Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Sudden death related to cardiovascular disorders is the most common cause of death among athletes during sports and exercise.
Genetic cardiovascular disorders, such as cardiomyopathies and ion channelopathies, are the primary causes of sudden cardiac death (SCD) in athletes.
Acquired cardiac conditions, such as myocarditis, also represent an important cause of SCD in young athletes.
Most of these disorders can be identified through characteristic changes on a resting 12-lead electrocardiogram (ECG).
Analyzing the ECG of an athlete requires careful interpretation to distinguish normal physiologic changes related to regular exercise from pathologic patterns suggestive of underlying disease.
The most current ECG interpretation guideline, the “International Criteria,” were published in 2017 and provide a framework of three different categories of ECG findings consisting of normal physiologic changes (“green zone”), borderline ECG abnormalities (“yellow zone”), and potential pathologic ECG findings (“red zone”) ( Fig. 34.1 ).
The International Criteria have greatly improved the specificity (i.e., lowered the false-positive rate) while maintaining sensitivity to detect cardiovascular conditions that are associated with an increased risk for SCD.
Utilization of the International Criteria by centers with experience in ECG interpretation in athletes has decreased the false-positive rate to as low as 1.3% in college athletes and 1.5% in elite adolescent soccer players.
The secondary investigation of an abnormal ECG requires additional cardiac testing directed at the specific ECG abnormality, as recommended in the International Criteria.
Informed decisions regarding sports participation after the diagnosis of a pathologic cardiac disorder should include a multidisciplinary team consisting of the patient, their family/support network, a cardiologist, the sports medicine physician, and others at the discretion of the patient.
The heart undergoes physiologic changes in response to regular exercise, termed exercise-induced cardiac remodeling (EICR) , or the “athlete’s heart.”
EICR can consist of increased cardiac chamber size, wall thickness, and/or vagal tone, all of which can lead to unique ECG changes.
ECG findings related to increased cardiac chamber size or thickness include voltage criteria for left ventricular hypertrophy (LVH), right ventricular hypertrophy (RVH), and incomplete right bundle branch block (RBBB), all of which are considered normal findings on an athlete’s ECG.
Findings consistent with increased vagal tone include early repolarization, sinus bradycardia, sinus arrhythmia, first-degree atrioventricular (AV) block, Mobitz type I second-degree AV block, and junctional and ectopic atrial rhythms.
QRS voltage criteria for LVH:
Definition: Sokolow–Lyon criteria: sum of the S wave in V 1 and the R wave in V 5 or V 6 (using the largest R wave) as >3.5 mV.
Present in up to 55% of male and 10% of female athletes.
When pathologic LVH is present, such as in hypertrophic cardiomyopathy (HCM), the voltage criteria for LVH are usually associated with other abnormal ECG findings (i.e., T-wave inversion, ST-segment depression, and/or pathologic Q waves).
QRS voltage criteria for RVH:
Definition: Sokolow–Lyon criteria: R wave in V 1 + largest S wave in V 5 or V 6 >10.5 mV.
Up to 13% of athletes meet these criteria.
Very poor correlation with arrhythmogenic cardiomyopathy (AC), pulmonary hypertension, or other right ventricle (RV) disorders.
Incomplete RBBB:
Definition: QRS duration 100–120 ms with terminal R wave in lead V 1 (commonly characterized as an rSR′ pattern) and wide terminal S wave in leads I and V 6 .
Present in up to 40% of highly trained athletes (particularly endurance and mixed-sport athletes).
Caused by delayed conduction given RV dilation from sustained exercise.
Early repolarization:
Definition: elevation of the QRS-ST junction (J-point) by ≥0.1 mV often associated with late QRS slurring or notching (J wave), most commonly found in the inferior (II, III, aVF) and/or lateral leads (V 5 –V 6 , I, aVL) ( Fig. 34.2 ).
