Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
fatty acid oxidation defects
carnitine cycle
newborn screening
hypoglycemia
medium-chain acyl-CoA dehydrogenase deficiency
liver disease
cardiomyopathy
rhabdomyolysis
fasting intolerance
mitochondrial metabolism
Reye syndrome
Mitochondrial β-oxidation of fatty acids is an essential energy-producing pathway. It is particularly important during prolonged periods of starvation and during periods of reduced caloric intake caused by gastrointestinal illness or increased energy expenditure during febrile illness. Under these conditions, the body switches from using predominantly carbohydrate to predominantly fat as its major fuel. Fatty acids are also important fuels for exercising skeletal muscle and are the preferred substrate for normal cardiac metabolism. In these tissues, fatty acids are completely oxidized to carbon dioxide and water. The end products of hepatic fatty acid oxidation are the ketone bodies β- hydroxybutyrate and acetoacetate. These cannot be oxidized by the liver but are exported to serve as important fuels in peripheral tissues, particularly the brain, where ketone bodies can partially substitute for glucose during periods of fasting.
Genetic defects have been identified in almost all the known steps in the fatty acid oxidation pathway; all are recessively inherited ( Table 104.1 ).
| ENZYME DEFICIENCY | GENE | CLINICAL PHENOTYPE | LABORATORY FINDINGS |
|---|---|---|---|
| Carnitine transporter | OCTN2 SLC22A5 |
Cardiomyopathy, skeletal myopathy, liver disease, sudden death, endocardial fibroelastosis, prenatal and newborn screening diagnosis reported | ↓ Total and free carnitine, normal acylcarnitines, acylglycine, and organic acids |
| Long-chain fatty acid transporter | FATP1-6 | Rare, acute liver failure in childhood requiring liver transplantation | ↓ intracellular C 14 -C 18 fatty acids, ↓ fatty acid oxidation |
| Carnitine palmitoyl transferase-I | CPT-IA | Liver failure, renal tubulopathy, and sudden death. Prenatal and newborn screening diagnosis reported, maternal preeclampsia, HELLP syndrome association described in a few patients. | Normal or ↑ free carnitine, normal acylcarnitines, acylglycine, and organic acids |
| Carnitine acylcarnitine translocase | CACT SLC25A20 |
Chronic progressive liver failure, persistent ↑ NH 3 , hypertrophic cardiomyopathy. Newborn screening diagnosis reported. | Normal or ↓ free carnitine, abnormal acylcarnitine profile |
| Carnitine palmitoyl transferase-II | CPT-II | Early and late onset types. Liver failure, encephalopathy, skeletal myopathy, cardiomyopathy, renal cystic changes, newborn screening diagnosis reported. Adult form with acute rhabdomyolysis, myoglobinuria. | Normal or ↓ free carnitine, abnormal acylcarnitine profile |
| Short-chain acyl-CoA dehydrogenase | SCAD ACADS |
Clinical phenotype unclear. Many individuals appear to be normal. Others have a variety of inconsistent signs and symptoms. Subset may have severe manifestations of unclear relationship to biochemical defects. Newborn screening diagnosis reported; significance being questioned. | Normal or ↓ free carnitine, elevated urine ethylmalonic acid, inconsistently abnormal acylcarnitine profile |
| Medium-chain acyl-CoA dehydrogenase | MCAD ACADM |
Hypoglycemia, hepatic encephalopathy, sudden death. Newborn screening diagnosis possible, maternal preeclampsia, HELLP syndrome association described rarely, possible long Qt interval. | Normal or ↓ free carnitine, ↑ plasma acylglycine, plasma C 6 -C 10 free fatty acids, ↑ C 8 -C 10 acyl-carnitine |
| Very long-chain acyl-CoA dehydrogenase | VLCAD ACADVL |
Dilated cardiomyopathy, arrhythmias, hypoglycemia, and hepatic steatosis. Late-onset, stress-induced rhabdomyolysis, episodic myopathy. Prenatal and newborn screening diagnosis possible. | Normal or ↓ free carnitine, ↑ plasma C 14:1 , C 14 acylcarnitine, ↑ plasma C 10 -C 16 free fatty acids |
| ETF dehydrogenase * | ETF-DH | Nonketotic fasting hypoglycemia, congenital anomalies, milder forms of liver disease, cardiomyopathy, and skeletal myopathy. Newborn screening diagnosis reported. | Normal or ↓ free carnitine, increased ratio of acyl:free carnitine, ↑ acylcarnitine, urine organic acid and acylglycines |
| ETF-α * | α-ETF | Nonketotic fasting hypoglycemia, congenital anomalies, liver disease, cardiomyopathy, and skeletal myopathy also described. Newborn screening diagnosis reported. | Normal or ↓ free carnitine, increased ratio of acyl:free carnitine, ↑ acylcarnitine, urine organic acid and acylglycines |
| ETF-β * | β-ETF | Fasting hypoglycemia, congenital anomalies, liver disease, cardiomyopathy, and skeletal myopathy also described. Newborn screening diagnosis reported. | Normal or ↓ free carnitine, increased ratio of acyl:free carnitine, ↑ acylcarnitine, urine organic acid and acylglycines |
| Short-chain l -3-hydroxyacyl-CoA dehydrogenase | SCHAD HAD1 |
Hyperinsulinemic hypoglycemia, cardiomyopathy, myopathy. Newborn screening diagnosis reported. | Normal or ↓ free carnitine, elevated free fatty acids, inconsistently abnormal urine organic acid, ↑ 3-OH glutarate, ↑ plasma C 4 -OH acylcarnitine |
| Long-chain l -3-hydroxyacyl-CoA dehydrogenase | LCHAD HADH-A | Newborn screening diagnosis reported, maternal preeclampsia, HELLP syndrome, and AFLP association described frequently. See also MTP below for clinical manifestations. | Normal or ↓ free carnitine, increased ratio of acyl:free carnitine, ↑ free fatty acids, ↑ C 16 -OH and C 18 -OH carnitines |
| MTP | HADH-A, HADH-B | Severe cardiac and skeletal myopathy, hypoglycemia, acidosis, hyper NH 3 , sudden death, elevated liver enzymes, retinopathy. Maternal preeclampsia, HELLP syndrome, and AFLP association described frequently. | Normal or ↓ free carnitine, increased ratio of acyl:free carnitine, ↑ free fatty acids, ↑ C 16 -OH and C 18 -OH carnitines |
| Long-chain 3-ketoacyl-CoA thiolase | LKAT HADH-B |
Severe neonatal presentation, hypoglycemia, acidosis, ↑ creatine kinase, cardiomyopathy, neuropathy, and early death | Normal or ↓ free carnitine, increased ratio of acyl:free carnitine, ↑ free fatty acids, ↑ 2- trans , 4- cis -decadienoylcarnitine |
| Short-chain 2,3-enoyl-CoA hydratase | ECHS1 | Leigh disease, lactic acidosis, seizures, cystic degeneration of white matter, microcephaly, metabolic acidosis, extrapyramidal dystonia, dilated cardiomyopathy | Abnormal organic acids, 2-methacrylglycine, 2-methyl-2,3 dihydroxybutyrate, also S-(2-carboxypropyl)cysteine, S-(2-carboxyethyl) cysteamine. Acylcarnitine shows ↑ C4OH (inconsistently). |
| 2,4-Dienoyl-CoA reductase | DECR1 | Only 1 patient described, hypotonia in the newborn, mainly severe skeletal myopathy and respiratory failure. Hypoglycemia rare. | Normal or ↓ free carnitine, ↑ acyl:free carnitine ratio, normal urine organic acids and acylglycines |
| HMG CoA synthetase | HMGCS2 | Hypoketosis and hypoglycemia, rarely myopathy | ↑ total plasma fatty acids, enzyme studies in biopsied liver may be diagnostic, genetic testing preferred |
| HMG CoA lyase | HMGCL | Hypoketosis and hypoglycemia, rarely myopathy | Normal free carnitine, ↑ C 5 -OH, and methylglutaryl-carnitine, enzymes studies in fibroblasts may be diagnostic |
| Monocarboxylate transporter 1 (MCT1) | SLC16A1 | Severe fasting induced ketoacidosis, rarely hypoglycemia | Profound ketoacidosis; no specific biomarkers yet identified |
* Also known as glutaric acidemia type II or multiple acyl-CoA dehydrogenase defect (MADD).
