Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Blunt and penetrating abdominal trauma are two of the more common indications for radiologic investigation in the emergency department setting and are common causes of morbidity and mortality among patients admitted to trauma centers. Many of these patients have multisystem injuries resulting from high-velocity mechanisms, and the full extent of these injuries is often difficult to detect clinically. A variety of imaging and nonimaging methods have been used to aid the clinician in the evaluation of patients with abdominal trauma, but in recent years ultrasonography (US) and computed tomography (CT) have become the cornerstones of diagnosis and management. The almost universal use of CT, and of US in specific settings or circumstances, has modified the diagnostic approach of the polytrauma patient and has relegated diagnostic peritoneal lavage to virtually a historical procedure. This state-of-the-art imaging allows rapid detection of potentially life-threatening injuries that can be difficult or impossible to detect clinically, especially when the presence of associated injuries may mask overt clinical manifestations or divert the attention of the admitting physician away from major intraabdominal bleeding.
Blunt trauma mechanisms leading to significant intraabdominal injuries often include compression and deceleration forces. Motor vehicle collisions are the leading cause of injury, both in the United States and throughout the world. Other common mechanisms include falls from high altitudes, assaults, projectile injuries, and sports-related trauma. Although the likelihood of injuring an individual organ depends on the specific mechanism of trauma and the vulnerability of the patient at the time of the event, the data in the trauma literature have repeatedly demonstrated that the liver and spleen are the most frequently injured organs. Other potentially injured organs include the kidneys, bowel and mesentery, pancreas, adrenals, diaphragm, intraabdominal vessels, and bladder.
During the past 2 decades, improvements in imaging technology have considerably improved our ability to detect intraabdominal injuries from blunt trauma. Of the available imaging modalities, CT and US are the two most commonly used imaging techniques when evaluating for acute traumatic abdominal injury. Other modalities such as plain film radiography, magnetic resonance imaging (MRI), nuclear scintigraphy, and catheter angiography are typically used in specific circumstances for further characterization of injuries, detection of complications from the initial injury, and treatment of such injuries.
Typically, US is used during the initial assessment of the patient who has sustained polytrauma, and CT images are obtained once the patient has been stabilized. Rapid assessment of the trauma patient can be performed at the bedside by experienced sonographers as part of the focused abdominal sonogram for trauma (FAST). Such scans can readily identify free fluid within the abdomen or pelvis and can help triage patients at the time of initial assessment. FAST evaluation consists of visualization of the following five spaces:
Pericardium (subxiphoid view)
Splenorenal and hepatorenal (i.e., the Morison pouch)
Right paracolic gutter ( Fig. 3-1 )
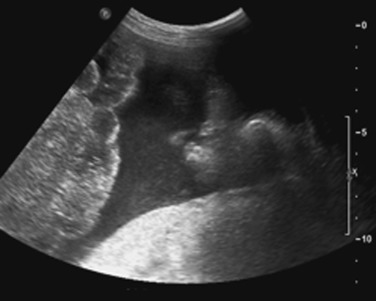
Left paracolic gutter (see Fig. 3-1 )
The Douglas pouch
Multiple studies have demonstrated the benefit of a FAST study in the emergent decision-making process for acutely traumatized patients. FAST is mainly used to detect free fluid (acute hemorrhage in this setting) in the peritoneal, pericardial, or pleural spaces to direct immediate therapeutic interventions by the trauma surgeons in unstable or marginally stable patients. More recently, the development of contrast-enhanced US has improved detection of solid organ injuries and active bleeding. This technique, although not commonly used in the United States, has potential as a means of evaluating trauma patients without the use of ionizing radiation.
Once the patient with abdominal trauma is stabilized, more complete imaging can be obtained with a CT examination. With multidetector CT (MDCT) scanners, imaging of the head, cervical spine, chest, abdomen, and pelvis is performed as a single examination (“panscan”). Although adequate evaluation is possible with 4- and 8-row detector scanners, most large trauma centers now use 64-row or higher detector scanners for trauma and other emergency department applications. Specific protocols regarding slice thickness, volume of the intravenous contrast material administered, timing of image acquisition, and use of an oral contrast agent are continually reconfigured and vary between institutions.
In general, optimal evaluation of the abdomen and pelvis is performed by acquiring the axial dataset of CT images after intravenous injection of iodinated contrast material during the portal venous phase of hepatic enhancement. Intravenous contrast material—100 mL injected at a rate of 3 to 5 mL per second—is routinely used for CT imaging of trauma patients. If a dual syringe power injector (the preferred system) is used, the contrast bolus is followed by a 30- to 50-mL bolus of normal saline solution, typically injected at the same rate as the contrast material. This saline “chaser” ensures delivery of the complete contrast bolus into the circulation so that it doesn’t remain in the tubing or the veins of the upper extremities or mediastinum.