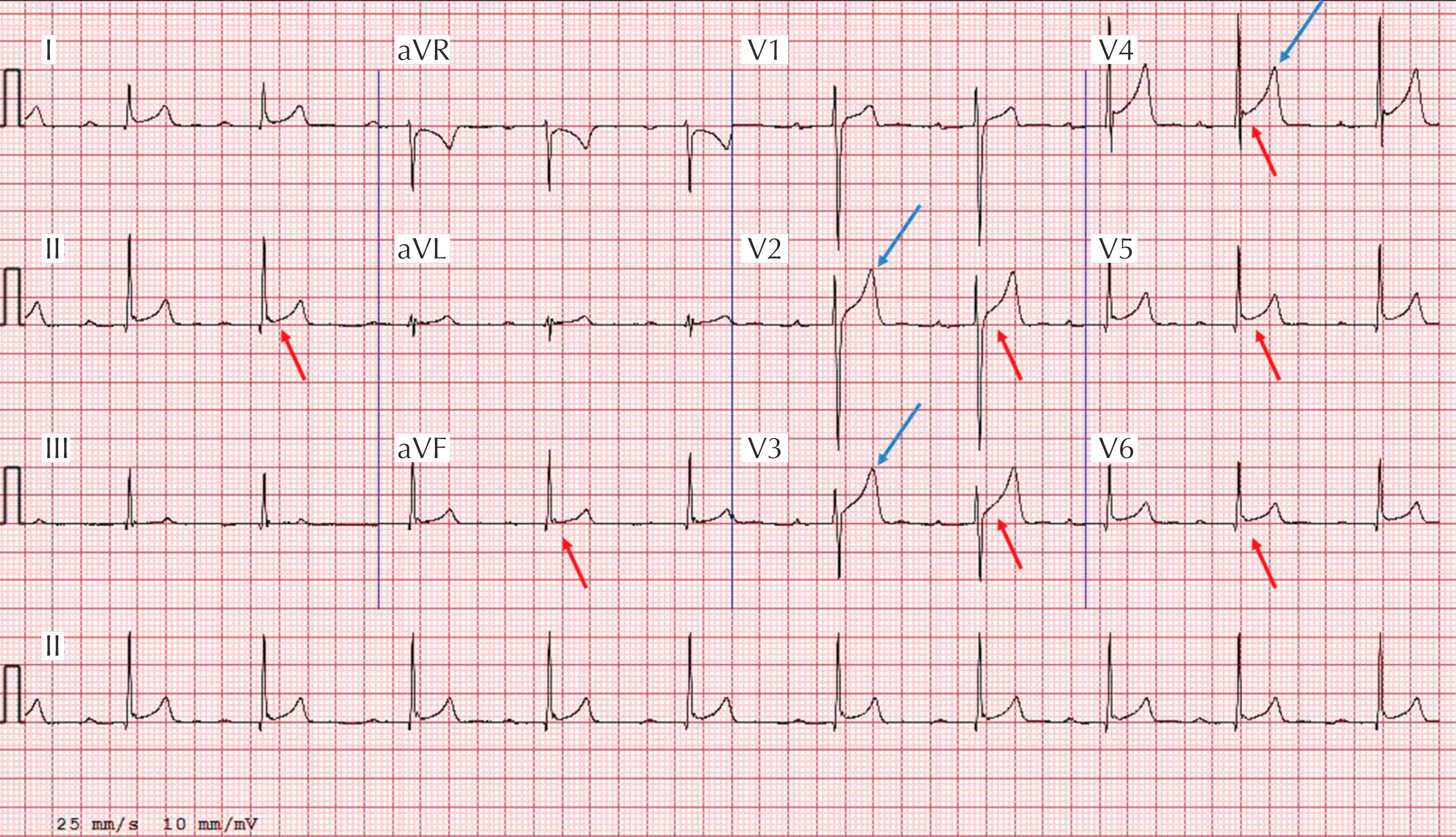
High prevalence in the general population and more common in young, male, and black individuals; seen in 45% of Caucasian athletes and 63%–91% of African-American athletes.
Available evidence suggests that all early repolarization patterns are normal in young athletes.
Early repolarization in black athletes
Five percent to fifteen percent of black/African athletes demonstrate a specific repolarization variant: J-point elevation with convex ST segment followed by T-wave inversion in the anterior leads (V 1 –V 4 ) ( Fig. 34.3 ).