Clinical manifestations characteristically involve tissues with a high β-oxidation flux, including liver, skeletal, and cardiac muscle. The most common presentation is an acute episode of life-threatening coma, hepatic encephalopathy, and hypoglycemia induced by a period of fasting resulting from defective hepatic ketogenesis. Other manifestations may include chronic cardiomyopathy and muscle weakness or exercise-induced acute rhabdomyolysis. The fatty acid oxidation defects can often be asymptomatic during periods when there is no fasting stress or increased energy demand. Acutely presenting disease may be misdiagnosed as Reye syndrome or, if fatal, as sudden unexpected infant death . Fatty acid oxidation disorders are easily overlooked because the only specific clue to the diagnosis may be the finding of inappropriately low concentrations of plasma or urinary ketones in an infant who has hypoglycemia, unless specialized metabolic testing is performed. Genetic defects in ketone body utilization may also be overlooked because ketonemia is an expected finding with fasting hypoglycemia. In some circumstances, clinical manifestations appear to arise from toxic effects of fatty acid metabolites rather than inadequate energy production. These circumstances include certain long-chain fatty acid oxidation disorders (deficiencies of long-chain 3-hydroxyacyl dehydrogenase [ LCHAD ], carnitine palmitoyltransferase-IA [ CPT-IA ], or mitochondrial trifunctional protein [ MTP ; also known as TFP]) in which the presence of a homozygous affected fetus increases the risk of a life-threatening illness in the heterozygote mother, resulting in acute fatty liver of pregnancy (AFLP) or preeclampsia with HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome. The mechanism of these obstetric complications is likely accumulation of toxic intermediates. Malformations of the brain and kidneys have been described in severe deficiencies of electron transfer flavoprotein ( ETF ), ETF dehydrogenase ( ETF-DH ), and carnitine palmitoyltransferase-II ( CPT-II ), which might reflect in utero toxicity of fatty acid metabolites or a developmental role for these enzymes. Progressive retinal degeneration, peripheral neuropathy, and chronic progressive liver disease have been identified in LCHAD and MTP deficiency. Newborn screening programs using tandem mass spectrometry detect characteristic plasma acylcarnitine profiles in most of these disorders, allowing early and presymptomatic diagnosis. Screening programs have demonstrated that all the fatty acid oxidation disorders combined are among the most common inborn errors of metabolism, at least in predominantly Caucasian populations.
Figs. 104.1 and 104.2 outline the steps involved in the oxidation of a typical long-chain fatty acid. In the carnitine cycle , long-chain fatty acids are transported across the barrier of the inner mitochondrial membrane as acylcarnitine esters. (Medium-chain fatty acids, which are commonly provided as medium-chain triglyceride supplementation in infants who are failing to thrive, can bypass the carnitine cycle and enter the mitochondrial β-oxidation cycle directly.) Within the mitochondria, successive turns of the 4-step β -oxidation cycle convert the coenzyme A (CoA) –activated fatty acids to acetyl-CoA units. Two or 3 different specific isoenzymes are needed for each of these β-oxidation steps to accommodate the different chain-length fatty acyl-CoA species. The electrons generated in the first β-oxidation step (acyl-CoA dehydrogenase) are carried by the electron transfer pathway to the electron transport chain at the level of coenzyme Q for adenosine triphosphate production; while electrons generated from the 3rd step (3-hydroxyacyl-CoA dehydrogenase) enter the electron transport chain at the level of complex 1. Most of the acetyl-CoA generated from fatty acid β-oxidation in the liver flows through the pathway of ketogenesis to form β-hydroxybutyrate and acetoacetate, whereas in muscle and heart the fatty acids are completely oxidized to CO 2 and water.
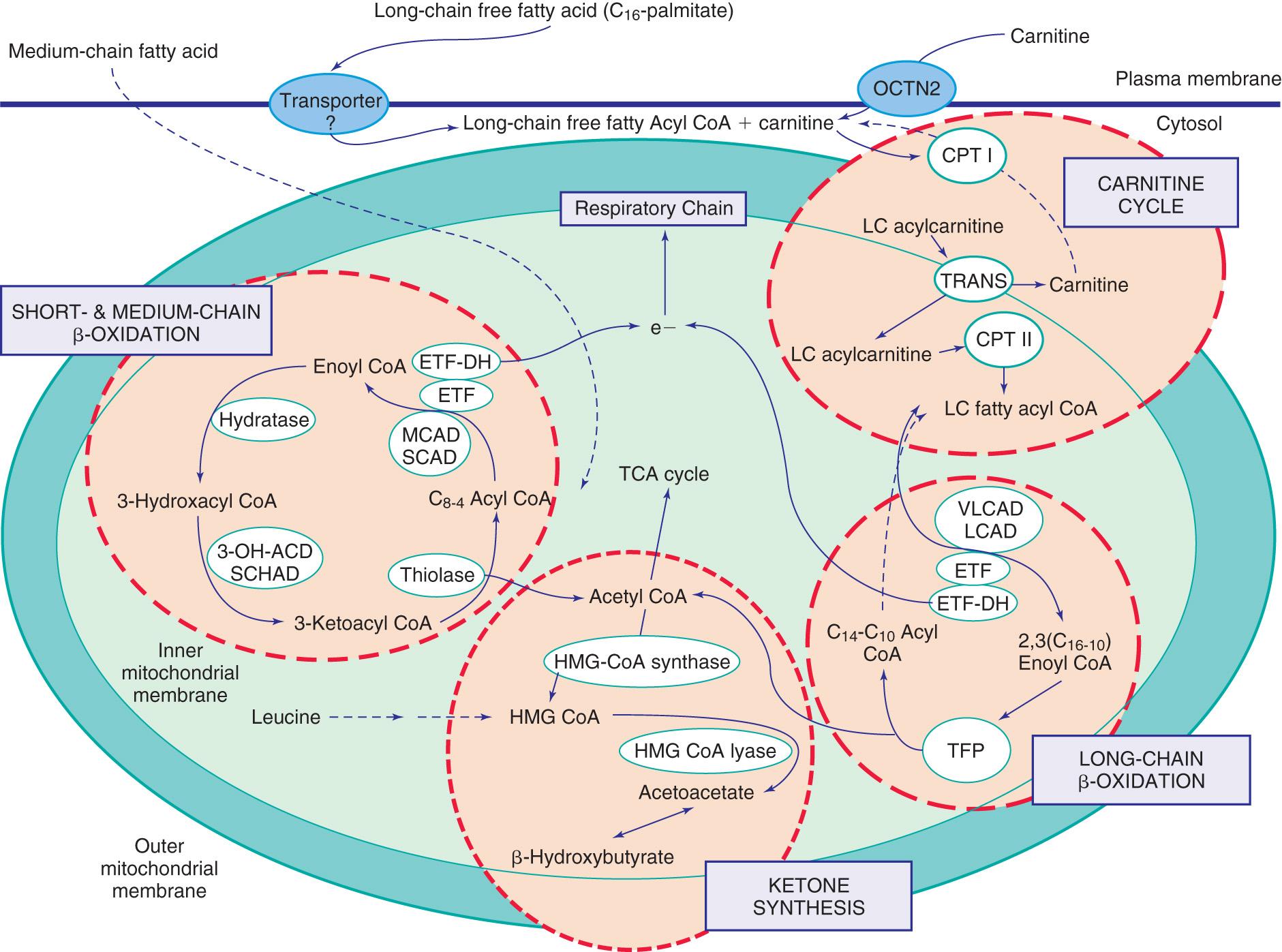

Medium-chain acyl-CoA dehydrogenase ( MCAD ) deficiency is the most common fatty acid oxidation disorder. The disorder shows a strong founder effect; most patients have a northwestern European ancestry, and the majority of these patients are homozygous for a single common MCAD missense mutation, an A-G transition at cDNA position 985 (c.985A>G) that changes a lysine to glutamic acid at residue 329 (p.K329E).