Regardless of the scanning protocol used, modern 64-row detector (or higher) scanners provide several definite advantages compared with earlier generation scanners. The most important of these advantages is their markedly improved temporal resolution. With the development of these multidetector-row scanners, thin images (1 to 2 mm) can be acquired easily while still keeping the scan time at 8 seconds or less per body part. To facilitate review at the interpretation workstations, it is advisable to reconstruct a separate set of thicker axial images by “fusing” the thin sections. For example, images acquired with 0.625- or 1.25-mm thickness can be reconstructed at 3.75- or 5-mm thickness. In addition, sagittal and coronal reformations are now routinely generated, taking advantage of the rapid scan times that nearly eliminate motion artifact. These sagittal and coronal reformations are ideal for adequate evaluation of the diaphragm, long vascular territories, and thoracic and lumbosacral spine, and they reduce the need for lumbosacral and thoracic spine radiographs in the vast majority of patients. All series are sent to the Picture Archival Computer System and are available at the time of interpretation and for further postprocessing, if necessary. Other benefits include the ability to combine routine protocols with CT angiograms of multiple body parts while still using a single bolus of contrast material. This procedure is possible because of the increased scanner table length (2 m) available with many of the 64-row MDCT scanners. With use of scout images of the entire body, multiple complex CT examinations (including CT angiograms of the neck or extremities) are planned and combined in succession into one scan using a single injection of contrast material ( Fig. 3-2 ).
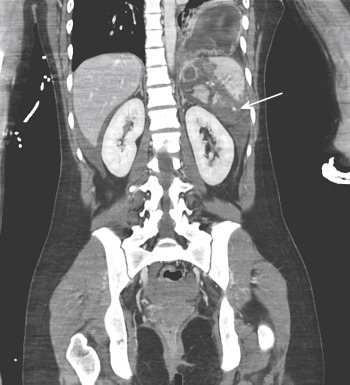
The increased temporal resolution and decreased scan times of 64-row or higher detector scanners have resulted in a modification in the time intervals applied between the start of contrast injection and image acquisition. To ensure that all body parts are scanned at the optimum peak of contrast enhancement, it is preferable to scan the chest (30 to 35 seconds) independently from the abdomen and pelvis (65 to 75 seconds), rather than with a continuous scan encompassing all three regions and starting at approximately 60 seconds. The split-scan method avoids the potential need for rescanning the chest to obtain true CT angiography images of the thoracic aorta, essentially eliminating the need for catheter angiography for suspected thoracic aortic injury. The scan time for each body part is approximately 4 to 8 seconds (for 64-row detector scanners), with a pause of 30 to 35 seconds between scans of the chest and abdomen. The only drawback of separating the thorax from the abdomen and pelvis is in the generation of multiplanar reformations. These acquisitions are, for all intents and purposes, two different studies and necessitate separate multiplanar reformats, which makes image analysis slightly more tedious. However, the benefits of an optimal study of the aortic arch and great vessels outweigh this drawback. Some centers advocate for the use of whole-body CT angiography when imaging traumatically injured patients to detect vascular injuries and to differentiate between arterial and venous hemorrhage—important findings that can reduce morbidity and mortality when detected early. In fact, certain injuries, including contained vascular injuries of the spleen, which have unfavorable outcomes with conservative management given their propensity to lead to active extravasation, can only be seen with arterial-phase imaging.
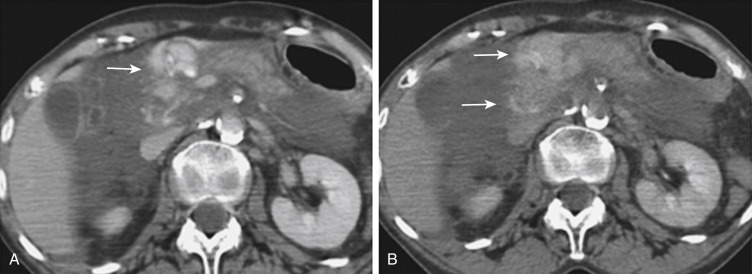
In addition to portal venous-phase imaging, the judicious acquisition of delayed images has become an increasingly important part of the trauma CT evaluation. Delayed images can be useful in evaluating vascular injuries, as well as injuries to both the solid organs and the bowel and mesentery. Delayed CT acquisitions allow for improved characterization of solid organ injuries by helping to differentiate contained injuries (such as arterial pseudoaneurysms and arteriovenous fistulas) from uncontrolled active extravasation of contrast-enhanced blood. On delayed images, areas of active extravasation persist as hyperattenuating foci (relative to the aorta) and change configuration as blood (with contrast material) diffuses into a potential space ( Fig. 3-3 ), whereas pseudoaneurysms show an attenuation coefficient that remains similar to the aorta, with no change in overall size or shape. Delayed images also improve detection and characterization of bladder and renal injuries, as discussed later in this chapter. Finally, delayed scans can help in the characterization of findings seen on portal venous-phase images that potentially represent foci of extravasation and could be related to the acute injury ( Fig. 3-4 ). However, the routine use of delayed images is unnecessary and should be discouraged, because the majority of trauma CT scans performed in emergency departments today show no evidence of abdominal injury, and the additional radiation dose is unnecessary. As an alternative, delayed images can be acquired selectively and used only when solid organ or bowel injury is detected or suspected on the initial CT acquisition. Additionally, because the sole purpose of this delayed scan is to characterize an injury seen or suspected on the initial scan, techniques using a reduced radiation dose are employed, including reducing the milliamperes and increasing the noise index. Iterative reconstruction techniques have led to substantial advancements in radiation dose reduction with use of CT.
