Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
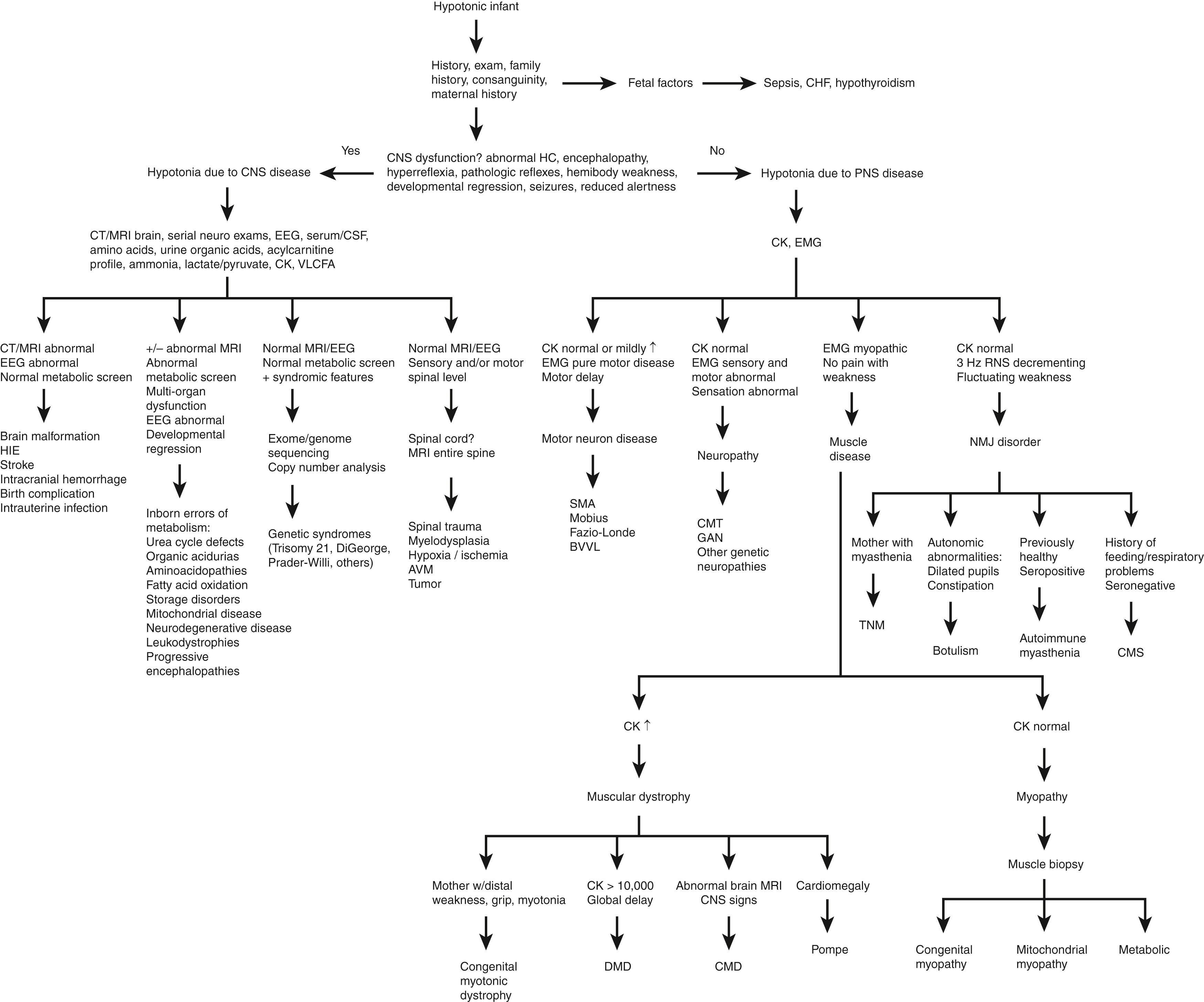
As a symptom, hypotonia may be overt in its presentation, with the manifestations of low muscle tone apparent to family and the medical team alike. Other times, hypotonia may be insidious, subtly presenting to an examiner during a medical evaluation and only rising to the awareness of family when delays or regressions in milestones are pointed out in the process of obtaining a history. Hypotonia is defined as decreased resistance to passive movement of a muscle through its range of motion, while weakness consists of decreased maximal force of active muscle contraction. Depending on the underlying cause, hypotonia may be associated with either weakness or normal muscle strength; similarly, muscle weakness may be associated with hypotonia, hypertonia, or no apparent change in muscle tone. Hypotonia affects children of all ages and may be congenital or acquired, acute or chronic, progressive or static, isolated or part of a complex clinical situation ( Table 35.1 ). An analytical approach to children with hypotonia requires historical information, clinical observations, and detailed general physical and comprehensive neurologic examinations to localize the source of hypotonia to a specific lesion ( Fig. 35.1 ). This neuraxial localization is then combined with laboratory, imaging, and genetic studies as indicated to arrive at a diagnosis ( Tables 35.2 and 35.3 ).
| Systemic | Connective Tissue | Cerebral | Spinal Cord | Anterior Horn Cell | Peripheral Nerve | Neuromuscular Junction | Muscle |
|---|---|---|---|---|---|---|---|
| Common | |||||||
| Sepsis Heart failure Acidosis Hypoxia Renal failure Hypoglycemia Trisomy 21 Prader-Willi syndrome Fragile X syndrome Hypothyroidism Other chromosomal disorders Maternal-fetal drug effects |
Stickler syndrome Marfan syndrome Achondroplasia |
Hypoxic-ischemic brain injury Intracranial hemorrhage Brain malformation ∗ Intrauterine infection Postnatal brain injury |
Myelodysplasia Spinal cord tumor Epidural abscess Transverse myelitis Acute flaccid myelitis Trauma (transection or compression) Syringomyelia |
Spinal muscular atrophy | Postinfectious polyneuropathy (Guillain-Barré syndrome) Toxic neuropathies (isoniazid, vincristine, platinum-based antineoplastic medications, nitrofurantoin) |
Botulism Infantile myasthenia Transient acquired neonatal myasthenia |
Duchenne muscular dystrophy Becker muscular dystrophy Myotonic dystrophy Dermatomyositis |
| Uncommon | |||||||
| Disorders of amino acid metabolism Urea cycle disorders Peroxisomal disorders Scurvy Rickets Sotos syndrome Angelman syndrome Rett syndrome Smith-Lemli-Opitz syndrome |
Ehlers-Danlos syndrome Osteogenesis imperfecta Velocardiofacial syndrome |
Progressive encephalopathies Mitochondrial disease |
Neonatal spinal cord transection Hypoxic-ischemic myelopathy Arteriovenous malformation |
Möbius syndrome | Chronic inflammatory demyelinating polyneuropathy Charcot-Marie-Tooth disease Hereditary sensory and autonomic neuropathies |
Toxic (organophosphate poisoning, aminoglycosides, magnesium) Postneuromuscular blocking agents (vecuronium) |
Pompe disease |
| Rare | |||||||
| Lowe syndrome Zellweger syndrome Neonatal adrenoleukodystrophy Mucolipidosis type IV Tay-Sachs disease Gangliosidosis Mannosidosis Infantile neuroaxonal dystrophy |
Miller-Dieker syndrome Congenital muscular dystrophy Metachromatic leukodystrophy Krabbe disease |
Poliomyelitis Incontinentia pigmenti Fazio-Londe disease Brown–Vialetto–Van Laere syndrome Juvenile amyotrophic lateral sclerosis |
Refsum disease Giant axonal neuropathy Metachromatic leukodystrophy Krabbe disease |
Congenital myasthenic syndromes | Other muscular dystrophies Congenital myopathies Metabolic myopathies Mitochondrial myopathies |
||
∗ Examples of brain malformations include agenesis of the corpus callosum, lissencephaly, Joubert syndrome, and Dandy-Walker malformations.