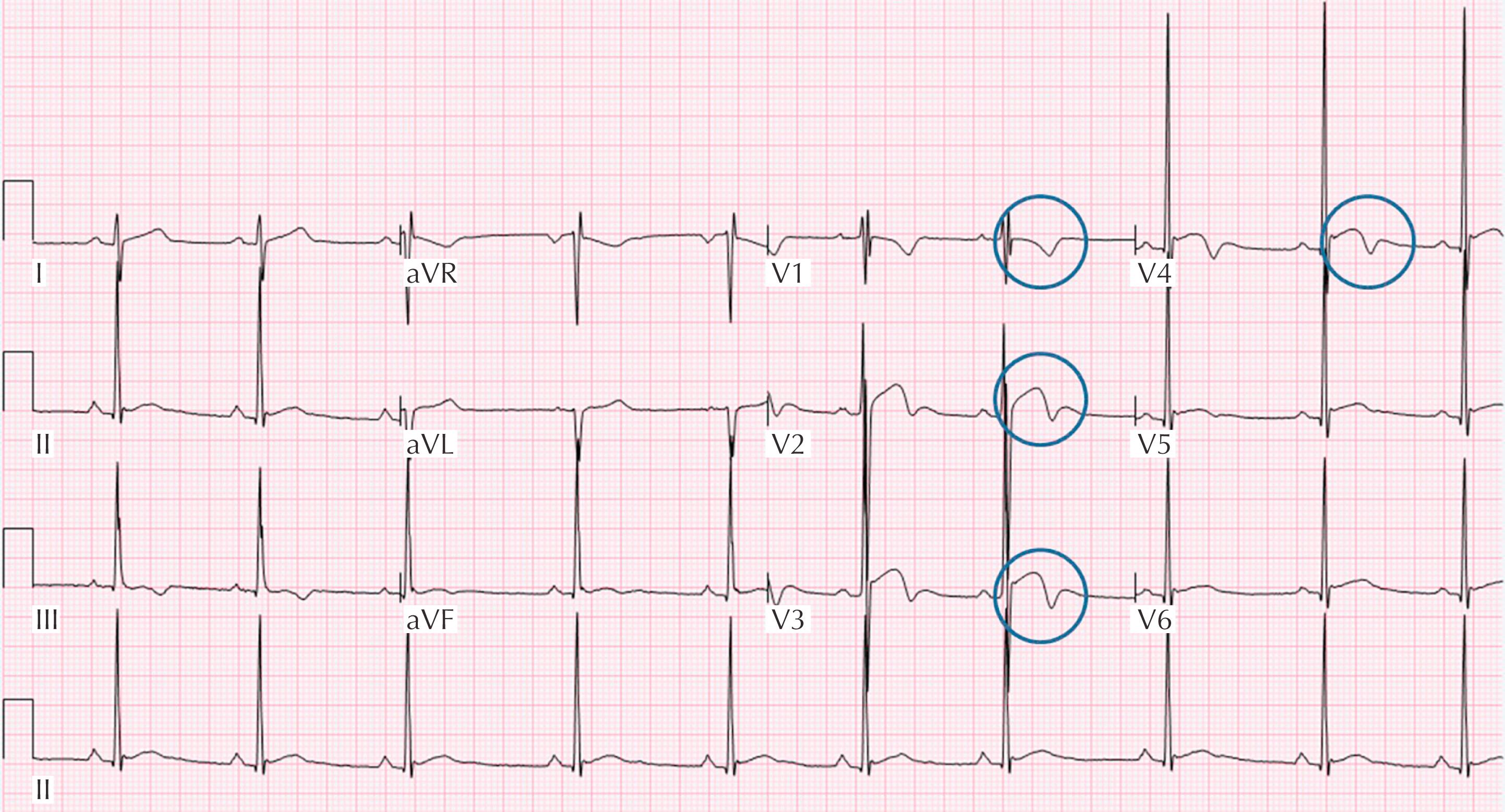
No association with cardiomyopathy with long-term follow-up.