In the past, oral contrast material was considered a mandatory component of trauma CT protocols. However, reports in the radiologic and surgical literature have concluded that imaging by MDCT without use of oral contrast material is similarly accurate for the detection of bowel and mesenteric injuries at the time of the initial CT evaluation. Occasionally, the initial scan may be inconclusive with regard to the presence of a bowel injury. In this case, and if there is a specific question of bowel injury on the initial scan, a follow-up CT (typically 12 hours later) with oral contrast material can be performed at the discretion of the interpreting radiologist. If oral contrast material is used to opacify the bowel in the setting of acute trauma, patients generally receive water-soluble contrast agents because of the potential for contrast extravasation into the peritoneal cavity from an acute bowel injury. Oral contrast material can be given by mouth or inserted into an indwelling nasogastric tube. The method chosen depends on the level of consciousness of the patient and associated injuries. CT imaging should not be delayed while waiting for the contrast material to migrate into the distal small bowel.
With isotropic voxel scanning, CT has truly become a multiplanar modality. As previously described, sagittal and coronal reformations have become a common component of trauma CT protocols. As the number of images generated for trauma patients increases, review of multiplanar images is one possible solution for improving interpretation efficiency. Coronal and sagittal reformations are also a useful, intuitive tool for communicating with clinical colleagues, because large amounts of information are represented on fewer images. Multiplanar image review is particularly useful for several trauma applications, including evaluation of potential spine injuries, a more complete appreciation of often-complex injuries of solid organs, hollow viscera, and the diaphragm, and for CT angiography, where longer segments of vessels may be visualized in sagittal and coronal planes.
The liver is one of the most commonly injured organs in the abdomen, with the prevalence of injury in patients who have sustained multiple blunt trauma between 1% to 8%. Despite the wide array of hepatic injuries that occur, including extensive lacerations and parenchymal hematomas, the vast majority of hemodynamically stable patients are treated conservatively with observation and supportive measures alone, without the need for direct therapeutic intervention. This advancement has led to significant improvements in mortality from severe liver trauma; however, nonoperative management is associated with morbidity such as bleeding, infection, abdominal compartment syndrome, and complications from biliary injury. Endovascular therapy with embolization is reserved for definitive treatment of patients with significant vascular injury and active bleeding. The growing trend toward successful nonsurgical management of liver injuries is in part related to major advances in imaging technology and CT techniques in the past decade. Specifically, the focus is to rapidly demonstrate areas of potentially life-threatening active bleeding that require immediate attention. MDCT and US are the main modalities used for the initial detection and characterization of hepatic injuries. Other modalities, such as MRI with MR cholangiopancreatography (MRCP), hepatobiliary scintigraphy, and endoscopic retrograde cholangiopancreatography (ERCP), are reserved for detecting subacute or delayed complications, such as bile duct leaks, bilomas, and biliary strictures. Because CT with proper technique is sufficient to demonstrate the extent and significance of essentially all liver injuries, catheter angiography is reserved for confirmation of CT findings that suggest major vascular injury and, especially, as a means to treat lesions that require intervention via embolization.
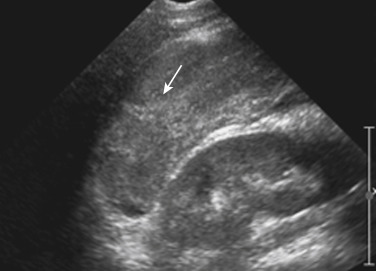
Sonographic evaluation of hepatic injuries is mostly limited to screening the trauma patient for indirect signs of injury, such as free fluid adjacent to the liver (as part of the FAST scan). When fluid is detected along the margin of the liver, it can appear complex and can contain an echogenic clot because of its hemorrhagic nature. Although a careful inspection of the liver can demonstrate lacerations and contusions as focal areas of parenchymal distortion, various factors limit the use of US beyond the search for free peritoneal fluid. These factors include technical limitations such as difficult access to appropriate sonographic windows and the variability in operator experience and availability. However, advances in US technology and the development of sonographic contrast agents has led to increased use of this modality for direct evaluation of the solid parenchymal organs, including the liver, especially in European countries. On noncontrast US examinations, hepatic parenchymal injuries can produce three different morphologic patterns. The most common pattern is a focal area of increased echogenicity with respect to the background liver, which is thought to correspond to the focal lacerations or hematomas seen on CT. The other two patterns are a more diffuse area of increased echogenicity and focal areas of decreased echogenicity. Liver lacerations can be difficult to detect on initial examinations; they often appear more prominent in the days after the initial injury ( Fig. 3-5 ). The advent of sonographic contrast agents has increased the ability of ultrasound to detect acute hepatic injuries. Generally, the contrast agent is given in a bolus and the area of interest is scanned continuously for 4 to 6 minutes. On contrast-enhanced US, liver injuries are best seen during the portal venous phase of imaging. Liver lacerations can also appear as focal linear or branching hypoechoic areas, often oriented perpendicular to the liver surface. Contusions may appear as geographic areas of decreased echogenicity, often with ill-defined borders. Similar to active contrast extravasation on CT, active bleeding can be detected by the presence of microbubbles (contrast material) extending into a hematoma. In general, despite recent advances in technology, US is still considered an adjunctive test.