Previously undiagnosed affected patients usually present in the 1st 3 mo to 5 yr of life with episodes of acute illness triggered by prolonged fasting (>12-16 hr). Signs and symptoms include vomiting and lethargy, which rapidly progress to coma or seizures and cardiorespiratory collapse. Sudden unexpected infant death may occur. The liver may be slightly enlarged with fat deposition. Attacks are rare until the infant is beyond the 1st few mo of life, presumably because of more frequent feedings at a younger age. Affected older infants are at higher risk of illness as they begin to fast through the night or are exposed to fasting stress during an intercurrent childhood illness. Presentation in the 1st days of life with neonatal hypoglycemia has been reported in newborns who were fasted inadvertently or were being breastfed. Diagnosis of MCAD has occasionally been documented in previously healthy teenage and adult individuals, indicating that even patients who have been asymptomatic in infancy are still at risk for metabolic decompensation if exposed to sufficient periods of fasting. An unknown number of patients may remain asymptomatic. Prior to routine newborn screening testing, as many as 25% of MCAD-deficient patients died or suffered severe brain damage from their first episode. Most patients are now diagnosed in the newborn period by blood spot acylcarnitine screening , allowing the initiation of early treatment and prevention of many of the severe signs and symptoms. In some reports, newborns with MCAD deficiency presented acutely before newborn screening results were obtained; neonates who are exclusively breastfed are at higher risk because of early poor caloric intake.
During acute episodes, hypoglycemia is usually present. Plasma and urinary ketone concentrations are inappropriately low ( hypoketotic hypoglycemia ). Because of the hypoketonemia, there is little or no metabolic acidosis, which is expected to be present in many children with hypoglycemia. Liver function tests (LFTs) are abnormal, with elevations of liver enzymes (alanine transaminase, aspartate transaminase), elevated blood ammonia, and prolonged prothrombin and partial thromboplastin times. Liver biopsy at times of acute illness shows microvesicular or macrovesicular steatosis from triglyceride accumulation. During fasting stress or acute illness, urinary organic acid profiles by gas chromatography/mass spectrometry show inappropriately low concentrations of ketones and elevated levels of medium-chain dicarboxylic acids (adipic, suberic, and sebacic acids) that derive from microsomal and peroxisomal omega oxidation of accumulated medium-chain fatty acids. Plasma and tissue concentrations of total carnitine are reduced to 25–50% of normal, and the fraction of total esterified carnitine is increased. This pattern of secondary carnitine deficiency is seen in most fatty acid oxidation defects and reflects competition between increased acylcarnitine levels and free carnitine for transport at the renal tubular plasma membrane. Significant exceptions to this rule are the plasma membrane carnitine transporter, CPT-IA, and β-hydroxy-β-methylglutaryl-CoA (HMG-CoA) synthase deficiencies, which do not manifest secondary carnitine deficiency.
Diagnostic metabolite patterns for MCAD deficiency include increased plasma C 6:0 , C 8:0 , C 10:0 , and C 10:1 acylcarnitine species and increased urinary acylglycines, including hexanoylglycine, suberylglycine, and 3-phenylpropionylglycine. Newborn screening, which almost all babies born in the United States receive, can detect presymptomatic MCAD deficiency based on these abnormal acylcarnitines in filter paper blood spots. The diagnosis can be confirmed by finding the common A985G mutation or sequencing the MCAD gene. A 2nd common variant, T199C, has been detected in infants identified by newborn screening. Interestingly, this allele has not been seen to date in symptomatic MCAD patients; it may represent a milder mutation.
Acute illnesses should be promptly treated with intravenous (IV) fluids containing 10% dextrose to correct or prevent hypoglycemia and to suppress lipolysis as rapidly as possible (see Chapter 111 ). Chronic therapy consists of avoiding fasting. This usually requires simply adjusting the diet to ensure that overnight fasting periods are limited to <10-12 hr. Restricting dietary fat or treatment with carnitine is controversial. The need for active therapeutic intervention for individuals with the T199C variant has not yet been established.
Up to 25% of unrecognized patients may die during their first attack of illness. There is frequently a history of a previous sibling death that is presumed to be from an unrecognized MCAD deficiency. Some patients may sustain permanent brain injury during an attack of profound hypoglycemia. For survivors without brain damage, the prognosis is excellent because progressive cognitive impairment or cardiomyopathy does not occur in MCAD deficiency. Fasting tolerance improves with age, and the risk of illness decreases. Because as many as 35% of affected patients have never had an episode, testing of siblings of affected patients is important to detect asymptomatic family members.
Very-long-chain acyl-CoA dehydrogenase ( VLCAD ) deficiency is the second most commonly diagnosed disorder of fatty acid oxidation. It was originally termed “long-chain acyl-CoA dehydrogenase deficiency” before the existence of the inner mitochondrial membrane-bound VLCAD was known. All patients previously diagnosed as having long-chain acyl-CoA dehydrogenase deficiency have VLCAD gene defects. Patients with VLCAD deficiency have no ability to oxidize physiologic long-chain fatty acids and are usually more severely affected than those with MCAD deficiency, who have a milder oxidative defect. VLCAD deficiency presents earlier in infancy and has more chronic problems with muscle weakness or episodes of muscle pain and rhabdomyolysis. Cardiomyopathy may be present during acute attacks provoked by fasting. The left ventricle may be hypertrophic or dilated and may show poor contractility on echocardiography. Sudden unexpected death has occurred in several patients, but most who survive the initial episode show improvement, including normalization of cardiac function. Other physical and routine laboratory features are similar to those of MCAD deficiency, including secondary carnitine deficiency. The urinary organic acid profile shows a nonketotic dicarboxylic aciduria with increased levels of C 6-12 dicarboxylic acids. Diagnosis may be suggested by an abnormal acylcarnitine profile with plasma or blood spot C 14:0, 14:1, 14:2 acylcarnitine species. However, the specific diagnosis requires mutational analysis of the VLCAD gene. Treatment is based primarily on avoidance of fasts for >10-12 hr. Continuous intragastric feeding is useful in some patients.
A small number of patients with 2 null mutations in the short-chain acyl-CoA dehydrogenase (SCAD) gene have been described with variable phenotype. Most individuals classified as being SCAD deficient actually have polymorphic DNA changes in the SCAD gene; for example, 2 common polymorphisms are G185S and R147W, which are homozygously present in 7% of the population. Some investigators argue that these variants may be susceptibility factors, which require a 2nd, as yet unknown, genetic mutation to express a clinical phenotype; many other investigators believe that SCAD deficiency is a harmless biochemical condition. This autosomal recessive disorder presents with neonatal hypoglycemia and may have normal levels of ketone bodies. The diagnosis is indicated by elevated levels of butyrylcarnitine (C4-carnitine) on newborn blood spots or plasma and increased excretion of urinary ethylmalonic acid and butyrylglycine. These metabolic abnormalities are most pronounced in patients with null mutations and are variably present in patients who are homozygous for the common polymorphisms.
The need for treatment in SCAD deficiency has not yet been established. It has been proposed that long-term evaluation of asymptomatic individuals is necessary to determine whether this is or is not a real disease. Most individuals with SCAD deficiency remain asymptomatic throughout life, but there may be a subset of individuals with severe manifestations , including dysmorphic facial features, feeding difficulties/failure to thrive, metabolic acidosis, ketotic hypoglycemia, lethargy, developmental delay, seizures, hypotonia, dystonia, and myopathy.
The LCHAD enzyme is part of the MTP, which also contains 2 other steps in β-oxidation: long-chain 2,3-enoyl CoA hydratase and long-chain β-ketothiolase. MTP is a hetero-octameric protein composed of 4 α and 4 β chains derived from distinct contiguous genes sharing a common promoter region. In some patients, only the LCHAD activity of the MTP is affected ( LCHAD deficiency ), whereas others have deficiencies of all 3 activities ( MTP deficiency ).