T-wave inversion that extends to the lateral leads (V 5 or V 6 ) should always be considered abnormal and warrants careful evaluation to exclude cardiomyopathy.
“Juvenile” ECG pattern
Definition: T-wave inversion in V 1 –V 3 in asymptomatic athletes aged <16 years (or prepubertal) and independent of race.
T-wave inversion beyond V 2 is rare in Caucasian athletes age ≥16 or postpubertal athletes (0.1%), so warrants further investigation when seen.
Sinus bradycardia
Definition: heart rate (HR) <60 beats per minute (bpm).
Present in up to 80% of highly trained athletes.
Sinus bradycardia should resolve with exertion.
HR ≥30 bpm is considered normal in trained athletes.
Sinus arrhythmia
Definition: subtle oscillation in HR with breathing related to elevated vagal tone.
Should resolve with exertion and is not associated with symptoms.
Junctional escape rhythm
Occurs when increased vagal tone decreases the resting sinus rate below the resting pacemaker cells within the AV junction.
Narrow complex QRS with QRS morphology similar as sinus rhythm but no preceding P waves.
HR <100 and regular RR interval.
Similar to other vagally mediated rhythms; will resolve with exercise.
Ectopic atrial rhythm
P waves are present but different in morphology compared with normal sinus rhythm.
Inverted P waves in the inferior leads (II, III, aVF) suggest a “low atrial” ectopic rhythm.
Eight percent of athletes have ectopic atrial rhythm or junctional escape rhythm.
First-degree AV block
Definition: PR interval >200 ms.
Occurs in 4.5%–7.5% of athletes.
Vagally mediated and should resolve with exertion.
Mobitz type I (Wenckebach) second-degree AV block
Definition: progressive PR lengthening until a nonconducted P wave occurs (no QRS after P wave).
The first PR interval after a nonconducted P wave is shorter than the PR interval for the last conducted P wave.
Exertion will decrease vagal tone, which should resume normal conduction.
Cardiomyopathies are a heterogeneous group of heart muscle diseases.
Collectively, they are the leading identified cause of SCD in young athletes.
Individuals with cardiomyopathies may be asymptomatic, with the presenting sign often being SCD.
The ECG is abnormal in 85%–95% of individuals with cardiomyopathies.
Common ECG findings found in cardiomyopathies are listed in Table 34.1 .
| Pathologic Q Waves | ST-Segment Depression | T-Wave Inversion | Other Findings | |
|---|---|---|---|---|
| HCM | 32%–42% of individuals | ∼50% of individuals | Lateral (V 5 –V 6 , I, aVL) or inferior (II, aVF) (52%–64% of individuals) | Left atrial enlargement Voltage criteria for LVH Multiple PVCs Complete LBBB (∼2% of individuals) Nonspecific IVCD Prolonged QTc |
| AC | Uncommon | Uncommon | Anterior (V 1 –V 4 ) with a flat or depressed ST segment (85% of individuals) | Multiple PVCs Epsilon wave = small negative deflection just after QRS in leads V 1 –V 3 Delayed S-wave upstroke ≥55 ms from nadir of S wave to the end of the QRS complex (in absence of RBBB) Low limb lead voltage (<5 mV) |
| DCM | 10%–25% of individuals | Uncommon | Anterior, lateral, and inferolateral leads (25%–45% of individuals) | Complete LBBB (9%–25% of individuals) Nonspecific IVCD Goldberger triad (criteria suggestive of DCM) = deep S waves in the anterior precordial leads V 1 –V 3 , low limb lead voltage, and poor precordial R-wave progression |
| LVNC | Variable | ∼50% of individuals | Lateral, inferolateral (41% of individuals) | Complete LBBB Nonspecific IVCD |
| Myocarditis | Variable | Variable | Variable | Sinus tachycardia Diffuse ST-segment elevation PR depression Multiple PVCs |
Unexplained, asymmetric LVH (≥16 mm) associated with nondilated ventricular chambers and the absence of another cardiac or systemic disease that could be responsible for the increase in wall thickness.
Genetic disorder with autosomal-dominant inheritance pattern.