CT is the dominant imaging modality in emergency departments in the United States and most other Western countries. Improvements in the rate of CT detection of liver injuries, as well as in the proper characterization of most injuries, are some of the reasons that support the trend toward conservative management of such injuries. As previously mentioned, liver injuries are optimally seen on CT that is performed during the portal venous phase of contrast enhancement. Once liver injuries are identified, it is important to document the type and location of such injuries. In addition, it is especially important to note the presence of active extravasation of contrast-enhanced blood and the potential for injury to central hepatic vessels such as the hepatic veins and inferior vena cava. Hepatic injuries are typically characterized as either lacerations or hematomas (subcapsular or parenchymal). Although many radiologists rely exclusively on morphologic descriptors in their report, it is useful to understand the liver injury scale that has been developed by the American Association for the Surgery of Trauma (AAST; Table 3-1 ). This grading scale takes into account features such as the size of subcapsular or parenchymal hematomas and lacerations, as well as evidence of active extravasation and major vascular injuries of the liver; these findings are all readily identified on well-performed CT examinations. The value of this scale lies more in the ability to communicate properly with trauma surgeons about the extent of the injury than in the ability to predict individual patient prognosis or the type of therapy necessary.
| Grade | Description |
|---|---|
| I | Hematoma: Subcapsular, <10% surface area Laceration: Capsular tear, <1 cm in parenchymal depth |
| II | Hematoma: Subcapsular, 10%-50% surface area; intraparenchymal, <10 cm in diameter Laceration: 1-3 cm in parenchymal depth, <10 cm in length |
| III | Hematoma: Subcapsular, >50% surface area or expanding or ruptured subcapsular hematoma with active bleeding; intraparenchymal, >10 cm or expanding or ruptured Laceration: >3 cm in parenchymal depth, <10 cm in length |
| IV | Hematoma: Ruptured intraparenchymal hematoma with active bleeding Laceration: Parenchymal disruption involving 25%-75% of a hepatic lobe or 1 to 3 Couinaud segments within a single lobe |
| V | Laceration: Parenchymal disruption involving >75% of a hepatic lobe or more than 3 Couinaud segments within a single lobe Vascular: Juxtahepatic venous injuries (i.e., retrohepatic vena cava or central major hepatic veins) |
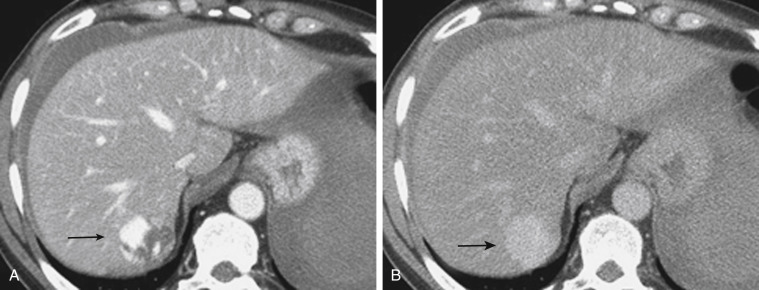
A subcapsular hematoma is typically hypodense to the enhancing liver parenchyma and appears elliptical, conforming to the confines of the liver capsule. Such collections are usually easily distinguished from perihepatic fluid. An intraparenchymal hematoma appears as an ill-defined hypoattenuating region within the liver. When seen on noncontrast CT, hematomas are typically hyperdense to the background liver parenchyma. Liver lacerations appear as hypoattenuating linear, often branching, and complex regions within the parenchyma of the liver ( Fig. 3-6 ). Extension to the hepatic surface is very common. Even small lacerations can be associated with perihepatic blood. It is important to identify lacerations that extend to the periportal region, because patients with these injuries are at an increased risk for the development of delayed bile leaks as a result of injury of the biliary ductal system.
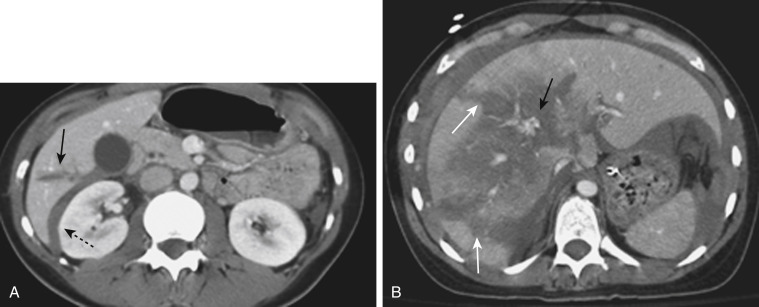
CT can also readily identify hepatic vascular injuries. Active extravasation of intravenous contrast material, when seen during routine portal venous phase images, suggests ongoing hemorrhage from a hepatic arterial or portal venous source. Active extravasation may be confined to the hepatic parenchyma or may be seen as hyperattenuating collections of contrast-enhanced blood accumulating in the perihepatic spaces. On delayed CT images, the focus of active extravasation typically increases in size as the material continues to diffuse throughout the area of the expanding hematoma ( Fig. 3-7 ). In the past, active extravasation was considered an indication of the need for prompt surgical management. Currently, the demonstration of a sizable focus of active extravasation is more likely to trigger a response from the vascular interventional team for catheter angiography and coil embolization (see Fig. 3-7 ). Even patients with high-grade injuries can be managed conservatively using such techniques.