| Localization | Cause | History and Exam Findings | Investigation to Aid in Diagnosis |
|---|---|---|---|
| Brain | HIE Intracerebral hemorrhage |
Prematurity, difficult delivery | Brain MRI |
| Brain malformations | Cranial nerve abnormalities, Babinski sign, gradual development of hypertonia (especially axial), respiratory or feeding difficulties, global delay, micro-macrocephaly | Cerebral ultrasound Brain MRI |
|
| Intrauterine infection | Fever, altered mental status | Microbial cultures/evaluations, CSF evaluation | |
| Postnatal birth injury | Seizures, focal neurologic deficits | Brain MRI, EEG | |
| Progressive encephalopathies (leukodystrophies, progressive myoclonic epilepsies, Lennox-Gastaut syndrome, infantile spasms) | Seizures, developmental regression, ataxia, focal neurologic deficits, visual loss | Brain MRI, EEG, EMG/NCS (useful in adrenoleukodystrophy, Krabbe disease, and metachromatic leukodystrophy), specific genetic testing | |
| Mitochondrial disease | Seizures, focal neurologic deficits, global delay, visual loss, hyper- or hyporeflexia | Brain MRI, lactate, pyruvate, creatine kinase, GDF-15, muscle biopsy, mitochondrial DNA sequencing and deletion/duplication analysis on muscle or affected tissue, EMG/NCS | |
| Brainstem | Joubert syndrome Pontocerebellar hypoplasia Cobblestone malformations |
Multiple cranial nerve abnormalities, breathing and feeding difficulties, possible intellectual delay, nystagmus, possible hyperreflexia, ataxia | Brain MRI (molar tooth sign in Joubert syndrome), genetic panels for specific disorders |
| Spinal cord | Myelodysplasia Spinal cord tumor Syringomyelia Hypoxia-ischemia Trauma AVM |
Spinal level on exam, weakness below a defined spinal level, absent reflexes (acutely) or hyperreflexia (chronically) below the level, may have Babinski sign, history of trauma | Brain MRI, complete spinal MRI |
| Motor neuron | Spinal muscular atrophy | Absence of antigravity movements, tongue fasciculations, absent reflexes to hyporeflexia, normal cognition, breathing/feeding difficulties; weakness in legs more than arms in SMA types II–III | SMN1 genetic analysis |
| Poliomyelitis | Neck stiffness, muscle spasms, areflexia, asymmetric flaccid paralysis of a limb, respiratory distress, muscle atrophy, normal sensation | Isolation of poliovirus from stool, confirmation using RT-PCR, acute and convalescent serology showing fourfold increase in titer, EMG/NCS showing pure motor neuronopathy | |
| Incontinentia pigmenti | Skin blistering, verrucous skin lesions, hyperpigmented streaks, pale/hairless atrophic linear streaks that respect Blaschko lines, dental abnormalities, intellectual delay | DNA analysis, EMG/NCS showing pure motor neuronopathy | |
| Fazio-Londe disease Brown–Vialetto–Van Laere syndrome (BVVL) |
Optic atrophy, nystagmus, bulbar palsy, facial weakness, hearing loss (BVVL only), tongue fasciculations, ptosis, respiratory compromise, muscle weakness | DNA analysis | |
| Nerve | Guillain-Barré syndrome (GBS) Chronic inflammatory demyelinating polyneuropathy (CIDP) |
Sensory ataxia with walking difficulties, rapidly (GBS) or slowly (CIDP) progressive weakness, absent reflexes or hyporeflexia, autonomic dysfunction, antecedent gastrointestinal or respiratory illness in GBS | EMG/NCS with absent or prolonged F-waves, prolonged distal latencies, conduction block, demyelinating nerve conduction velocities, CSF showing cytoalbuminologic dissociation, MRI with edematous enhancing nerve roots |
| Toxic neuropathies | History and temporal correlation with exposure to a neurotoxic drug, distal then proximal muscle weakness, absent reflexes or hyporeflexia, sensory ataxia with walking difficulties | EMG/NCS showing mixed axonal/demyelinating features, plasma drug levels | |
| Charcot-Marie-Tooth disease | Family history of similar disease, pes cavus and hammer toe foot deformities, ataxic gait, foot drop, absent reflexes or hyporeflexia | EMG/NCS to determine if axonal or demyelinating subtypes, DNA analysis | |
| Hereditary sensory and autonomic neuropathies | Sensory loss in a stocking/glove distribution, chronic skin ulceration and poor wound healing, distal muscle weakness with foot deformity, absent reflexes or hyporeflexia, variable anhidrosis | EMG/NCS showing normal or mildly abnormal motor responses and abnormal sensory responses, nerve biopsy showing reduced myelinated and unmyelinated fibers, DNA analysis | |
| Nerve—cont’d | Refsum disease | Autosomal recessive inheritance, stocking/glove distribution of sensory and motor weakness, anosmia, hearing loss, ataxia, ichthyosis, short metacarpals and metatarsals, cardiac arrhythmia, and cardiomyopathy | Elevated plasma phytanic acid concentration, DNA analysis |
| Giant axonal neuropathy | Stocking/glove distribution of sensory loss and motor weakness, cerebellar ataxia, absent reflexes or hyporeflexia, kinky hair (tightly curled), nystagmus, dysarthria, pyramidal tract signs, optic neuropathy, seizures | Brain MRI with white matter abnormalities, axonal sensorimotor polyneuropathy on EMG/NCS, nerve biopsy showing giant axons (axonal swelling) and disorganized neurofilaments, DNA analysis | |
| Metachromatic leukodystrophy Krabbe disease Adrenoleukodystrophy |
Developmental regression, absent reflexes or hyporeflexia, Babinski signs | EMG/NCS showing demyelinating neuropathy, brain MRI showing white matter disease, DNA analysis | |
| Neuromuscular junction | Botulism | Sudden poor feeding, constipation, weak cry, gradual muscle weakness, dilated poorly reactive pupils, exposure to soil/dust with bacterium or honey consumption | Presence of toxin in stool/serum, culture bacterium from stool, EMG/NCS showing low-amplitude motor responses or decrement on repetitive nerve stimulation in a weak muscle |
| Transient acquired neonatal myasthenia | Ptosis, feeding and respiratory difficulties, aspiration, mother with signs or symptoms of autoimmune myasthenia | Maternal history of myasthenia, EMG/NCS showing decrement on repetitive nerve stimulation in a weak muscle, good response to acetylcholinesterase inhibitors | |