Clinical manifestations include attacks of acute hypoketotic hypoglycemia similar to MCAD deficiency; however, patients often show evidence of more severe disease, including cardiomyopathy, muscle cramps and weakness, and abnormal liver function (cholestasis). Toxic effects of fatty acid metabolites may produce pigmented retinopathy leading to blindness, progressive liver failure, peripheral neuropathy, and rhabdomyolysis. Life-threatening obstetric complications (AFLP, HELLP syndrome) have been observed in heterozygous mothers carrying homozygous fetuses affected with LCHAD/MTP deficiency. Sudden unexpected infant death may occur. The diagnosis is indicated by elevated levels of blood spot or plasma 3-hydroxy acylcarnitines of chain lengths C 16 -C 18 . Urinary organic acid profile in patients may show increased levels of 3-hydroxydicarboxylic acids of chain lengths C 6 -C 14 . Secondary carnitine deficiency is common. A common mutation in the α subunit, E474Q, is seen in more than 60% of LCHAD-deficient patients. This mutation in the fetus is especially associated with the obstetric complications, but other mutations in either subunit may also be linked to maternal illness.
Treatment is similar to that for MCAD or VLCAD deficiency; that is, avoiding fasting stress. Some investigators have suggested that dietary supplements with medium-chain triglyceride oil to bypass the defect in long-chain fatty acid oxidation and docosahexaenoic acid (for protection against the retinal changes) may be useful. Liver transplantation has been attempted in patients with severe liver failure, but does not ameliorate the metabolic abnormalities or prevent the myopathic or retinal complications.
Only 14 patients with proven mutations of short-chain 3-hydroxyacyl-CoA dehydrogenase ( SCHAD ) have been reported. Most cases with recessive mutations of the SCHAD gene have presented with episodes of hypoketotic hypoglycemia that was caused by hyperinsulinism. In contrast to those with other forms of fatty acid oxidation disorders, these patients required specific therapy with diazoxide for hyperinsulinism to avoid recurrent hypoglycemia. A single patient with compound heterozygous mutations presented with fulminant hepatic failure at age 10 mo. The SCHAD protein has a nonenzymatic function in which it directly interacts with glutamate dehydrogenase (GDH) to inhibit its activity. In the absence of SCHAD protein, this inhibition is removed, leading to upregulation of GDH enzyme activity, a recognized cause of hyperinsulinism usually from activating mutations of the GDH gene. Severe deficiency of SCHAD protein often presents predominantly as protein-sensitive hypoglycemia rather than as fasting hypoglycemia. It appears that if SCHAD protein is present, inhibition of GDH is maintained even when there is no SCHAD enzyme activity; these patients may present with a more traditional fatty acid oxidation defect. Specific metabolic markers for SCHAD deficiency include elevated plasma C4-hydroxy acylcarnitine and urine 3-hydroxyglutaric acid. Successful newborn screening for SCHAD deficiency has been recorded, but the sensitivity of the process has not yet been established.
Treatment of SCHAD-deficient patients with hyperinsulinism is with diazoxide. There is insufficient experience with the nonhyperinsulinemic form of SCHAD deficiency at present to recommend treatment modalities, but prevention of fasting seems advisable.
This disorder, resulting from mutations in the ECHS1 gene, has only recently been defined. Many patients were identified through exome sequencing, and currently there are approximately 20 cases in the literature. The disorder affects a shared pathway of short-chain fatty acid and valine metabolism. The clinical phenotypes are more characteristic of mitochondrial disorders of pyruvate metabolism with predominantly a Leigh-like disease (see Chapter 616.2 ) with profound and often-fatal lactic acidosis. Currently, no treatment modalities or specific biomarkers have been established. Several patients were found to excrete increased levels of methacrylylglycine, a highly reactive and potentially toxic intermediate; 2-methyl-2.3 dihydroxybutyrate; S -(2-carboxypropyl) cysteine; and S -(2-carboxpropyl) cysteamine.
Primary carnitine deficiency is the only genetic defect in which carnitine deficiency is the cause, rather than the consequence, of impaired fatty acid oxidation. The most common presentation is progressive cardiomyopathy with or without skeletal muscle weakness beginning at age 1-4 yr. A smaller number of patients may present with fasting hypoketotic hypoglycemia in the 1st yr of life, before the cardiomyopathy becomes symptomatic. The underlying defect involves the plasma membrane sodium gradient–dependent carnitine transporter that is present in heart, muscle, and kidney. This transporter is responsible both for maintaining intracellular carnitine concentrations 20-50–fold higher than plasma concentrations and for renal conservation of carnitine.
Diagnosis of the carnitine transporter defect is aided by patients having extremely reduced carnitine levels in plasma and muscle (1–2% of normal). Heterozygote parents have plasma carnitine levels approximately 50% of normal. Fasting ketogenesis may be normal because liver carnitine transport is normal, but it may become impaired if dietary carnitine intake is interrupted. The fasting urinary organic acid profile may show a hypoketotic dicarboxylic aciduria pattern if hepatic fatty acid oxidation is impaired, but is otherwise unremarkable. The defect in carnitine transport can be demonstrated clinically by the severe reduction in renal carnitine threshold or by in vitro assay of carnitine uptake using cultured fibroblasts or lymphoblasts. Mutations in the organic cation/carnitine transporter (OCTN2) underlie this disorder. Treatment with pharmacologic doses of oral carnitine (100-200 mg/kg/day) is highly effective in correcting the cardiomyopathy and muscle weakness, as well as any impairment in fasting ketogenesis. Muscle total carnitine concentrations remain <5% of normal on treatment.
Several dozen infants and children have been described with a deficiency of the liver and kidney CPT-I isozyme (CPT-IA). Clinical manifestations include fasting-induced hypoketotic hypoglycemia, occasionally with extremely abnormal LFTs and rarely with renal tubular acidosis. The heart and skeletal muscle are not involved because the muscle isozyme is unaffected. Fasting urinary organic acid profiles sometimes show a hypoketotic C 6 -C 12 dicarboxylic aciduria but may be normal. Plasma acylcarnitine analysis demonstrates mostly free carnitine with very little acylated carnitine. This observation has been used to identify CPT-IA deficiency on newborn screening by tandem mass spectrometry. CPT-IA deficiency is the only fatty acid oxidation disorder in which plasma total carnitine levels may be elevated, often to 150–200% of normal. This phenomenon is explained by the absence of inhibitory effects of long-chain acylcarnitines on the renal tubular carnitine transporter in CPT-IA deficiency. The enzyme defect can be demonstrated in cultured fibroblasts or lymphoblasts. CPT-IA deficiency in the fetus has been associated in a single case report with AFLP in the mother. A common variant in the CPTIA gene (c.1436C>t, p.P479L) has been identified in individuals of Inuit background in the United States, Canada, and Greenland. This variant is associated with an increased risk for sudden infant death syndrome (SIDS) in the Inuit population. The variant can be detected by newborn screening; enzyme activity is reduced by 80%, and regulation by malonyl-CoA is lost. It has not been established whether CPT-IA (c.1436C>t, p.P479L) is a pathologic enzyme variant or an adaptation to ancient Inuit high-fat diets. Treatment for the severe form of CPT-IA deficiency that is found in non-Inuit populations is similar to that for MCAD deficiency, with avoidance of situations where fasting ketogenesis is necessary. The need for treatment of the Inuit variant has not yet been determined.
This defect of the inner mitochondrial membrane carrier protein for long-chain acylcarnitines blocks the entry of long-chain fatty acids into the mitochondria for oxidation. The clinical phenotype of this disorder is characterized by a severe and generalized impairment of fatty acid oxidation. Most newborn patients present with attacks of fasting-induced hypoglycemia, hyperammonemia, and cardiorespiratory collapse. All symptomatic newborns have had evidence of cardiomyopathy and muscle weakness. Several patients with a partial translocase deficiency and milder disease without cardiac involvement have also been identified. No distinctive urinary or plasma organic acids are noted, although increased levels of plasma long-chain acylcarnitines of chain lengths C 16 -C 18 are reported. Diagnosis can be confirmed using genetic analysis. Functional carnitine:acylcarnitine translocase activity can be measured in cultured fibroblasts or lymphoblasts. Treatment is similar to that of other long-chain fatty acid oxidation disorders.