Common associations include diastolic dysfunction, mitral regurgitation, myocyte disarray on histopathology, and LV outflow tract obstruction (at rest and increased with exercise).
The most common site of asymmetric LVH is the intraventricular septum.
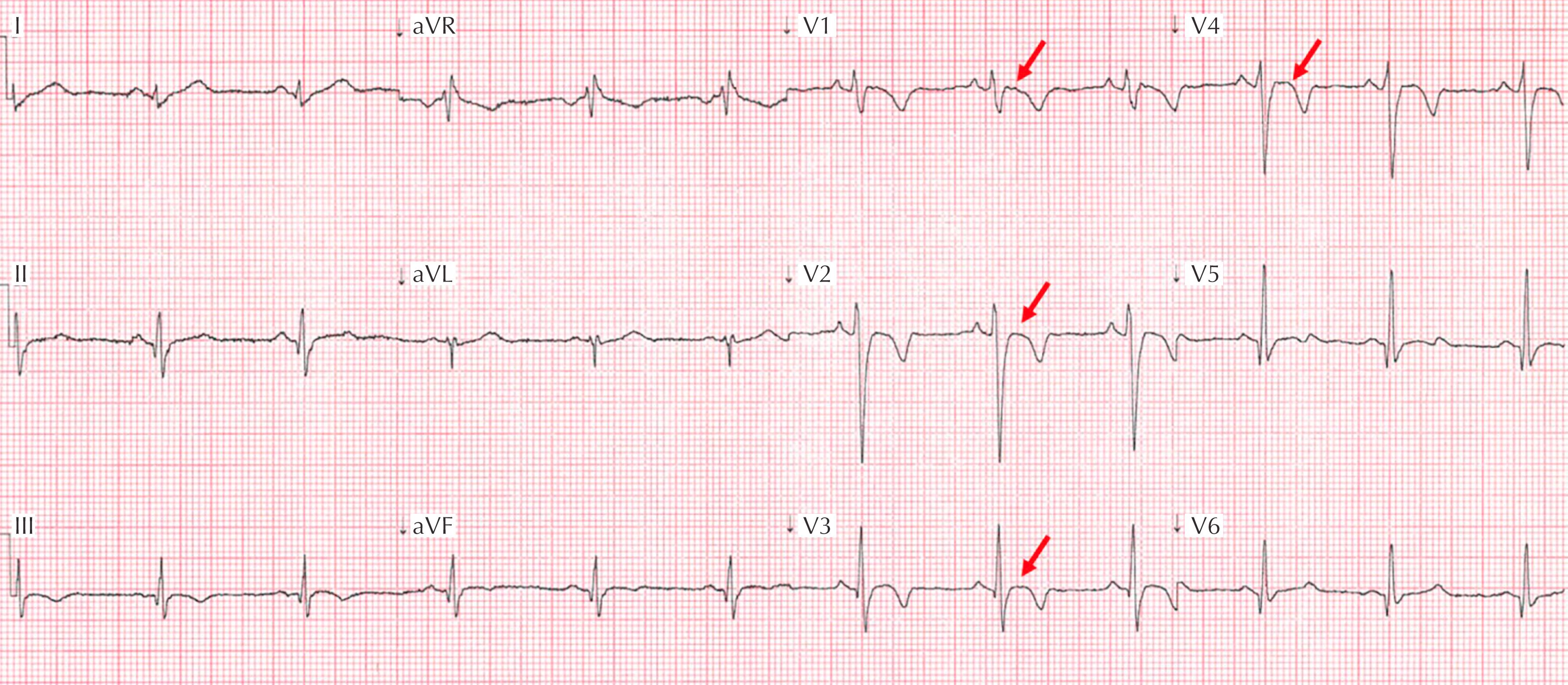
Apical-variant HCM is a variation that accounts for one-third of HCM found in competitive athletes and presents with a markedly abnormal ECG pattern consisting of deep T-wave inversions (>2 mm) and ST-segment depression in the lateral or inferolateral leads ( Fig. 34.4 ).