| Infantile (autoimmune) myasthenia | Ptosis, episodic weakness, recurrent feeding and respiratory difficulties, easy fatigability | EMG/NCS showing decrement on repetitive nerve stimulation in a weak muscle, good response to acetylcholinesterase inhibitors, antiacetylcholine receptor antibody serology | |
| Congenital myasthenic syndrome | Ptosis, episodic weakness, recurrent feeding and respiratory difficulties, easy fatigability | EMG/NCS showing decrement on repetitive nerve stimulation in a weak muscle, DNA analysis, negative antiacetylcholine receptor antibody serology | |
| Muscle | Duchenne/Becker muscular dystrophy | X-linked pattern of inheritance, enlarged calves, proximal muscle weakness with a Gower maneuver | Markedly elevated CK, DNA analysis |
| Congenital myotonic dystrophy | Autosomal dominant pattern of inheritance, frog-leg position, open down-turned mouth, minimal antigravity movements in infants, distal > proximal weakness in children, impaired relaxation of grip, dysarthria, myopathic facies with temporal wasting | Test mother (then father) for clinical myotonia or electrical myotonic discharges, EMG/NCS with myopathy in newborn period and myotonic discharges in older children, normal to mildly elevated CK, DPMK gene CTG repeat analysis | |
| Pompe disease | Absence of antigravity movements, severe cardiomegaly, feeding/respiratory difficulties, hepatomegaly | GAA enzyme activity in dried blood spot, lymphocytes or fibroblasts, GAA gene analysis | |
| Congenital muscular dystrophy | Family history, proximal > distal muscle weakness, feeding and respiratory difficulties, early-onset contractures in specific subtypes, keloids/hyperkeratosis pilaris in specific subtypes, CNS dysfunction in specific subtypes | Brain MRI, mild to markedly elevated CK, muscle biopsy showing dystrophic changes, muscle MRI, EMG/NCS to assess for demyelinating neuropathy component and myopathy, DNA analysis | |
| Congenital myopathies | Family history, proximal > distal muscle weakness, feeding and respiratory difficulties, ptosis and ophthalmoparesis in specific subtypes | Normal to mildly elevated CK, muscle biopsy showing specific changes (nemaline rods, cores, centrally placed nuclei), DNA analysis | |
| Metabolic myopathies | Family history, proximal muscle weakness, history of rhabdomyolysis or myoglobinuria, second-wind phenomenon in some subtypes | Normal to markedly elevated CK, EMG/NCS usually myopathic, metabolic evaluation (lactate, pyruvate, acylcarnitine profile, plasma amino acids, urine organic acids), muscle biopsy, DNA analysis | |
| Mitochondrial myopathies | Maternal inheritance pattern, proximal > distal weakness, ptosis, ophthalmoparesis, short stature, variable cardiac and CNS involvement, recurrent rhabdomyolysis | Normal to moderately elevated CK, abnormal GDF-15, EMG/NCS showing myopathy and variable neuropathy, muscle biopsy with ragged red fibers, mitochondrial DNA analysis |
| Upper Motor Unit | Lower Motor Unit | |||||
|---|---|---|---|---|---|---|
| Brain | Spinal Cord | Alpha Motor Neuron ∗ | Peripheral Nerve | Neuromuscular Junction | Muscle | |
| Level of consciousness | ↓ | Normal | Normal | Normal | Normal | Normal |
| Strength | Mild to moderate ↓ | Mild to moderate ↓ | Marked ↓ | Marked ↓ | Marked ↓ | Marked ↓ |
| Tone | Spastic (hypotonia at onset possible) | ↓ Acutely; ↑ | ↓, flaccid | ↓ | ↓ | ↓ |
| Deep tendon reflexes | Normal to ↑ | ↓ Acutely; ↑ | ↓ to absent | ↓ to absent (lost early) | Normal | Normal to ↓ to absent |
| Babinski | Present | Present usually | Absent | Absent | Absent | Absent |
| Fasciculations | Absent | Absent | Present | Rarely | Absent | Absent |
| Atrophy | Mild to moderate | Mild to moderate | Present | Present | Absent | Present pseudohypertrophy |
| Sensation | Normal | Absent below level of lesion | Normal | Abnormal in defined peripheral nerve distribution or glove/stocking | Normal | Normal |
| Creatine kinase level | Normal | Normal | Normal to moderately elevated (several 1,000s IU/L) | Normal or mildly elevated (100s IU/L) | Normal | Normal to severely elevated |
| Overall pattern | Hemibody deficits | Spinal level present | Proximal weakness in SMA; asymmetric weakness in other diseases | Distal, length-dependent usually, defined nerve territory | Symmetric, painless weakness of tonically active muscles | Proximal > distal weakness |
| Other | Seizures Developmental delay Regression Cortical signs (e.g., language) |
Radicular back pain, bowel/bladder dysfunction | Fluctuating diurnal variation Fatigability |
Myalgia, Gower sign | ||
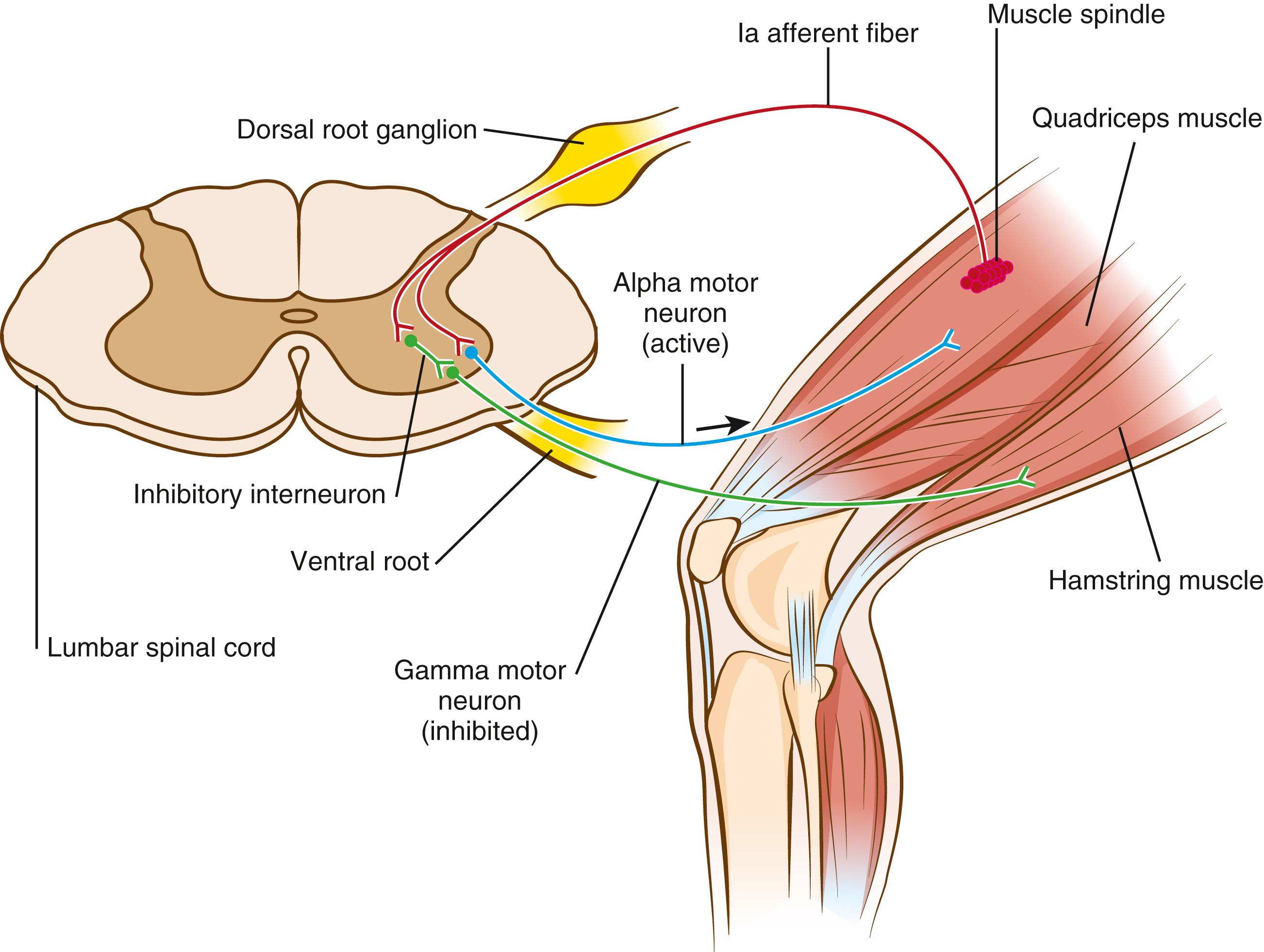
The generation of normal muscle tone requires the integrity of the entire central and peripheral nervous systems, from the cerebral cortex to cortical white matter pathways, basal ganglia, cerebellum, brainstem, spinal cord, peripheral nerve, neuromuscular junction (NMJ), and muscle. Diseases that affect the function of the nervous system at any of these levels may result in abnormal muscle tone (see Tables 35.2 and 35.3 ). The primary structure responsible for regulating muscle tone is the muscle spindle , a sensory apparatus within the muscle that detects stretching of muscle fibers and that, in response, sends impulses to the spinal cord via sensory afferent pathways. These sensory afferent fibers bifurcate in the spinal cord, with one branch synapsing directly onto anterior horn α motor neurons, producing contraction in the agonist muscle that underwent stretching, while the other branch synapses onto an inhibitory interneuron, inactivating the antagonist muscle. When engaged by stretching of the muscle, this pathway is referred to as the stretch reflex , which works to oppose changes in muscle length ( Fig. 35.2 ). Additional inputs to the muscle include excitatory input traveling via α motor neurons that end at the NMJ to produce voluntary contraction, as well as γ motor neurons that end at the muscle spindle, providing inhibitory input to the muscle spindle, setting the level of resting muscle tone. This lower motor neuron pathway of α motor neurons and γ motor neurons is closely monitored and influenced by descending central pathways from the cerebral cortex, basal ganglia, brainstem, and cerebellum. These descending pathways constitute the upper motor neuron pathways that influence resting muscle tone.