Three forms of CPT-II deficiency have been described. A severe neonatal lethal presentation associated with a profound enzyme deficiency and early death has been reported in several newborns in association with dysplastic kidneys, cerebral malformations, and mild facial anomalies. A milder defect is associated with an adult presentation of episodic rhabdomyolysis. The first episode usually does not occur until late childhood or early adulthood. Attacks are frequently precipitated by prolonged exercise. There is aching muscle pain and myoglobinuria that may be severe enough to cause renal failure. Serum levels of creatine kinase are elevated to 5,000-100,000 units/L. Hypoglycemia has not been described, but fasting may contribute to attacks of myoglobinuria. Muscle biopsy shows increased deposition of neutral fat. This adult myopathic presentation of CPT-II deficiency is associated with a common mutation, c.338C>T, p.S113L. This mutation produces a heat-labile protein that is unstable to increased muscle temperature during exercise resulting in the myopathic presentation. The 3rd, intermediate form of CPT-II deficiency presents in infancy or early childhood with fasting-induced hepatic failure, cardiomyopathy, and skeletal myopathy with hypoketotic hypoglycemia, but is not associated with the severe developmental changes seen in the neonatal lethal presentation. This pattern of illness is similar to that seen in VLCAD deficiency, and management is identical.
Diagnosis of all forms of CPT-II deficiency can be made by a combination of molecular genetic analysis and demonstrating deficient enzyme activity in muscle or other tissues and in cultured fibroblasts.
ETF and ETF-DH function to transfer electrons into the mitochondrial electron transport chain from dehydrogenation reactions catalyzed by VLCAD, MCAD, and SCAD, as well as by glutaryl-CoA dehydrogenase and 4 enzymes involved in branched-chain amino acid (BCAA) oxidation. Deficiencies of ETF or ETF-DH produce illness that combines the features of impaired fatty acid oxidation and impaired oxidation of several amino acids. Complete deficiencies of either protein are associated with severe illness in the newborn period, characterized by acidosis, hypoketotic hypoglycemia, coma, hypotonia, cardiomyopathy, and an unusual odor of sweaty feet caused by isovaleryl-CoA dehydrogenase inhibition. Some affected neonates have had congenital facial dysmorphism and polycystic kidneys similar to that seen in severe CPT-II deficiency, which suggests that toxic effects of accumulated metabolites may occur in utero.
Diagnosis can be made from the newborn blood spot acylcarnitine profile and urinary organic acids; both tests show abnormalities corresponding to blocks in the oxidation of fatty acids (ethylmalonate and C 6 -C 10 dicarboxylic acids), lysine (glutarate), and BCAAs (isovaleryl-, isobutyryl-, and α-methylbutyryl-glycine). The diagnosis can be confirmed by genetic testing for ETF (2 genes, A and B) and ETF dehydrogenase. Most severely affected infants do not survive the neonatal period.
Partial deficiencies of ETF and ETF-DH produce a disorder that may mimic MCAD deficiency or other milder fatty acid oxidation defects. These patients have attacks of fasting hypoketotic coma. The urinary organic acid profile reveals primarily elevations of dicarboxylic acids and ethylmalonate, derived from short-chain fatty acid intermediates. Secondary carnitine deficiency is present. Some patients with mild forms of ETF/ETF-DH deficiency may benefit from treatment with high doses of riboflavin, a precursor of the various flavoproteins involved in electron transfer.
The final steps in production of ketones from mitochondrial fatty acid β-oxidation convert acetyl-CoA to acetoacetate through 2 enzymes of the HMG-CoA pathway ( Fig. 104.2 ).
See Chapter 103.6 .
HMG-CoA synthase is the rate-limiting step in the conversion of acetyl-CoA derived from fatty acid β-oxidation in the liver to ketones. Several patients with this defect have been identified. The presentation is one of fasting hypoketotic hypoglycemia without evidence of impaired cardiac or skeletal muscle function. Urinary organic acid profile shows only a nonspecific hypoketotic dicarboxylic aciduria. Plasma and tissue carnitine levels are normal, in contrast to all the other disorders of fatty acid oxidation. A separate synthase enzyme, present in cytosol for cholesterol biosynthesis, is not affected. The HMG-CoA synthase defect is expressed only in the liver (and kidney) and cannot be demonstrated in cultured fibroblasts. The diagnosis can be made by genetic mutation analysis. Avoiding fasting is usually a successful treatment.
See Chapter 103.6 .
The ketone bodies, β-hydroxybutyrate and acetoacetate, are the end products of hepatic fatty acid oxidation and are important metabolic fuels for the brain during fasting. Three defects in utilization of ketones in brain and other peripheral tissues present as episodes of hyperketotic coma , with or without hypoglycemia.
About 10 patients have been described with recurrent episodes of potentially lethal ketoacidosis, with or without hypoglycemia, caused by deficiency of monocarboxylate transporter 1 (MCT1), a plasma membrane carrier encoded by SLC16A1 that is required to transport ketones into tissues from plasma. Although the first cases identified were homozygous for inactivating mutations of MCT1, heterozygous carriers can also be affected. Affected patients developed severe ketoacidosis provoked by fasting or infections in their 1st years of life; hypoglycemia was not always present. The differential includes ketotic hypoglycemia associated with milder forms of glycogen storage disease, such as phosphorylase or phosphorylase kinase deficiency (see Chapter 105 ). Treatment for acute episodes includes IV dextrose to suppress lipolysis and inhibit ongoing ketogenesis. Long-term treatment includes avoidance of prolonged fasting stress. The diagnosis can be suspected by unusually severe ketosis and delayed suppression of ketones after starting treatment with dextrose. There are no specific metabolic markers or newborn screening methods. The diagnosis can be established by genetic sequencing of SLC16A1 .
See Chapter 103.6 .
Several patients with succinyl-CoA:3-ketoacid-CoA transferase ( SCOT ) deficiency have been reported. The characteristic presentation is an infant with recurrent episodes of severe ketoacidosis induced by fasting. Plasma acylcarnitine and urine organic acid abnormalities do not distinguish SCOT deficiency from other causes of ketoacidosis. Treatment of episodes requires infusion of glucose and large amounts of bicarbonate until metabolically stable. Patients usually exhibit inappropriate hyperketonemia even between episodes of illness. SCOT is responsible for activating acetoacetate in peripheral tissues, using succinyl CoA as a donor to form acetoacetyl-CoA. Deficient enzyme activity can be demonstrated in the brain, muscle, and fibroblasts from affected patients. The gene has been cloned, and numerous mutations have been characterized.
See Chapter 103.6 .
very-long-chain fatty acids
VLCFAs
peroxisome
Zellweger syndrome
adrenoleukodystrophy
Disorders of very-long-chain fatty acids (VLCFAs) fall within the broader group of peroxisomal diseases. The peroxisomal diseases are genetically determined disorders caused either by the failure to form or maintain the peroxisome or by a defect in the function of a single protein that is normally located in this organelle. These disorders cause serious disability in childhood and occur more frequently and present a wider range of phenotypes than recognized in the past. Many, but not all, peroxisomal disorders are associated with elevations of VLCFAs. This discussion addresses the broader group of peroxisomal disorders with a focus on pediatric presentations.
