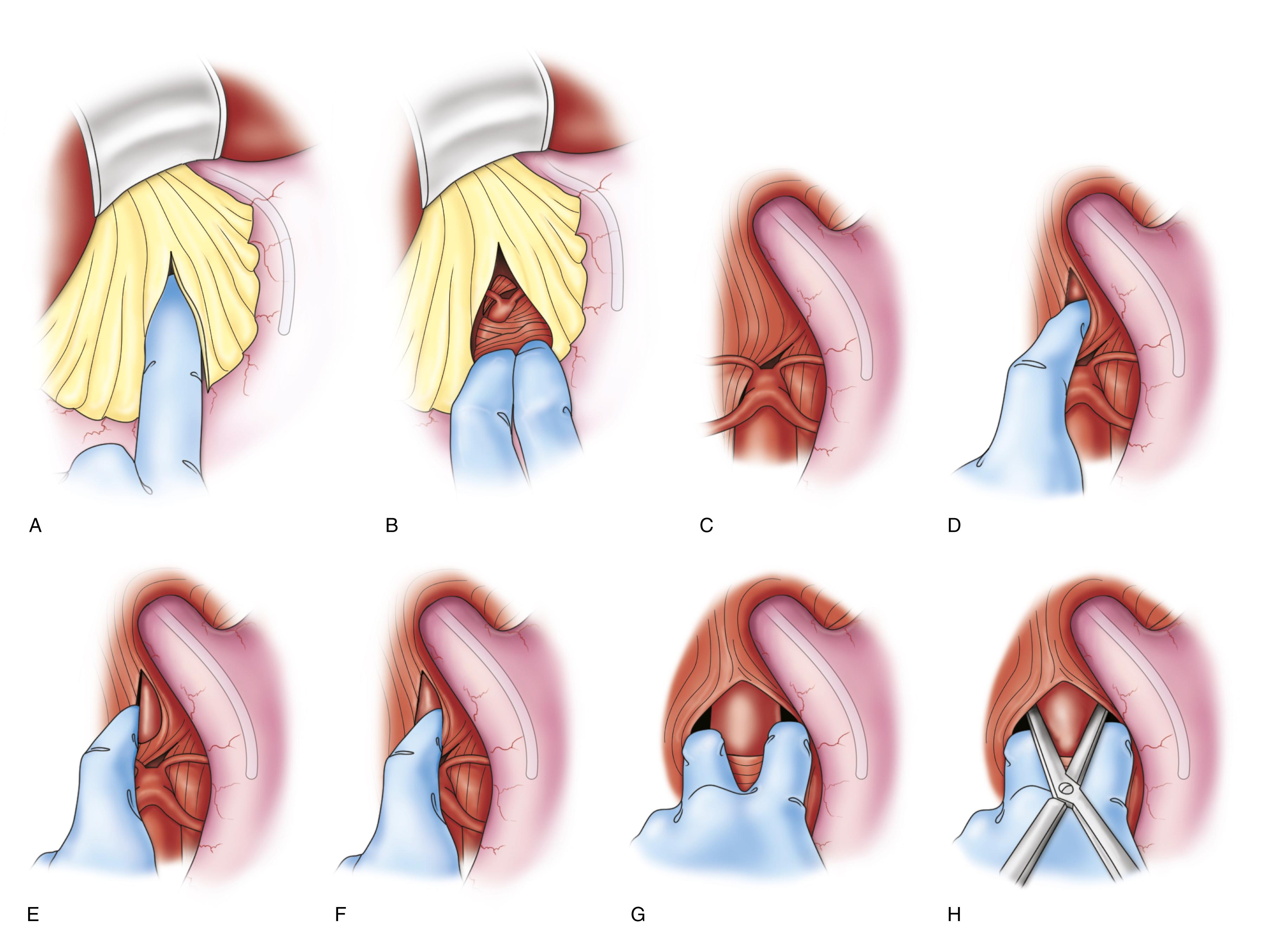
An important technique for rapid supraceliac aortic control is blind digital dissection and incision of the right diaphragmatic crus to allow clamp application ( Fig. 73.7 ). Another helpful rapid proximal control technique is introduction of occlusion balloon through a small sac aortotomy. Supraceliac control can also be achieved endovascularly through a transfemoral approach using a variety of tools and devices, akin to the approaches used in EVAR or vascular trauma. These maneuvers can be lifesaving when faced with rapid exsanguination until secure proximal aortic control approach is established.