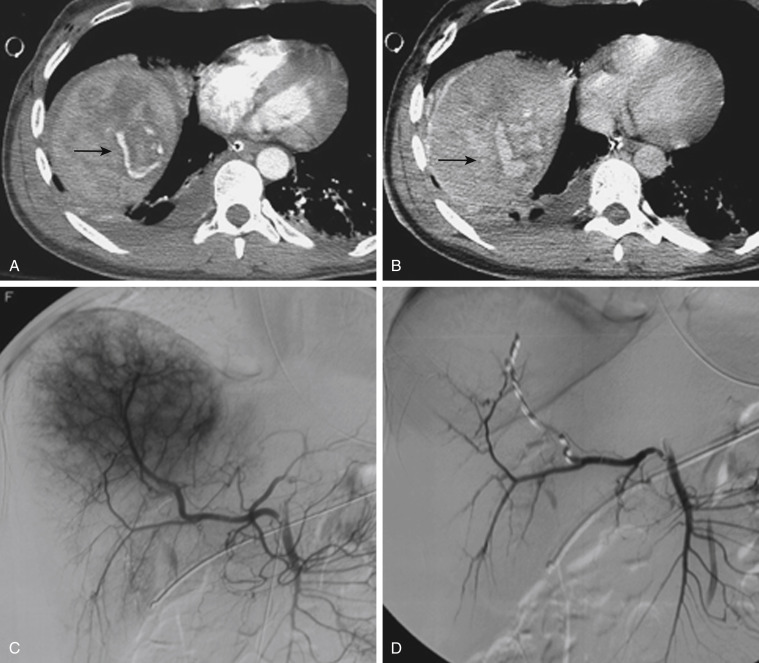
Injuries to major hepatic vessels also may be directly depicted with CT. For example, direct evidence of portal venous injury may be seen as abrupt termination of a branch of an intrahepatic portal vein. Parenchymal injuries may extend centrally to involve the hepatic veins and inferior vena cava, seen on CT as abrupt termination of the hepatic veins, which may just begin to enhance during routine portal venous phase images. Such major venous injuries are more likely to require surgical management, because they can cause continued bleeding and hemodynamic instability and are not readily treated by interventional radiology techniques ( Fig. 3-8 ). Major venous injuries are also commonly associated with hepatic arterial trauma. Complications of hepatic vascular injuries include traumatic fistulas between various hepatic structures, including arterioportal structures; fistulas between hepatic arteries and biliary ducts; and, rarely, fistulas between a hepatic artery and adjacent bowel. Hepatic pseudoaneurysms also can occur as a result of hepatic injury and are extremely important to detect and document because they are at risk for delayed rupture, a potentially lethal complication. Hepatic artery pseudoaneurysms used to be considered rare, but they are now detected more frequently as a result of improvements in the spatial resolution of CT and the ability to routinely scan at the peak of contrast enhancement throughout the scan ( Fig. 3-9 ). Pseudoaneurysms appear as hyperattenuating foci on early-phase images and demonstrate washout on delayed-phase images.
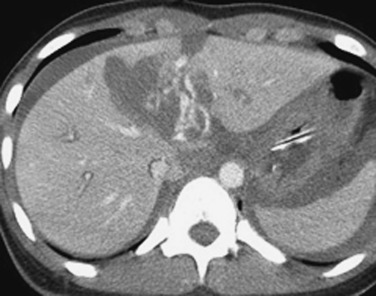
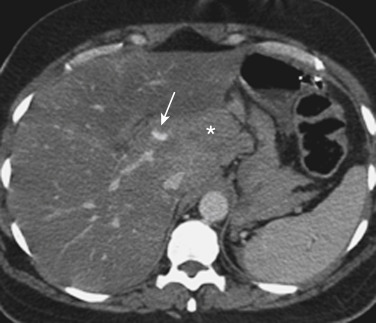
If delayed rupture and hemorrhage are suspected on the basis of clinical or laboratory parameters, CT is also the best method for detecting a subacute hemorrhage. On follow-up CT, a delayed hemorrhage presents as an increase in the size of a previous hematoma. The more acute hemorrhage appears as focal hyperattenuating material in a previously documented hematoma or along the margin of the liver, the so-called “sentinel clot” sign. In addition to pseudoaneurysm formation and delayed hemorrhage, other complications of hepatic trauma result from associated bile duct injury and include the development of bilomas and abscess collections, persistent bile leaks with bile peritonitis, and bile duct strictures.
Blunt trauma may result in injury to the biliary tract, including the gallbladder and intrahepatic or extrahepatic bile ducts. The gallbladder and extrahepatic ducts are protected by the liver, which may explain the low frequency of injury to these structures with blunt trauma. The most common location of extrahepatic biliary tract injuries is the gallbladder, followed by the common bile duct. Gallbladder injury is almost invariably associated with additional significant injuries. Liver, splenic, and duodenal injuries are most common, occurring in up to 91%, 54%, and 54% of cases, respectively. Extrahepatic bile duct injuries are very rare and also are usually associated with injuries to other organs.
Gallbladder and bile duct injury may occur as a result of torsion, shearing, or compression forces. Certain factors may predispose to gallbladder injury, such as distention of the gallbladder in a preprandial state, which makes the gallbladder more vulnerable to compression injury. Injuries to the gallbladder are classified into three main categories: contusion, laceration/perforation, and complete avulsion. In general, contusions are considered to represent intramural hematomas, are the mildest form of gallbladder injury, and are treated conservatively. Lacerations and perforations are full-thickness wall injuries that require a cholecystectomy. Avulsion of the gallbladder may involve variable portions of the gallbladder and cystic duct. Any of these lesions can be associated with transection of the cystic artery and major blood loss.