Peroxisomal disorders are subdivided into two major categories ( Table 104.2 ). In the peroxisomal biogenesis disorders (PBDs) the basic defect is the failure to import 1 or more proteins into the organelle. In the other group, defects affect a single peroxisomal protein ( single-enzyme defects ). The peroxisome is present in all cells except mature erythrocytes and is a subcellular organelle surrounded by a single membrane; >50 peroxisomal enzymes are identified. Some enzymes are involved in production and decomposition of hydrogen peroxide and others in lipid and amino acid metabolism. Most peroxisomal enzymes are first synthesized in their mature form on free polyribosomes and enter the cytoplasm. Proteins that are destined for the peroxisome contain specific peroxisome targeting sequences (PTSs). Most peroxisomal matrix proteins contain PTS1 , a 3-amino acid sequence at the carboxyl terminus. PTS2 is an aminoterminal sequence that is critical for the import of enzymes involved in plasmalogen and branched-chain fatty acid metabolism. Import of proteins involves a complex series of reactions that involves at least 23 distinct proteins. These proteins, referred to as peroxins, are encoded by PEX genes.
| PEROXISOMAL BIOGENESIS DISORDERS | SINGLE-ENZYME DEFECTS |
|---|---|
| Zellweger spectrum disorder | X-linked adrenoleukodystrophy Acyl-CoA oxidase deficiency Bifunctional enzyme deficiency 2-Methylacyl-CoA racemase deficiency DHAP acyltransferase deficiency Alkyl-DHAP synthase deficiency Adult Refsum disease |
| Zellweger syndrome Neonatal adrenoleukodystrophy (ALD) Infantile Refsum disease |
|
| Rhizomelic chondrodysplasia punctata (RCDP) and other PEX7 conditions |
Except for X-linked adrenoleukodystrophy (ALD) , all the peroxisomal disorders listed in Table 104.2 are autosomal recessive diseases . ALD is the most common peroxisomal disorder, with an estimated incidence of 1 in 17,000 live births. The combined incidence of the other peroxisomal disorders is estimated to be 1 in 50,000 live births, although with broader newborn screening it is expected that the actual incidences of all of the disorders of very-long-chain fatty acids will be more accurately established.
Absence or reduction in the number of peroxisomes is pathognomonic for disorders of peroxisome biogenesis. In most disorders, membranous sacs contain peroxisomal integral membrane proteins, which lack the normal complement of matrix proteins; these are peroxisome “ghosts.” Pathologic changes are observed in most organs and include profound and characteristic defects in neuronal migration, micronodular cirrhosis of the liver, renal cysts, chondrodysplasia punctata, sensorineural hearing loss, retinopathy, congenital heart disease, and dysmorphic features.
All pathologic changes likely are secondary to the peroxisome defect. Multiple peroxisomal enzymes fail to function in the PBDs ( Table 104.3 ). The enzymes that are diminished or absent are synthesized but are degraded abnormally fast because they may be unprotected outside the peroxisome. It is not clear how defective peroxisome functions lead to the widespread pathologic manifestations.
Peroxisomes absent to reduced in number
Catalase in cytosol
Deficient synthesis and reduced tissue levels of plasmalogens
Defective oxidation and abnormal accumulation of very-long-chain fatty acids
Deficient oxidation and age-dependent accumulation of phytanic acid
Defects in certain steps of bile acid formation and accumulation of bile acid intermediates
Defects in oxidation and accumulation of l -pipecolic acid
Increased urinary excretion of dicarboxylic acids
Mutations in 12 different PEX genes have been identified in PBDs. The pattern and severity of pathologic features vary with the nature of the import defects and the degree of import impairment. These gene defects lead to disorders that were named before their relationship to the peroxisome was recognized, namely, Zellweger syndrome, neonatal ALD, infantile Refsum disease, and rhizomelic chondrodysplasia punctata ( RCDP ). The first 3 disorders are considered to form a clinical continuum , with Zellweger syndrome the most severe, infantile Refsum disease the least severe, and neonatal ALD intermediate. They can be caused by mutations in any of the 11 genes involved in peroxisome assembly. The specific gene defects cannot be distinguished by clinical features. The clinical severity varies with the degree to which protein import is impaired. Mutations that abolish import completely are often associated with the Zellweger syndrome phenotype, whereas a missense mutation, in which some degree of import function is retained, leads to the somewhat milder phenotypes. A defect in PEX7, which involves the import of proteins that utilize PTS2, is associated with RCDP. PEX7 defects that leave import partially intact are associated with milder phenotypes, some of which resemble classic (adult) Refsum disease.
The genetic disorders that involve single peroxisomal enzymes usually have clinical manifestations that are more restricted and relate to the single biochemical defect. The primary adrenal insufficiency of ALD is caused by accumulation of VLCFAs in the adrenal cortex, and the peripheral neuropathy in adult Refsum disease is caused by the accumulation of phytanic acid in Schwann cells and myelin.
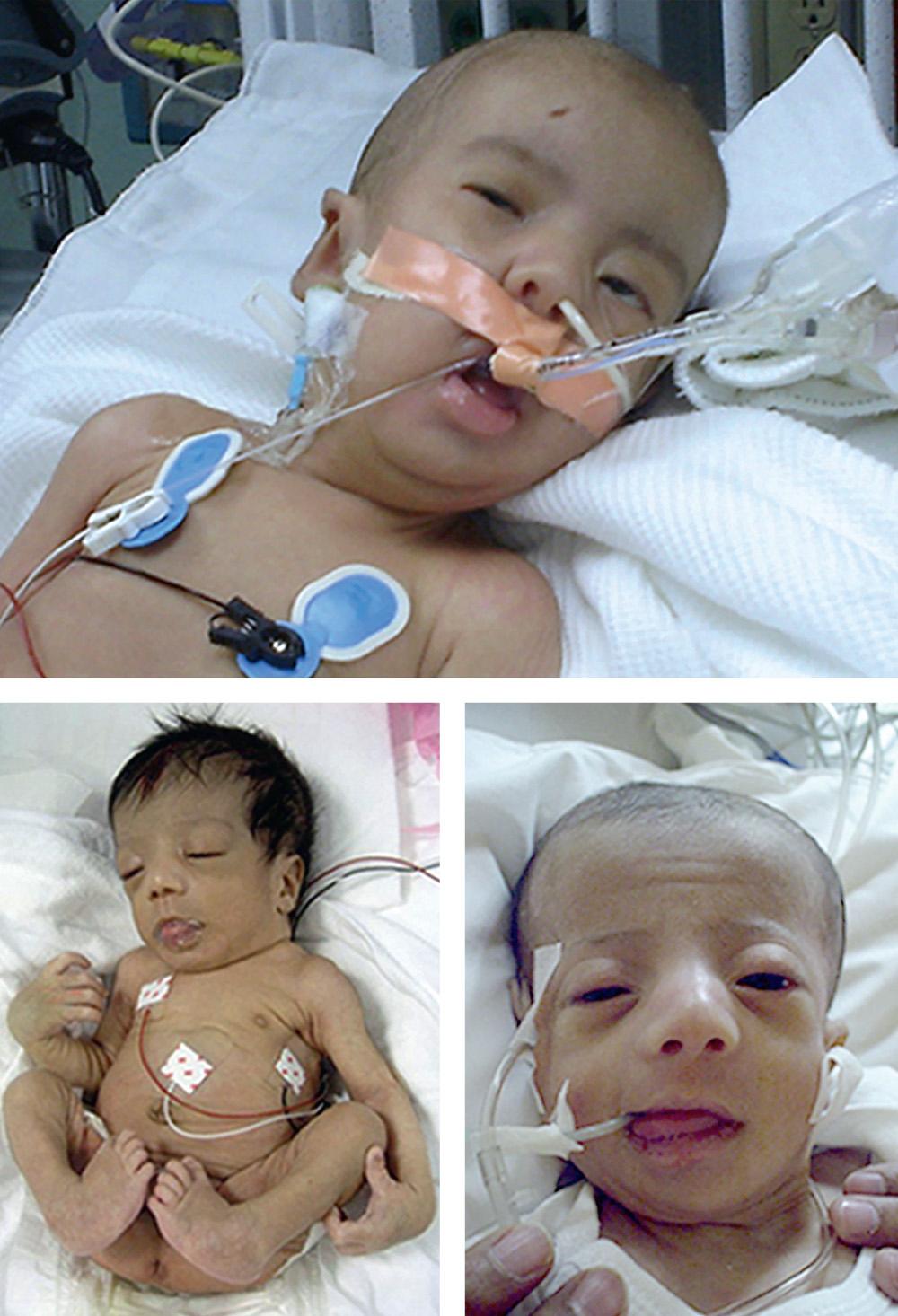
Newborn infants with Zellweger syndrome show striking and consistent recognizable abnormalities. Of central diagnostic importance are the typical facial appearance (high forehead, unslanting palpebral fissures, hypoplastic supraorbital ridges, and epicanthal folds; Fig. 104.3 ), severe weakness and hypotonia, neonatal seizures, and eye abnormalities. Because of the hypotonia and craniofacial appearance, Down syndrome may be suspected. Infants with Zellweger syndrome rarely live more than a few months. More than 90% show postnatal growth failure. Table 104.4 lists the main clinical abnormalities.