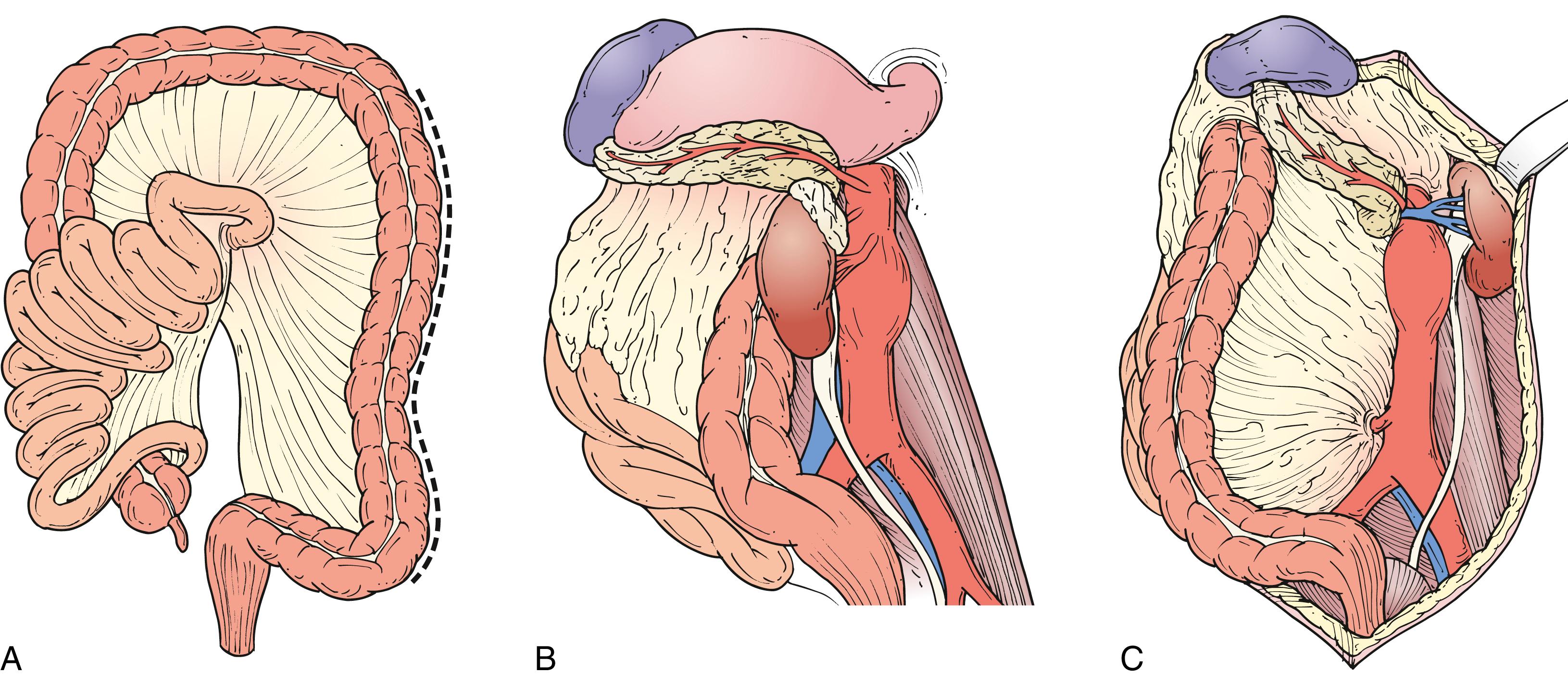
Suprarenal aneurysms are best approached using a retroperitoneal exposure. However, the visceral segment of the aorta can be accessed transperitoneally using a left medial visceral rotation ( Fig. 73.8A ). The left colon is mobilized by incising the posterior peritoneum just lateral to the line of Toldt. This incision is carried cephalad through the phrenicocolic ligament and then medially toward the aortic hiatus along the underside of the diaphragm. The plane separating the posterior aspect of Gerota’s fascia and posterior abdominal wall is developed, allowing the colon, pancreas, spleen, and left kidney to be reflected medially en bloc ( Fig. 73.8B ). As the kidney is mobilized, the lumbar branch of the left renal vein should be identified and ligated to prevent avulsion. Alternatively, the kidney can be left in the renal fossa, which requires dividing the linorenal and linophrenic ligaments and developing a plane between the anterior surface of Gerota’s fascia and the posterior surface of the colonic mesentery and pancreas ( Fig. 73.8C ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here