Extrahepatic duct injuries may occur at sites of anatomic fixation, such as the intrapancreatic portion of the common bile duct, and are frequently caused by blunt impact or acute deceleration, possibly with compression against the spine. Elevation of the liver after blunt trauma may cause stretching of the relatively fixed common duct. Injuries to the intrahepatic bile ducts are seen in patients with severe liver lacerations.
Delayed complications of gallbladder or bile duct injury, such as sepsis, may result from leakage of bile into the peritoneal cavity and subsequent infection. Sterile bile within the peritoneum undergoes continuous peritoneal reabsorption and initially may lead to surprisingly few symptoms. Because bile in the peritoneum usually does not cause symptoms until infection occurs, bile leakage may occur for weeks or months before it is detected clinically. When signs and symptoms are present, they are nonspecific and include vague abdominal pain, nausea, vomiting, and occasionally jaundice. With an extrahepatic bile duct injury, diagnosis may be particularly difficult; up to 20% of such injuries are not detected at surgery. Injury to either extra- or intrahepatic bile ducts may also result in biliary strictures. Patients may present weeks, months, or even years later with signs of biliary obstruction or infection as a result of focal strictures that have developed at the site of the injury.
Acute gallbladder trauma is only rarely detected by US. Gallbladder wall thickening or complex echogenic fluid within the gallbladder may be present, which might suggest the presence of intraluminal blood. However, sonographic examination may be limited by underdistention of the gallbladder in a nonfasting patient. Ultrasound plays only a very small role in the detection of acute injuries of the biliary system.
Gallbladder injuries are usually diagnosed at the time of the initial trauma CT scan. Contusions appear as diffuse gallbladder wall thickening. The presence of pericholecystic fluid is not specific but may be an associated finding. High-attenuation fluid within the gallbladder lumen suggests hemorrhage and is a good indicator of acute injury. However, differentiation between high-attenuation sludge and blood may be difficult. Lacerations of the gallbladder wall are seen as focal disruption of the normal mural enhancement of the gallbladder wall. The presence of dense contrast material in the gallbladder lumen or in the gallbladder fossa suggests active bleeding from injury to the cystic artery. If the gallbladder is avulsed from its pedicle, it may be displaced from the gallbladder fossa ( Fig. 3-10 ). Injury of the extrahepatic bile ducts can be difficult to diagnose with CT, because perihepatic fluid is often caused by injury to other organs in the abdomen. An intrahepatic biliary ductal injury may be suggested on follow-up CT by the development or persistence of low-attenuation perihepatic fluid collections, usually with an obvious associated hepatic injury.
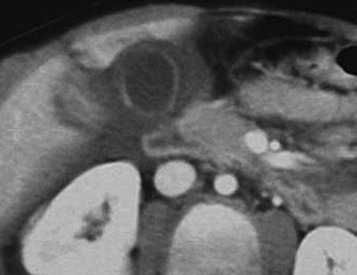
Once a patient with complex liver trauma has survived the acute phase of hepatic trauma, when bleeding and possible exsanguination are the main concerns, the possibility of the development of bile leaks with complicating abscesses and sepsis must be considered and treatment provided. A recent study evaluating patients who had sustained either blunt or penetrating trauma demonstrated a high incidence of bile leaks in the setting of liver laceration (31%). An increased propensity for bile leaks was seen in these patients with increasing AAST liver injury grade and central location of the liver laceration. Free bile leaks in the setting of hepatic trauma are important because they portend a higher morbidity, with these patients undergoing more imaging studies and therapeutic procedures while having longer lengths of hospital stay when compared with posttraumatic patients who have either no biliary injury or contained bile leaks. Persistent perihepatic fluid collections and increasing low-attenuation intraperitoneal fluid are common indicators of bile leaks that require direct therapy.
Biliary scintigraphy is a simple and useful method for detecting and characterizing bile duct injuries. Hepatobiliary radiopharmaceutical agents are taken up by hepatocytes and excreted into the bile ducts. Sequential imaging over 1 to 2 hours identifies extraluminal collections that develop as the radiotracer is excreted into the biliary system and drains into the small bowel lumen. In some cases, images delayed 4 hours are necessary when no evidence of injury is found on the initial image acquisition. With hepatobiliary scintigraphy, accumulation of the radiopharmaceutical agent outside the bile ducts is indicative of a bile leak, which can be either contained (see Fig. 3-12 ) or free if it extends into the peritoneal cavity.
Small bilomas can be treated conservatively and monitored, whereas larger collections may require percutaneous drainage, especially in the presence of superimposed infection. Early detection of bile leaks allows proper treatment through the use of either percutaneous drainage or ERCP with a sphincterotomy and placement of a stent ( Fig. 3-11 ). A possible delayed complication of bile duct injury is the development of a bile duct stricture with obstruction and infection. MRCP is an ideal method for following up on hepatobiliary injuries to assess for the possible development of strictures. An emerging tool for biliary imaging in trauma patients is MRI with use of hepatobiliary agents such as gadoxetic acid (Eovist), which provides both anatomic and functional evaluation of the hepatobiliary system.