| ABNORMAL FEATURE | PATIENTS IN WHOM THE FEATURE WAS PRESENT | |
|---|---|---|
| Number | % | |
| High forehead | 58 | 97 |
| Flat occiput | 13 | 81 |
| Large fontanelle(s), wide sutures | 55 | 96 |
| Shallow orbital ridges | 33 | 100 |
| Low/broad nasal bridge | 23 | 100 |
| Epicanthus | 33 | 92 |
| High-arched palate | 35 | 95 |
| External ear deformity | 39 | 97 |
| Micrognathia | 18 | 100 |
| Redundant skin fold of neck | 13 | 100 |
| Brushfield spots | 5 | 83 |
| Cataract/cloudy cornea | 30 | 86 |
| Glaucoma | 7 | 58 |
| Abnormal retinal pigmentation | 6 | 40 |
| Optic disc pallor | 17 | 74 |
| Severe hypotonia | 94 | 99 |
| Abnormal Moro response | 26 | 100 |
| Hyporeflexia or areflexia | 56 | 98 |
| Poor sucking | 74 | 96 |
| Gavage feeding | 26 | 100 |
| Epileptic seizures | 56 | 92 |
| Psychomotor retardation | 45 | 100 |
| Impaired hearing | 9 | 40 |
| Nystagmus | 30 | 81 |
Patients with neonatal ALD show fewer, less prominent craniofacial features. Neonatal seizures occur frequently. Some degree of psychomotor developmental delay is present; function remains in the range of severe intellectual disability, and development may regress after 3-5 yr of age, probably from a progressive leukodystrophy. Hepatomegaly, impaired liver function, pigmentary degeneration of the retina, and severely impaired hearing are invariably present. Adrenocortical function is usually impaired and may require adrenal hormone replacement. Chondrodysplasia punctata and renal cysts are absent.
Patients with infantile Refsum disease have survived to adulthood. They can walk, although gait may be ataxic and broad based. Cognitive function is generally impaired, but accurate assessment is limited, usually by the presence of both vision and hearing impairment. Almost all have some degree of sensorineural hearing loss and pigmentary degeneration of the retina. They have moderately dysmorphic features that may include epicanthal folds, a flat nose bridge, and low-set ears. Early hypotonia and hepatomegaly with impaired function are common. Levels of plasma cholesterol and high-density and low-density lipoprotein are often moderately reduced. Chondrodysplasia punctata and renal cortical cysts are absent. Postmortem study in infantile Refsum disease reveals micronodular liver cirrhosis and small, hypoplastic adrenals. The brain shows no malformations, except for severe hypoplasia of the cerebellar granule layer and ectopic locations of the Purkinje cells in the molecular layer. The mode of inheritance is autosomal recessive.
Some patients with PBDs have milder and atypical phenotypes. They may present with peripheral neuropathy or with retinopathy, impaired vision, or cataracts in childhood, adolescence, or adulthood and have been diagnosed to have Charcot-Marie-Tooth disease or Usher syndrome . Some patients have survived to the fifth decade. Defects in PEX7, which most frequently lead to the RCDP phenotype, may also lead to a milder phenotype with clinical manifestations similar to those of adult Refsum disease.
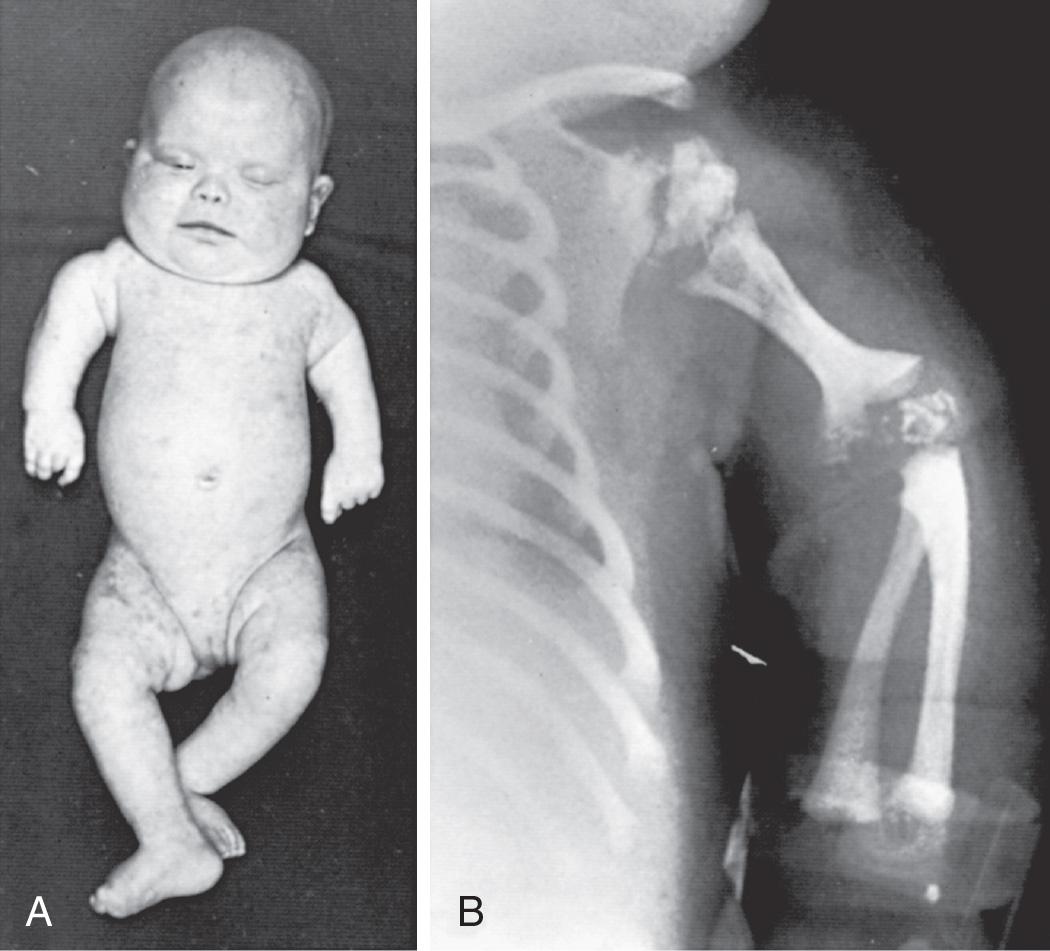
RCDP is characterized by the presence of stippled foci of calcification within the hyaline cartilage and is associated with dwarfing, cataracts (72%), and multiple malformations caused by contractures. Vertebral bodies have a coronal cleft filled by cartilage that is a result of an embryonic arrest. Disproportionate short stature affects the proximal parts of the extremities ( Fig. 104.4 A ). Radiologic abnormalities consist of shortening of the proximal limb bones, metaphyseal cupping, and disturbed ossification ( Fig. 104.4 B ). Height, weight, and head circumference are less than the 3rd percentile, and these children have a severe intellectual disability. Skin changes such as those observed in ichthyosiform erythroderma are present in approximately 25% of patients.
In the group of single-enzyme defects, acyl-CoA oxidase and bifunctional enzyme deficiency involve a single enzymatic step in peroxisomal fatty acid oxidation. Defects of bifunctional enzyme are common and are found in approximately 15% of patients who are initially suspected of having Zellweger spectrum disorder. Patients with isolated acyl-CoA oxidase deficiency have a somewhat milder phenotype that resembles, and comes to attention because of the development of, an early childhood leukodystrophy.