Associated symptoms and physical findings in hypotonia vary depending on whether the hypotonia is caused by lesions of the central nervous system (CNS), as opposed to the peripheral nervous system (PNS) ( Table 35.4 ). An estimated 80–90% of infantile hypotonia is central in origin, with the remaining 10–20% being peripheral. Most cases of acute lateralized body weakness result from abnormalities of the blood supply to a portion of the CNS. Stroke serves as a term to denote the sudden onset of symptoms attributable to such an interruption of cerebral or spinal perfusion and is discussed in Chapter 37 .
| Finding | Central | Peripheral |
|---|---|---|
| Seizures | Present | Absent |
| Altered mental status | Present | Absent |
| Delayed cognitive milestones | Present | Absent |
| Deep tendon reflexes | Normal or increased | Absent or decreased usually |
| Babinski sign | Present | Absent |
| Infantile reflexes | Persistent | Not persistent |
| Pull-to-sit | Minor head lag | Marked head lag |
| Tongue fasciculations without other cranial nerve deficits | Unlikely | Very likely |
| Ophthalmoparesis | Present in brainstem disease | Present in some myopathic diseases |
| Ptosis | Present in some brainstem diseases | Present in some myopathic and neuromuscular junction diseases |
| Weakness | Mild to moderate | Severe |
| Antigravity movements | Present | Absent usually |
| Arthrogryposis | Less common | More common |
| Muscle atrophy | None to mild | Moderate to severe |
The systematic evaluation of muscle tone consists first of making the following clinical observations:
Characterization of spontaneous posture
Response to postural changes
Extent of joint mobility
Response to flapping of distal extremities
Muscle tone is divided into postural and phasic. Postural tone is the steady contraction of muscles in uniform resistance to passive movement. Antigravity resistance is an example of postural tone. Phasic tone is the catch experienced when an extremity is rapidly flexed or extended across a joint. The method of evaluating muscle tone and strength depends on the age of the patient.
In an infant, historical information must include a complete obstetric history, including the amount and quality of fetal movements, amniotic fluid volume, and intrauterine growth restriction, as well as accurate data about perinatal events, diet, toxic exposures, and family history. The muscle strength, passive tone, joint extensibility, and postural reflexes, including responses to traction, axillary suspension, and ventral suspension of the hypotonic infant, should be compared to those of the normal infant ( Fig. 35.3 ).
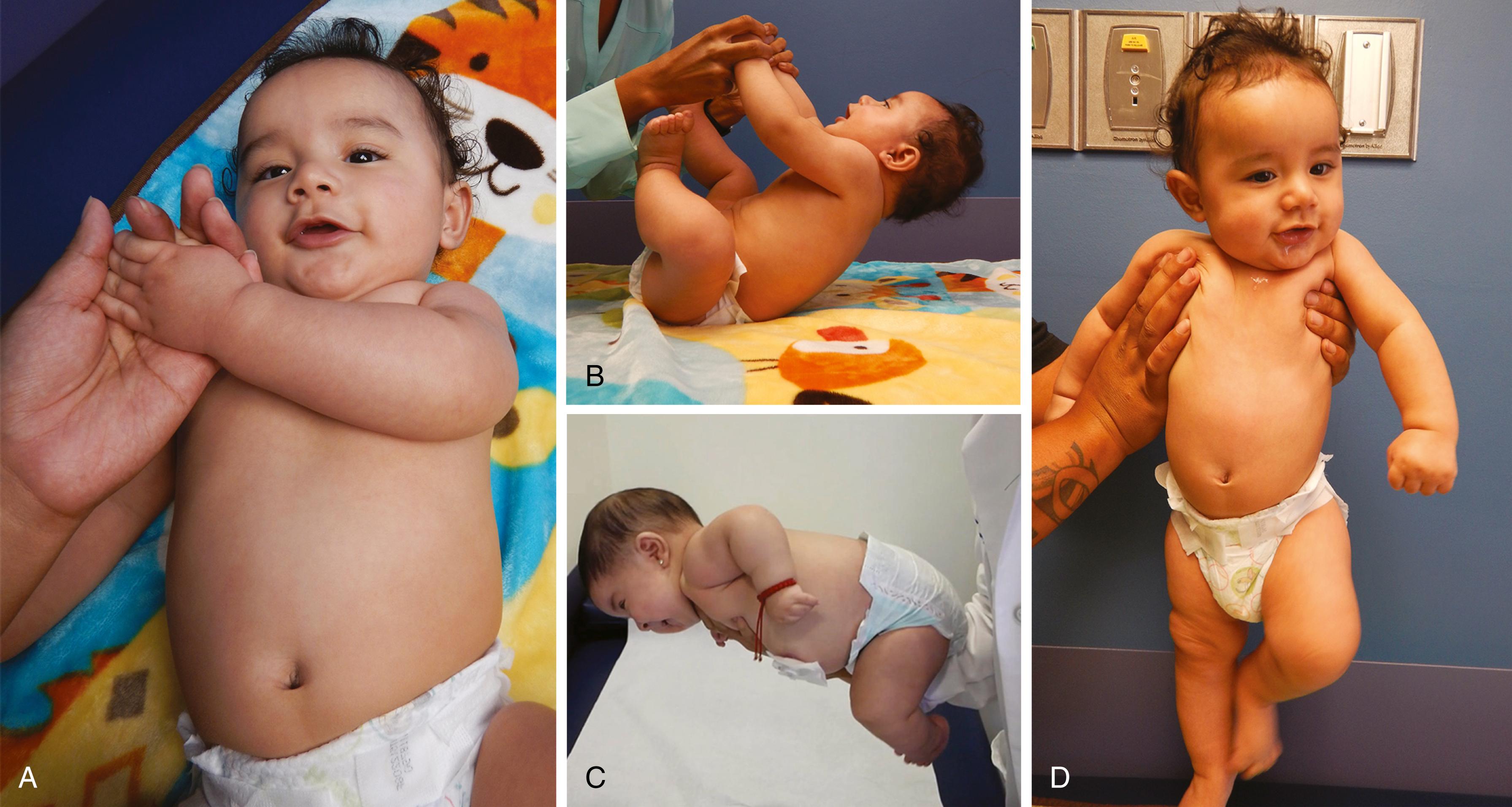
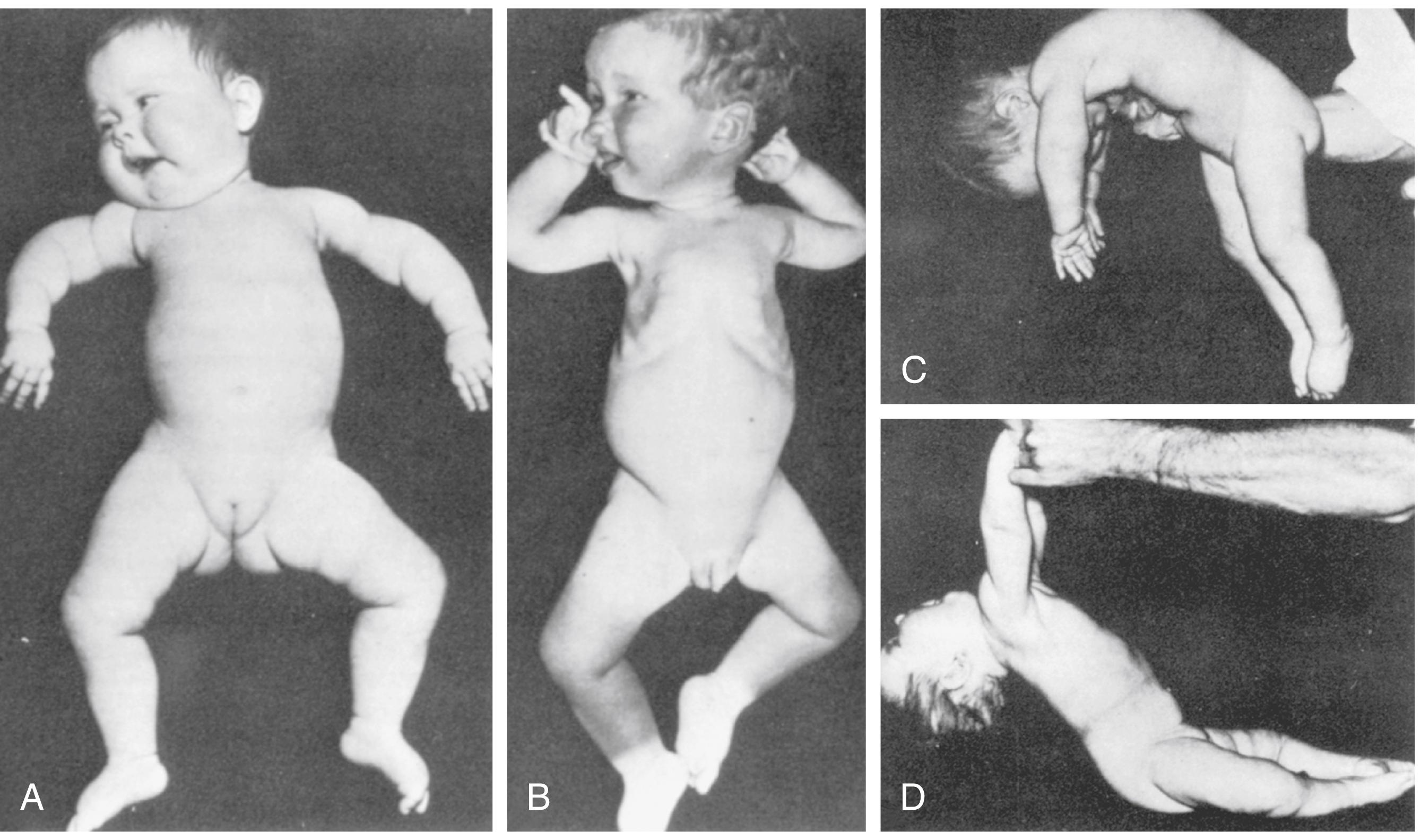