The spleen is the most commonly injured organ in the abdomen when blunt trauma is sustained. In the past, an exploratory laparotomy with a splenectomy was the dominant treatment for splenic injuries. However, improvements in our understanding of the natural history of splenic injuries and in the quality of and access to imaging methods have modified treatment algorithms. Nonoperative management is now used initially for the vast majority of splenic injuries. Splenectomy is reserved for the most complex injuries in unstable patients who do not respond to resuscitative efforts and for patients in whom conservative therapy fails. Patients who would otherwise be candidates for conservative management but who require a laparotomy for other abdominal injuries may still undergo a splenectomy.
Splenic trauma is typically difficult to detect clinically. Patients can present with left upper quadrant pain, although associated severe injuries may confound the clinical picture or distract attention from the spleen or abdomen. Instead, most injuries are detected with either US or CT imaging studies performed in these trauma patients. The portable radiograph performed upon admission may demonstrate left rib fractures and alert trauma surgeons to the possibility of an underlying splenic injury. However, plain film radiographs have no role in the direct demonstration of splenic injury. Once an injury is detected, and while the resuscitation process is ongoing, the interventional radiology team should be alerted, because catheter angiography may become necessary for treating vascular injuries, thus avoiding the need for a splenectomy. However, a splenectomy may still become necessary if the injury is severe and bleeding cannot be controlled by nonoperative means.
Splenic injuries are characterized as either hematomas or lacerations. As with the liver, the AAST has developed a scale for grading splenic trauma that is still commonly used for describing specific patterns of injury ( Table 3-2 ). The prognostic implications of this scale are limited, because even complex injuries can heal without specific therapy. It is important to describe the type of injury, the location (parenchymal vs. subcapsular), the size of the hematoma or laceration, and all associated complications. Severe injuries can affect the hilar vascular structures, leading to total or subtotal organ devascularization. Injuries to the splenic artery or branch vessels can cause active bleeding, which can be easily demonstrated with current MDCT examinations. Finally, splenic injuries can lead to the development of contained vascular injuries that are often seen only in an arterial phase; these injuries are extremely important to note, because patients with such injuries have an increased risk of delayed morbidity and mortality as a result of rupture.
| Grade | Description |
|---|---|
| I | Hematoma: Subcapsular, <10% of surface area Laceration: Capsular tear, <1 cm of parenchymal depth |
| II | Hematoma: Subcapsular, 10%-50% of surface area; intraparenchymal hematoma, <5 cm in diameter Laceration: 1-3 cm in parenchymal depth not involving a parenchymal vessel |
| III | Hematoma: Subcapsular, >50% of surface area or expanding or ruptured subcapsular or parenchymal hematoma; intraparenchymal hematoma, >5 cm in diameter Laceration of >3 cm parenchymal depth or involving trabecular vessels |
| IV | Laceration of segmental or hilar vessels producing major devascularization (>25% of the spleen) |
| V | Completely shattered spleen Vascular hilar injury with devascularized spleen |
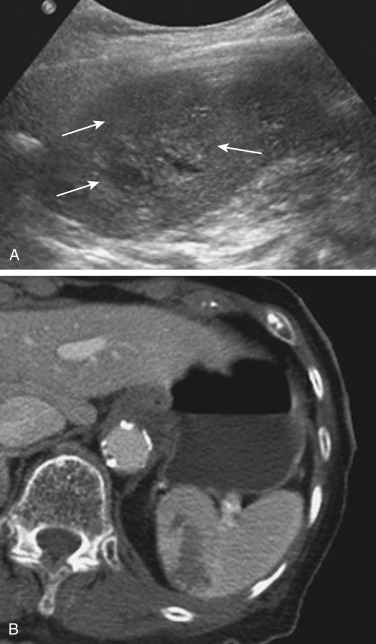
Splenic injuries can be detected by US evaluation of the abdomen and may be suspected based on the results of the initial FAST scan. However, the parenchymal injury can be difficult to detect. Instead, indirect evidence of injury is often identified, including a hemoperitoneum and a focal echogenic clot adjacent to the spleen. Splenic hematomas are heterogeneous and hypoechoic compared with the background spleen ( Fig. 3-12 ). The borders are ill defined, and no associated mass effect or vessel displacement is present. Lacerations appear as linear or branching areas of decreased echogenicity compared with the normal spleen and often extend to the splenic surface. Although they are not commonly used in many countries, sonographic contrast agents have been shown to improve the ability to detect splenic injuries. The spleen can be readily imaged with contrast-enhanced US because it retains the contrast agent for up to 5 to 7 minutes after intravenous injection. If the vascular pedicle is injured, there may be total or subtotal loss of enhancement in the spleen. Active contrast extravasation can be identified as a hyperechoic collection that develops in the early phase after injection of the contrast agent.