Plasmalogens are lipids in which the first carbon of glycerol is linked to an alcohol rather than a fatty acid. They are synthesized through a complex series of reactions, the first 2 steps of which are catalyzed by the peroxisomal enzymes dihydroxyacetone phosphate alkyl transferase (DHAPT) and synthase. Deficiency of either of these enzymes leads to a phenotype that is clinically indistinguishable from the peroxisomal import disorder RCDP. This latter disorder is caused by a defect in PEX7, the receptor for PTS2. RCDP shares the severe deficiency of plasmalogens with these single-enzyme disorders but also has defects of phytanic oxidation. The fact that these single genetic disorders are associated with the full phenotype of RCDP suggests that a deficiency of plasmalogens is sufficient to produce it.
The defective enzyme (phytanoyl-CoA hydroxylase) is localized to the peroxisome. The manifestation of Refsum disease includes impaired vision from retinitis pigmentosa, anosmia, ichthyosis, peripheral neuropathy, ataxia, and occasionally cardiac arrhythmias. In contrast to infantile Refsum disease, cognitive function is normal, and there are no congenital malformations. Refsum disease often does not manifest until young adulthood, but visual disturbances such as night blindness, ichthyosis, and peripheral neuropathy may already be present in childhood and adolescence. Early diagnosis is important because institution of a phytanic acid–restricted diet can reverse the peripheral neuropathy and prevent the progression of the visual and central nervous system (CNS) manifestations. The adult Refsum disease phenotype may also be caused by defects in PEX7.
This disorder is caused by an enzyme defect that leads to the accumulation of the branched-chain fatty acids (phytanic and pristanic acid) and bile acids. Individuals present with typically an adult-onset peripheral neuropathy and may also have pigmentary degeneration of the retina.
Diagnosis of a peroxisomal disorder often follows from a biochemical determination of an abnormality and then is confirmed through further genetic testing.
The biochemical characterization of peroxisomal disorders uses the generally available testing listed in Table 104.5 . Measurement of plasma VLCFA levels is the most common assay. It must be emphasized that although plasma VLCFA levels are elevated in many patients with peroxisomal disorders, this is not always the case. The most important exception is RCDP, in which VLCFA levels are normal, but plasma phytanic acid levels are increased and red blood cell (RBC) plasmalogen levels are reduced. In other peroxisomal disorders, the biochemical abnormalities are still more restricted. Therefore, a panel of tests is recommended and includes plasma levels of VLCFAs and phytanic, pristanic, and pipecolic acids and RBC levels of plasmalogens. Tandem mass spectrometry techniques also permit convenient quantitation of bile acids in plasma and urine. This panel of tests can be performed on very small amounts of venous blood and permits detection of most peroxisomal disorders. Furthermore, normal results make the presence of the typical peroxisomal disorder unlikely. Biochemical findings combined with the clinical presentation are often sufficient to arrive at a clinical diagnosis. Methods using dried blood spots of filter paper have been developed and are being incorporated into newborn screening assays.
| DISORDER | VLCFA | PHYTANIC ACID | PRISTANIC ACID | PLASMALOGENS |
|---|---|---|---|---|
| ZSD | ↑↑ | ↑ * | ↑ * | ↓ |
| RCDP | Nl | ↑ | Nl | ↓↓ |
| ALD | ↑ | Nl | Nl | Nl |
| ACoX | ↑ | Nl | Nl | Nl |
| Bifunctional enzyme deficiency | ↑ | ↑ | ↑ | Nl |
| AMACR | Nl | ↑ | ↑ | Nl |
| Refsum disease | Nl | ↑ | ↑ | Nl |
* Phytanic acid and pristanic acid accumulation is age dependent, and normal (Nl) levels may be seen in infants and young children.
The next step in diagnosis is generally to proceed to molecular DNA diagnosis, and many clinical laboratories provide a peroxisomal panel using next-generation technology. In some circumstances the diagnosis has been revealed through whole exome sequencing and the pathogenic nature of the alteration then confirmed through biochemical means.
Definition of the molecular defect in the proband is essential for carrier detection and speeds prenatal diagnosis . Characterization of the mutation may be of prognostic value in patients with PEX1 defects. This defect is present in approximately 60% of PBD patients, and about half the PEX1 defects have the G843D allele, which is associated with a significantly milder phenotype than found in other mutations.
Several noninvasive laboratory tests permit precise and early diagnosis of peroxisomal disorders (see Table 104.5 ). The challenge in PBDs is to differentiate them from the large variety of other conditions that can cause hypotonia, seizures, failure to thrive, or dysmorphic features. Experienced clinicians readily recognize classic Zellweger syndrome by its clinical manifestations. However, more mildly affected PBD patients often do not show the full clinical spectrum of disease and may be identifiable only by laboratory assays. Clinical features that warrant diagnostic assay include intellectual disability; weakness and hypotonia; dysmorphic features; neonatal seizures; retinopathy, glaucoma, or cataracts; hearing deficits; enlarged liver and impaired liver function; and chondrodysplasia punctata. The presence of 1 or more of these abnormalities increases the likelihood of this diagnosis. Atypical milder forms presenting as peripheral neuropathy have also been described.
Some patients with the isolated defects of peroxisomal fatty acid oxidation resemble those with Zellweger spectrum disorder and can be detected by the demonstration of abnormally high levels of VLCFAs.
Patients with RCDP must be distinguished from patients with other causes of chondrodysplasia punctata. RCDP is suspected clinically because of the shortness of limbs, developmental delays, and ichthyosis. The most decisive laboratory test is the demonstration of abnormally low plasmalogen levels in RBCs and an alteration in PEX7 .
Patients with Zellweger syndrome have multiple disabilities involving muscle tone, swallowing, cardiac abnormalities, liver disease, and seizures. These conditions are treated symptomatically, but the prognosis is poor, and most patients succumb in the 1st yr of life. Similarly, individuals with RCDP have multiple systemic and neurologic issues. In addition, they may develop quadriparesis from compression at the base of the brain.
The most effective therapy is the dietary treatment of adult Refsum disease with a phytanic acid–restricted diet. However, this only applies to this specific condition.
For patients with the somewhat milder variants of the peroxisome import disorders, success has been achieved with multidisciplinary early intervention, including physical and occupational therapy, hearing aids or cochlear implants, augmentative and alternative communication, nutrition, and support for the families. Although most patients continue to function in the impaired range, some make significant gains in self-help skills, and several are in stable condition in their teens or even early 20s.
Attempts to mitigate some of the secondary biochemical abnormalities include the oral administration of docosahexaenoic acid (DHA). DHA level is greatly reduced in patients with disorders of peroxisome biogenesis, and this therapy normalizes DHA plasma levels. Although there were anecdotal reports of clinical improvement with DHA therapy, a randomized placebo-controlled study failed to find benefit.
All the discussed peroxisomal disorders can be diagnosed prenatally. Prenatal testing using chorionic villi sampling or amniocentesis will usually rely on genetic testing when the alteration is known, but biochemical measurements may be made using the same tests as described for postnatal diagnosis (see Table 104.5 ). Because of the 25% recurrence risk, couples with an affected child should be advised about the availability of prenatal diagnosis.
ALD is an X-linked disorder associated with the accumulation of saturated VLCFAs and a progressive dysfunction of the adrenal cortex and nervous system. It is the most common peroxisomal disorder.
The key biochemical abnormality in ALD is the tissue accumulation of saturated VLCFAs, with a carbon chain length of 24 or more. Excess hexacosanoic acid (C 26:0 ) is the most striking and characteristic feature. This accumulation of fatty acids is caused by genetically deficient peroxisomal degradation of fatty acid. The defective gene (ABCD1) codes for a peroxisomal membrane protein (ALDP, the ALD protein). Many alterations in ABCD1 have been determined to be pathogenic, with over half these being private or unique to the kindred. A curated database of mutations is maintained ( www.x-ald.nl ). The mechanism by which the ALDP defect leads to VLCFA accumulation appears to be a disruption of transport of saturated fatty acids into the peroxisome, with resultant continued elongation of progressively longer fatty acids.
The minimum incidence of ALD in males is 1 in 21,000, and the combined incidence of ALD males and heterozygous females in the general population is estimated to be 1 in 17,000. All races are affected. The various phenotypes often occur in members of the same kindred. Increased implementation of newborn screening in the United States and other countries is expected to improve the accuracy of these incidence estimates.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here