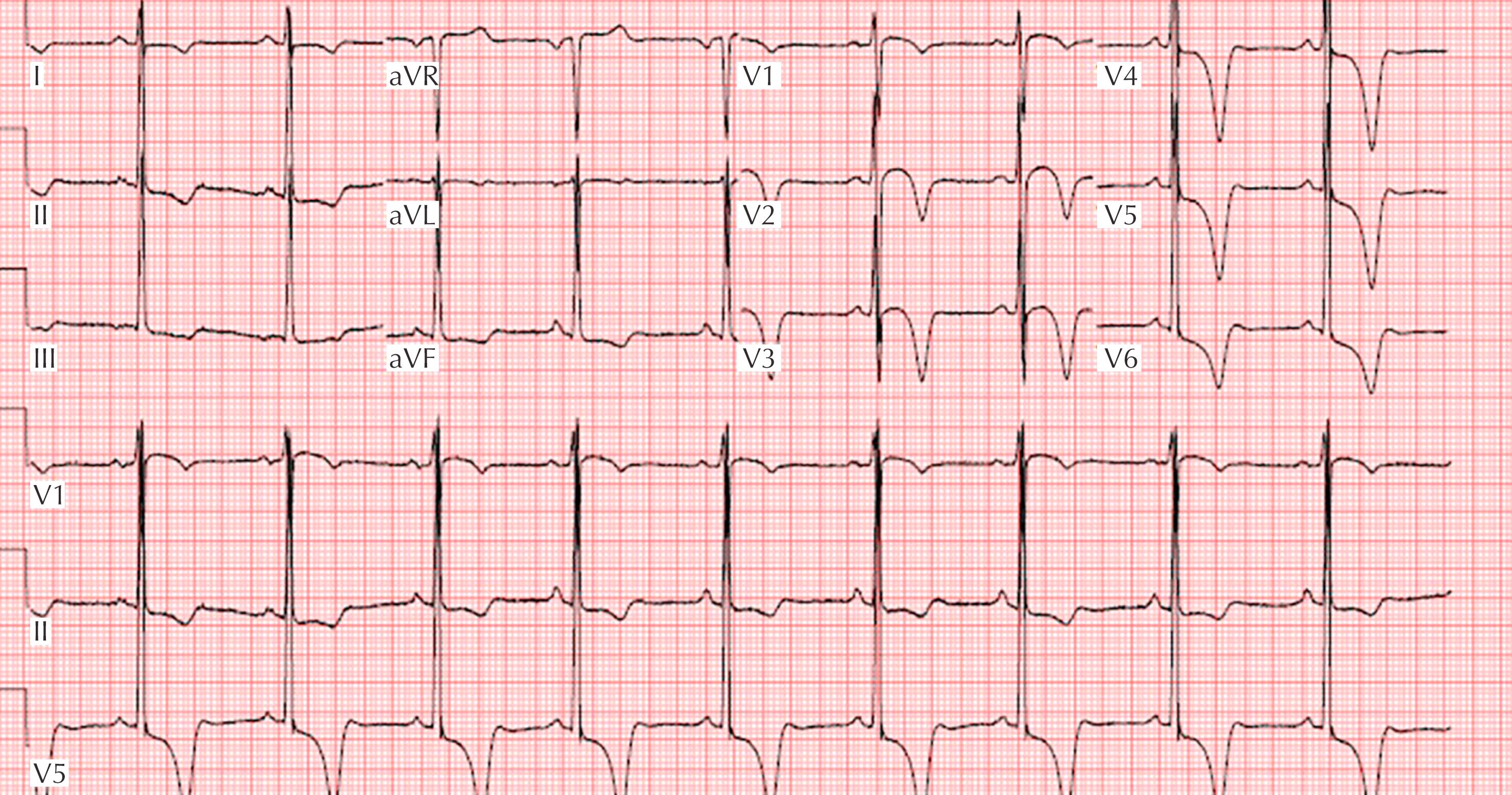
Prevalence of approximately 1:200–1:500 in adults (1:800–2600 in young competitive athletes).
Genetic disorder related to abnormal desmosomes (cell adhesion proteins) leading to subsequent fibrofatty replacement of the myocardium.
Predominately affects the RV free wall ( Fig. 34.5 ), but equal (RV and LV) or predominant LV involvement can occur in some cases.