Muscle strength ( Table 35.5 ) cannot be measured directly in infants, but numerous clinical clues allow the careful observer to identify weakness. The most important of these clues is the spontaneous posture. The weak infant has diminished or no spontaneous movement, often in striking contrast to the usual vigorous and plentiful movements of the infant with normal strength. The lower extremities are abducted and the lateral surfaces of the thighs lie against the examination table in a classic “frog leg” position, whereas the upper extremities lie extended alongside the body or flexed in a flaccid position beside the head ( Figs. 35.4 and 35.5A ). With marked weakness, there are no movements that overcome the pull of gravity. The immobility of the weak infant results in flattening of the occipital bone, which is often associated with occipital hair loss. When placed in a sitting posture, the infant droops forward, the shoulders sink, the head falls forward, and the arms hang limply.
|
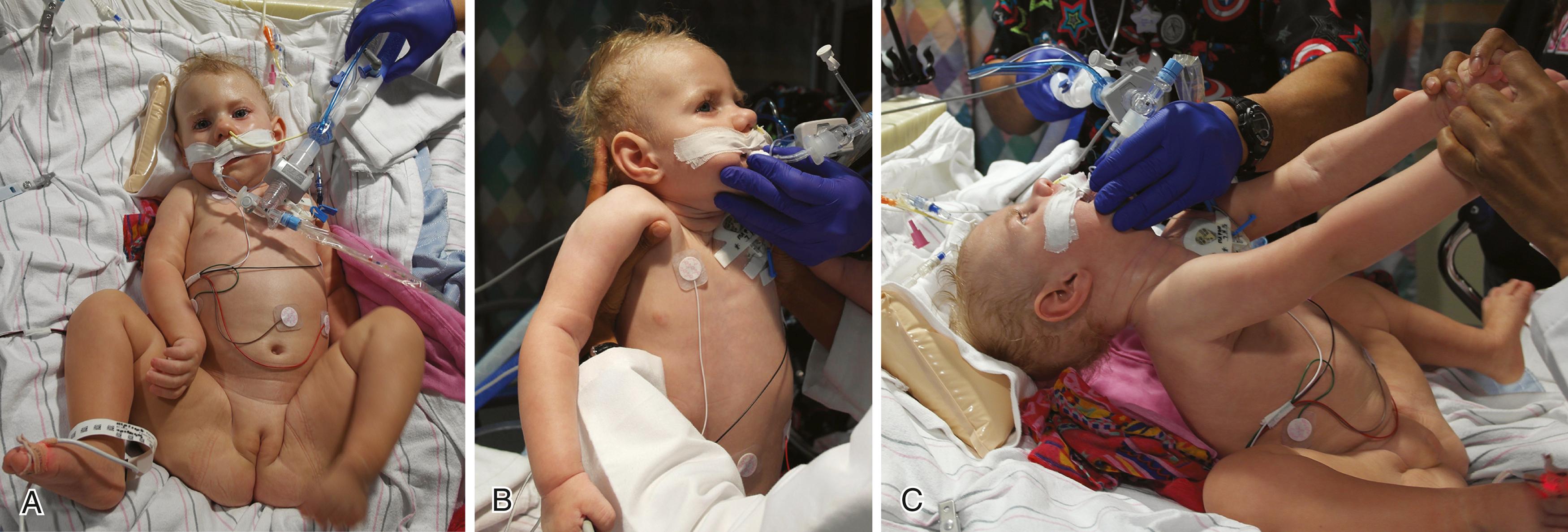
Passive tone can be assessed by evaluating the resistance to movement of the limbs through their range of motion. Evaluation of the shoulders, elbows, wrists, hips, knees, and ankles is especially helpful. In hypotonic infants, the examiner senses a looseness of the limbs as the limbs are moved. In addition, grasping the midportion of the infant’s limb and passively flapping the extremity allows the examiner to evaluate the degree of limpness of the distal extremity. In the hypotonic infant, the hands and feet wave limply; in the normal infant, the ankle and wrist are maintained fairly rigidly in line with the rest of the extremity.
Even in normal infants, there is a wide variation of muscle tone. Passive muscle tone varies and is particularly diminished after feeding and before sleep. There is profound hypotonia in all infants during sleep. Premature infants, even those without neurologic injury, have diminished tone relative to term infants, due to incomplete myelination of corticospinal and subcorticospinal tracts. Tone can also be affected by the position of the head. The infant whose head is turned to one side may manifest an asymmetric tonic neck response , with increased extensor tone on the side of the body to which the head is turned and increased flexor tone on the contralateral side, resulting in a “fencer’s posture” ( Fig. 35.6 ). This asymmetry of tone may be elicited even in the child who does not exhibit the typical fencer’s posture. Therefore, examination of an infant should always be conducted while the infant’s head is at the midline; the same is true for eliciting muscle stretch reflexes. Hypotonia can also be a secondary finding in a variety of systemic conditions, such as heart failure, sepsis, acidosis, or failure to thrive (see Table 35.1 ).
The extent to which the joints may be extensible provides an indirect clue to the presence of hypotonia. Examination of mobility at the elbows, wrists, hips, and knees is helpful. The hypotonic infant may assume unusual postures in the presence of joint hyperextensibility. The scarf sign is a useful sign of hyperextensibility in the young infant. With the infant in a semireclining position, the hand is pulled across the chest toward the opposite shoulder and the position of the elbow is noted (see Fig. 35.3A ). If the elbow passes the midline, there is hypotonia.