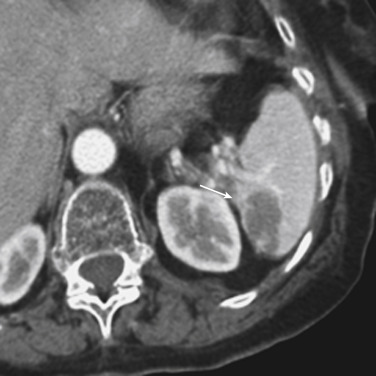
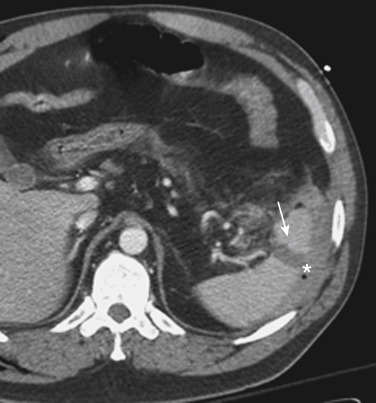
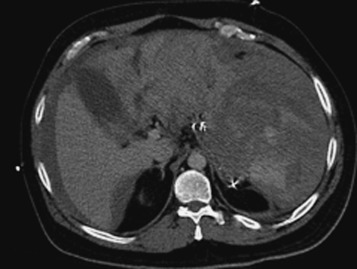
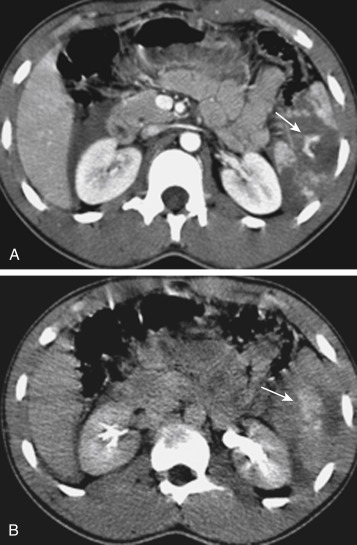
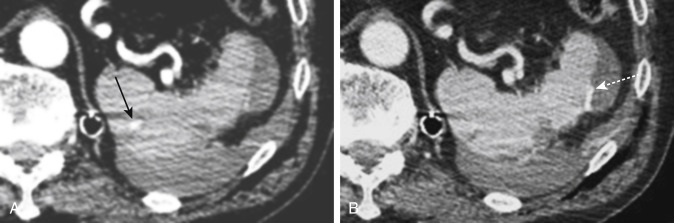
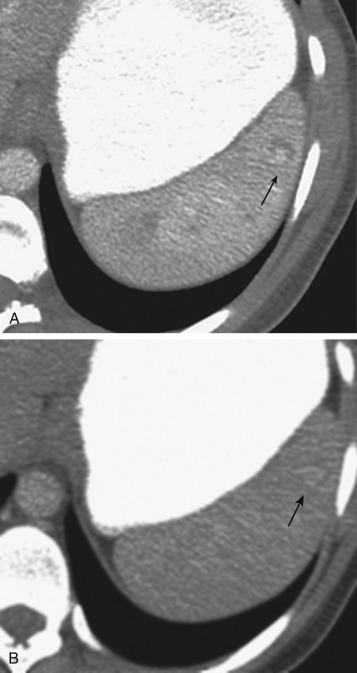
MDCT is the main imaging modality used to detect, characterize, and monitor splenic trauma. Most splenic injuries are optimally detected on portal venous-phase images of the abdomen after intravenous injection of contrast material. Splenic hematomas appear as focal areas of decreased attenuation compared with the background-enhancing splenic tissue ( Fig. 3-13 ). Hematomas can be intraparenchymal or subcapsular. Lacerations appear as linear, irregular, and often branching areas of decreased attenuation ( Fig. 3-14 ). Higher-grade injuries tend to be larger and involve more of the total volume of the spleen ( Fig. 3-15 ). Injuries of the vascular pedicle lead to focal wedge-shaped areas of decreased enhancement, whereas severe injuries of the vascular pedicle may produce a markedly decreased or absent enhancement of most or all of the spleen on CT. In addition to describing the type of injury, it is important to note the presence of either active contrast extravasation or contained vascular injury. Active contrast extravasation in the spleen is characterized on CT by contrast density outside the expected lumen of the vessel that is similar or higher in attenuation compared with the adjacent vessels ( Fig. 3-16 ). The shape is often irregular, with poorly defined margins as a result of the diffusion of the contrast material into the site of the injury. Typically, contrast extravasation is identified within the splenic parenchyma or in a hematoma adjacent to the site of laceration in the perisplenic spaces. In contrast, contained vascular injuries of the spleen are contained extraluminal collections that often have a more round or ovoid appearance ( Fig. 3-17 ). These injuries are usually confined to the splenic parenchyma. Recent literature reports that contained vascular injuries of the spleen are often only seen on an arterial phase. On delayed CT images, active contrast extravasation shows a change in shape and usually an increase in size as the material continues to diffuse into the site of injury (see Figs. 3-16 and 3-17 ). On the other hand, a contained vascular injury does not change in size or shape at different points in time, and the attenuation of the contained vascular injury tends to follow that of adjacent arteries such as the splenic artery or aorta (see Figs. 3-18 and 3-19 ). Of note, some contained vascular injuries of the spleen can only be detected on arterial phase images ( Fig. 3-18 ).
Other focal vascular injuries include the development of arteriovenous fistulas. Such injuries can be difficult to distinguish by CT alone and are better characterized with catheter angiography. Once active contrast extravasation or a focal vascular injury such as a pseudoaneurysm is detected, the need for catheter angiography and possible endovascular therapy should be carefully assessed. Although practices vary among institutions, patients with proved active extravasation are more likely to undergo a splenectomy, whereas contained vascular injuries are more amenable to endovascular therapy with coil embolization.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here