Often manifests in the second to fourth decade of life with a progressive nature characterized by four phases (concealed, overt electrical disorder, RV failure, and biventricular pump failure).
Athletic activity accelerates disease progression and arrhythmic triggers.
Heart muscle disorder wherein weakened contractile function leads to heart chamber enlargement without associated increase in wall thickness.
Etiologies include viral, toxin mediated (drug/alcohol), tachycardia induced (atrial fibrillation, frequent premature ventricular contractions), and familial dilated cardiomyopathies.
Heart muscle disorder characterized by failure of trabeculated structures to “compact” from spongy to solid myocardium. This noncompaction is phenotypically expressed as deep trabeculations and thinned compacted myocardium, often in a >2:1 ratio. Mutations in sarcomere genes are seen in ∼32% of cases; however, the final common pathway leading to LVNC remains unknown.
Evaluation requires multimodality imaging (e.g., echocardiogram and cardiac magnetic resonance imaging [MRI] and careful clinical evaluation for symptoms, as hypertrabeculation is more common in athletes and black individuals. Clinical correlation is important.
A diagnosis of pathologic LVNC usually requires concurrent systolic dysfunction.
AC (often viral or inflammatory) where ECG abnormalities often overlap with findings found in other myocardial diseases.
Acute presentation may include fever, chest pain, flulike viral syndrome, arrhythmias, syncope, and change in exercise tolerance.
ECG manifestations of myocarditis include sinus tachycardia, T-wave inversion, ST-segment depression, diffuse ST-segment elevation, pathologic Q waves, and PR depression.
In addition to the clinical history, other diagnostic considerations include reduction in left ventricular systolic function on echocardiography and/or supporting criteria on cardiac MRI. Other markers of inflammation (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP]) and high-sensitivity cardiac troponin can also be used during the diagnostic process.
>1 mm of depth in two or more anatomically contiguous leads V 2 –V 6 , II and aVF, or I and aVL (excludes leads III, aVR, and V 1 ); isolated T-wave inversion in V 5 or V 6 is also considered abnormal.
Directionality (i.e., positivity or negativity) refers to the direction of T-wave deflection with regard to the electrically neutral ECG baseline, conventionally defined as the PR segment.
Excludes T-wave inversion found in the repolarization variant in black athletes confined to V 1 –V 4 .
Excludes juvenile T-wave inversion in leads V 1 –V 3 when age is <16 years.
Recommended follow-up evaluation:
In lateral (V 5 –V 6 , I, aVL) or inferolateral leads: T-wave inversion in these territories is likely associated with quiescent cardiomyopathy and deserves full evaluation and follow-up.
Echocardiogram (if nondiagnostic, then cardiac MRI).
Cardiac MRI is indicated for any “markedly” abnormal ECG with >2 mm lateral or inferolateral T-wave inversion and ST-segment depression to exclude apical-variant HCM or other LV-dominant AC.
If imaging nondiagnostic or “gray-zone” hypertrophy (13–15 mm), then exercise ECG test and 24-hour ECG monitor to evaluate for nonsustained ventricular tachycardia.
Inferior leads (II and aVF) alone: T-wave inversion confined to the inferior leads is not a strong predictor of cardiomyopathy in the absence of other ECG criteria or clinical features, but has not been studied in detail.
Echocardiogram with additional evaluation based on abnormal echocardiographic findings or clinical suspicion.
Anterior leads (V 1 –V 4 ): After exclusion of the black athlete repolarization variant or juvenile pattern in athletes age <16 years, anterior T-wave inversion in the absence of J-point elevation or with coexistent ST depression is concerning for RV-dominant AC.
Evaluation includes an echocardiogram, cardiac MRI, exercise ECG test, and 24-hour ECG monitor.
Because of variable phenotypic expression, patients with abnormal T-wave inversion and initial normal cardiac imaging require serial (i.e., annual) re-evaluation, as multiple studies have found that approximately 6% of patients develop clinical cardiomyopathy. Thus, the ECG manifestations of cardiomyopathy can occur before the morphologic changes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here