The traction response is the most useful and most sensitive of the postural reflexes in infants. With the infant lying supine, the infant’s hands are grasped, and the infant is pulled up to a sitting position. Once the sitting posture is attained, the head is held erect in the midline. During the maneuver, the examiner notes the infant’s attempt to counter the traction by flexion of the arms (see Fig. 35.3B ).
In an infant younger than 3 months, the plantar grasp should also be evident when grasping the infant’s hands to assess the traction response. In addition, there should be flexion at the elbow, knee, and ankle in response to the maneuver. The degree to which the head and neck pull up along with the trunk depends on the child’s age.
In infants younger than 33 weeks’ gestation, there is no traction response. From 33 weeks to term, the infant has head lag but responds to the traction maneuver by flexing the neck flexors in an attempt to lift the head. The full-term infant exhibits a traction response with minimal head lag, and when the sitting posture is attained, the head may be held erect momentarily and then falls forward.
By age 3 months, there should be no head lag, and the head should be aligned with the plane of the back as the child is pulled to sitting. The absence of flexion of the limbs in response to the examiner’s pull and the presence of head lag inappropriate for age suggest hypotonia (see Figs. 35.4D and 35.5C ).
The response to axillary suspension allows assessment of generalized and shoulder girdle tone. The infant is held under the arms, lifted, and suspended from the axillae without the thorax being grasped. In infants with normal tone and strength, the shoulder girdle muscles exert enough strength to allow the infant to be suspended without slipping through the examiner’s grasp. In addition, the infant’s head is held midline and the legs are held with some flexion at the hips, knees, and ankles (see Fig. 35.3D ). The hypotonic infant droops with legs extended and head falling forward, and the absence of resistance of the muscles of the shoulder girdle allows the infant to slip through the grasp of the examiner as the baby’s arms fling upward (see Fig. 35.5B ).
The response to ventral suspension allows assessment of tone of the trunk, neck, and extremities. The examiner holds the infant, who is lying prone. The infant is supported only by the examiner’s hand on the abdomen. A normal infant holds the head erect and the back straight and holds the extremities with some flexion at the elbows, hips, knees, and ankles (see Fig. 35.3C ). A full-term neonate makes intermittent attempts to hold the head straight, maintains the back straight, and can flex the limbs. The hypotonic infant droops in the examiner’s palm, as if in the shape of an inverted “U,” with the head and legs dangling limply (see Fig. 35.4C ).
Observation of the child’s spontaneous posture may suggest the presence of weakness. Muscle strength can be observed as the child performs functional tasks, including moving from a supine to a seated position, arising to stand from a sitting or lying position, standing on one leg independently, hopping, walking, running, and climbing stairs. The wheelbarrow maneuver , in which the pelvis and lower extremities are supported by the examiner while the child propels themselves forward on the floor using only the arms, can be used to functionally assess strength in the upper extremities. In the child older than 5 years, manual muscle testing can be performed if the child is cooperative (see Table 35.5 ). The examiner evaluates each muscle group independently, comparing the child’s muscle strength in resistance to the examiner’s strength. Although a child’s strength is not expected to be equal to an adult, the child is given full strength scores if the degree of strength exhibited is substantial for age. The child with muscle weakness has difficulty performing motor tasks and may exhibit unusual postures (e.g., lordosis) or toe walking, and on manual muscle testing may be easily overcome by the examiner’s strength.
Passive muscle tone is more consistent during the waking hours in the child than in the infant. The major joints should be moved through their range of motion and the extent of resistance noted. Flapping the distal extremities provides a useful clue. Briskly lifting the lower extremity at the knee while the patient lies supine is another useful test of muscle tone. In the normal child, the foot briefly drags along the examination table and then rises with the leg. In the hypertonic child, the leg remains extended stiffly at the knee. In the hypotonic child, the lower leg hangs limply and the foot drags as the knee is raised.
The hypotonic child demonstrates hyperextensibility of joints, especially at the elbows, wrists, knees, and ankles. Examination of the small muscles of the fingers across distal joints may also be helpful ( Fig. 35.7 ).

The diagnosis of a particular neurologic disorder depends on the location of the lesion (i.e., which part of the nervous system is impaired or abnormal), the patient’s age, and whether the condition is progressive or static (see Tables 35.1 to 35.3 ).
A careful perinatal history is obtained to identify possible features suggestive of perinatal hypoxic-ischemic brain injury . The infant who has neurologic dysfunction attributable to perinatal asphyxia typically has a history of an acute encephalopathy during the neonatal period (e.g., disturbance of consciousness, poor feeding, seizures, autonomic dysfunction).
A CT study or MRI of the head is helpful to identify evidence of brain malformation, intrauterine infection, hypoxic brain injury, intracranial hemorrhage, or hydrocephalus. If the history suggests seizures, an EEG should be obtained.
An ophthalmologic evaluation may detect evidence of ocular malformation (cataracts, microphthalmia, optic hypoplasia), evidence of intrauterine infection (chorioretinitis), or retinal/macular abnormality (retinitis pigmentosa, cherry-red spot) (see Chapter 43 ).
In some cases, requesting a hearing evaluation or brainstem auditory evoked response may be appropriate. A lumbar puncture is necessary if acute or chronic (intrauterine) meningitis is suspected.
Fig. 35.1 summarizes the diagnostic approach to the hypotonic infant. After a thorough history and detailed general and neurologic examinations, the first priority is to determine if there are signs and symptoms of CNS dysfunction because the majority of hypotonia in infants is central in origin (see Table 35.4 ). Extraneural involvement such as the presence of multiorgan dysfunction, bone marrow suppression, organomegaly, or heart failure accompanying hypotonia is highly suggestive of a systemic disease, such as a storage disorder or an inborn error of metabolism. Particular attention should be given to family history, perinatal maternal illnesses and exposures, delivery complications, and the possibility of consanguinity. Signs and symptoms of CNS involvement in an infant include abnormal head circumference (either micro- or macrocephaly), reduced levels of alertness (i.e., encephalopathy), seizures, respiratory and feeding difficulties, hypotonia and later hypertonia, hemibody weakness, hyperreflexia, developmental cognitive regression, and pathologic reflexes such as persistence of the Babinski sign in an older infant or child. The hypotonic infant’s exam findings may change with age, even on the order of weeks to months. Many hypotonic neonates initially thought to have PNS dysfunction eventually develop hypertonia, hyperreflexia, and pathologic Babinski signs over time, indicative of CNS dysfunction; therefore, serial thorough neurologic exams are essential until confirmatory testing yields a firm diagnosis or until a clinical diagnosis is made with reasonable confidence. An MRI of the head is obtained to detect any anatomic abnormalities or other causative lesions. If CNS dysfunction is present, initial recommended testing includes EEG (which may show seizures or cortical slowing) and a complete metabolic screen—serum amino acids (aminoacidopathies, urea cycle disorders, some organic acidurias), urine organic acids (organic acidurias), ammonia (urea cycle disorders, organic acidurias), acylcarnitine profile (organic acidurias, fatty acid oxidation disorders, riboflavin transporter defect), serum and cerebrospinal fluid (CSF) lactate and pyruvate (mitochondrial disease), serum growth differentiation factor-15 (GDF-15) levels (mitochondrial disease), very long-chain fatty acids (peroxisomal disorders), and lysosomal enzyme panel (lysosomal storage disease), since some conditions are treatable and prompt initiation of therapy may halt or slow progression of disease.
An abnormal brain MRI and EEG with a normal metabolic screen suggests a possible static cause such as brain malformation, hypoxic-ischemic encephalopathy (HIE)/stroke, maternal or fetal infection, or a birth complication. Developmental regression is highly suggestive of inborn errors of metabolism, storage disorders, mitochondrial diseases, or neurodegenerative diseases (see Table 35.1 under “systemic” and “cerebral” for specific disorders) (see Chapter 28 ). Regression coupled with an abnormal metabolic screen may suggest urea cycle defects, organic acidurias, aminoacidopathies, fatty acid oxidation defects, lysosomal and peroxisomal disorders, mitochondrial disease, or certain neurodegenerative disorders and should prompt immediate referral to a metabolic specialist for rapid diagnosis and possible treatment. Many of the treatable metabolic disorders are diagnosed early in the United States via newborn screening programs.
In the setting of persistent hypotonia with normal MRI, EEG, and metabolic screening, syndromic genetic causes should be considered. Common congenital considerations such as Prader-Willi syndrome, spinal muscular atrophy, and myotonic dystrophy type 1 may lack other overt phenotypic features beyond reduced tone and require specific genetic testing to detect the underlying pathogenic genetic abnormalities, which may not be detected on exome or genome sequencing. In the case of Prader-Willi syndrome, DNA methylation testing detects >99% of affected individuals, whereas sequence analysis detects <1%. For patients not identified prenatally or on newborn screening, deletion/duplication analysis of the SMN1 gene detects >95% of patients with spinal muscular atrophy, whereas sequence analysis typically detects <5%. Targeted analysis of DMPK is required to identify pathologic expansion of a CTG trinucleotide repeat leading to myotonic dystrophy type 1. If these targeted analyses are negative in patients with a high suspicion of a genetic disorder, exome sequencing with copy number variant analysis should be pursued to detect other genetic etiologies of hypotonia. Detailed phenotyping is essential in interpreting the results of genetic sequencing. Some syndromes, such as trisomy 21 or 22q11.2 deletion syndrome, have characteristic features that may allow for presumptive clinical diagnosis while awaiting confirmatory genetic testing. For other disorders, dysmorphic facial features to note in infancy include low-set ears, large/small ears, hyper- or hypotelorism, epicanthal folds, asymmetric crying facies, short or webbed neck, cleft lip/palate, and maxillary or mandibular hypoplasia (see Chapter 29 ). Body dysmorphisms in infancy include abnormal limb length, palmar crease, club feet, polydactyly/syndactyly, genitourinary abnormalities, spinal abnormalities, and ophthalmologic abnormalities. These phenotypic findings may provide additional context for the interpretation of sequence or copy number variants of uncertain significance.
If a careful neurologic exam demonstrates a sensory or motor level, such as strong arms and flaccid legs with a pathologic Babinski sign and lower extremity hyperreflexia, then spinal cord pathology is suspected. MRI of the entire spine (with and without gadolinium) typically captures anatomic abnormalities as well as intra- and extramedullary spinal abnormalities.
If hypotonia persists and MRI of the brain and spine, EEG, metabolic screening, and preliminary genetic syndrome screens are negative, then motor neuron, nerve, muscle, or NMJ diseases should be considered (see Tables 35.1 and 35.3 ).
Profound and persistent hypotonia in the setting of normal intellectual function is the typical pattern for the majority of PNS diseases, and the physical examination does not tend to evolve as much during infancy as it does in CNS disease. In addition, hyporeflexia and absence of a pathologic Babinski sign are the norm with a few exceptions, such as in congenital muscular dystrophy. The most useful initial tests for these patients are a creatine kinase (CK), thyroid-stimulating hormone (TSH), and electromyography with nerve conduction study (EMG/NCS, or simply EMG). In motor neuron disease, CK is typically normal but occasionally might be slightly elevated, while the EMG shows a pure motor neuropathy (see Table 35.2 ). When obtaining EMG, at least one sensory and motor nerve conduction assessment should be done in an upper and lower extremity and needle EMG should be performed on at least one proximal and one distal muscle. If ptosis, ophthalmoparesis, episodic weakness, or feeding/breathing difficulties are present clinically, then doing a 3-Hz repetitive nerve stimulation (RNS) on an affected muscle may be informative to assess for fatigability suggestive of NMJ disorders. Needle EMG in newborns can be difficult to interpret; waiting for the child to be at least 2 months old might yield more meaningful results. In the meantime, other avenues of testing, such as muscle biopsy and genetic testing, should be pursued as appropriate.
Nerve disorders demonstrate involvement of both sensory and motor nerves either in an axonal or demyelinating pattern on EMG; CK is normal. Muscle diseases have a myopathic EMG, typically with normal sensory nerve conductions. An elevated CK is a hallmark of muscular dystrophies due to the continual degeneration and regeneration of muscle fibers, whereas myopathies typically have a normal CK. Duchenne muscular dystrophy is the prototypical muscular dystrophy with a markedly elevated CK >10,000 U/L in an infant. A parental history of distal weakness with grip myotonia suggests congenital myotonic dystrophy . The congenital muscular dystrophies have variable brain MRI findings ranging from normal to lissencephaly, with clinical findings of intellectual delay and seizures. Pompe disease is characterized by concomitant cardiomegaly, feeding difficulties, and failure to thrive. When EMG suggests a myopathy and the CK is normal, then a muscle biopsy can help distinguish between congenital myopathy, mitochondrial disorders, and metabolic myopathies.
If the 3-Hz RNS shows significant decrement and CK is normal, then an NMJ disorder is likely. Clinically, NMJ disorders appear similar to each other with fluctuating ptosis, ophthalmoparesis, feeding and respiratory difficulties, limb and trunk weakness, and poor head control, but key differences help narrow the differential diagnosis. If the mother is known to have autoimmune myasthenia, then a hypotonic infant may have transplacental-derived transient neonatal myasthenia. If the hypotonic infant has autonomic dysfunction such as dilated pupils or constipation, then botulism must be considered. If the infant was previously healthy and then rapidly developed symptoms that localize to the NMJ, autoimmune myasthenia is possible, and antibodies should be tested. A child with a history of persistent feeding and respiratory difficulties since birth, worsened by illness or suddenly without an identified cause, should prompt evaluation for congenital myasthenic syndromes, which are a heterogeneous group of genetic disorders.
Arthrogryposis multiplex congenita , the finding of multiple joint contractures affecting two or more body areas and present at birth, can be caused by lesions anywhere in the neural axis including the CNS and PNS, connective tissue and joint disorders, and maternal and fetal factors ( Table 35.6 ). In an infant with arthrogryposis, determining if the cause is central or peripheral or neither is often crucial to making a diagnosis.
| Site of Major Pathologic Findings | Disorder |
|---|---|
| Cerebrum, brainstem, cerebellum |
|
| Spinal cord |
|
| Motor neuron |
|
| Peripheral nerve |
|
| Neuromuscular junction |
|
| Muscle |
|
| Intrauterine/maternal factors |
|
| Joint and connective tissue abnormalities |
|
The diagnostic approach to the weak child is very similar to the hypotonic infant, with emphasis placed on identifying systemic disorders first and then determining if signs and symptoms localize to the CNS or PNS. Fig. 35.8 outlines an algorithm for determining the cause of weakness in a child.
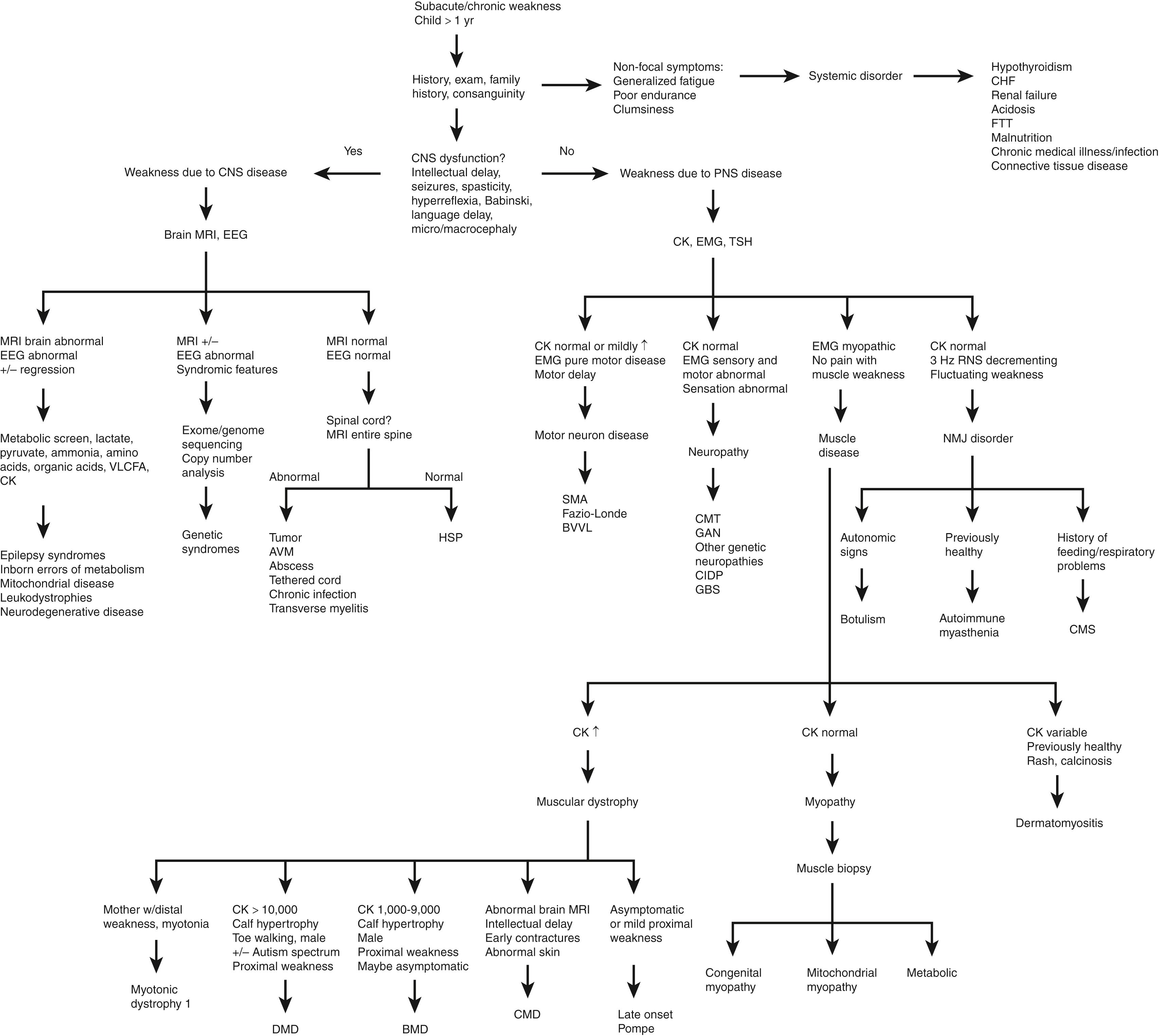
Disorders of the cerebral cortex commonly cause axial hypotonia but appendicular hypertonia in children. Some progressive neurologic disorders affect both the brain and peripheral nerves (metachromatic dystrophy, Krabbe disease, adrenoleukodystrophies, and some mitochondrial disorders). Other progressive disorders may affect both brain and muscle (Duchenne muscular dystrophy, myotonic dystrophy, dystroglycanopathies, and some mitochondrial disorders).
Sometimes disturbance of function at one site conveys a predilection for injury to another site in the nervous system, making neuraxial localization more challenging. For instance, children with congenital muscle weakness (e.g., congenital myopathy) are likely to have had severe respiratory impairment at birth that results in secondary anoxic injury to the brain. Because hypotonia itself is nonspecific with regard to localizing the site of nervous system dysfunction, the evaluation of the child with hypotonia must begin with a search for clues that might identify the location of the abnormality.
Systemic disorders are a common cause of generalized hypotonia in toddlers and children (see Table 35.1 ). Hypotonia is commonly seen in association with sepsis and other infections, heart failure, failure to thrive, hypercalcemia, renal failure, hypothyroidism, acidosis, hypoxia, hyperammonemia, hypoglycemia, rickets, scurvy, amino and organic acid disorders, severe malnutrition, and other chronic disorders. This observation warrants a careful search for a systemic or metabolic abnormality in children with hypotonia, particularly (but not exclusively) when the onset of hypotonia is acute ( Fig. 35.9 ).
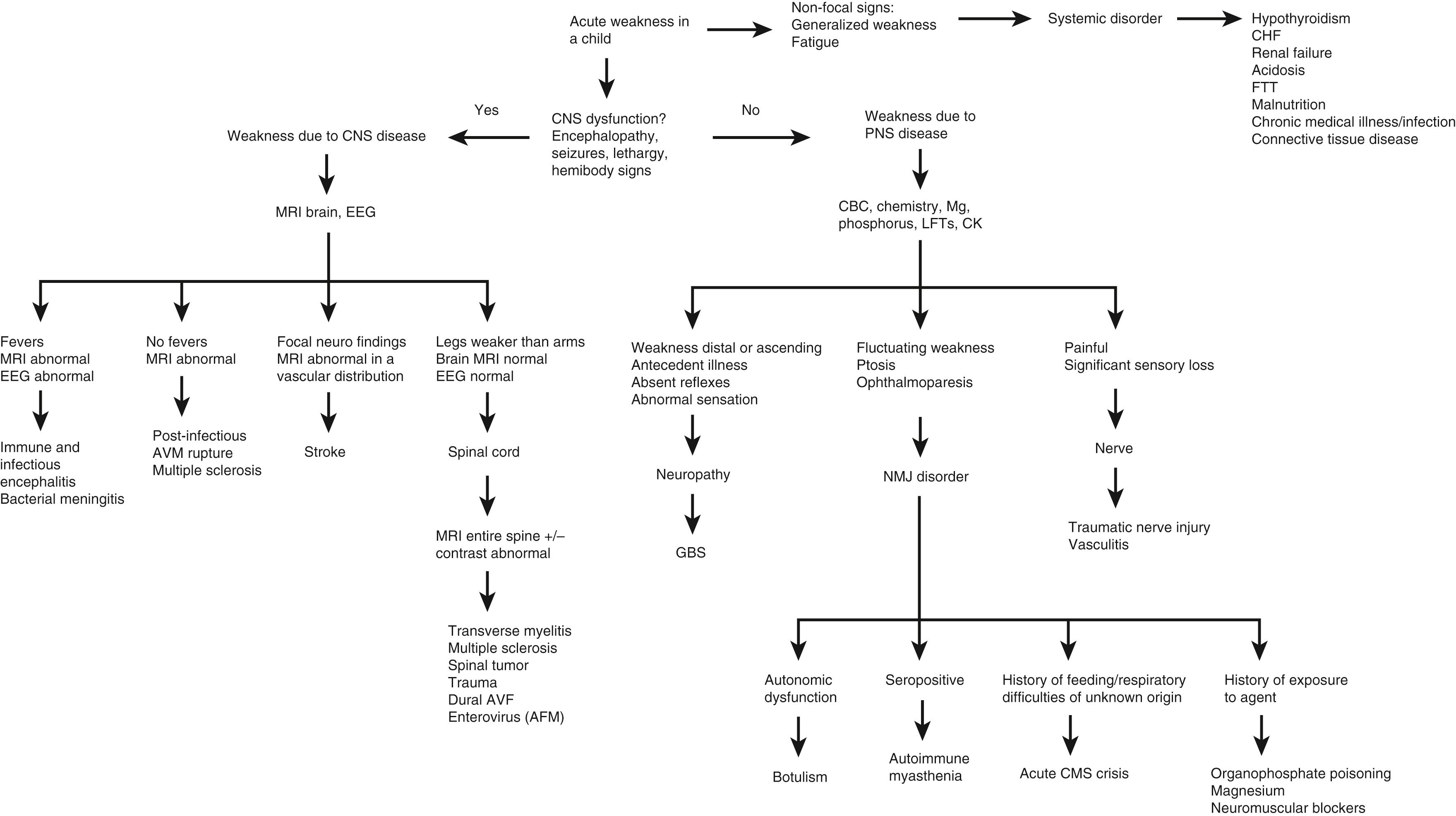
Frequently overlooked causes of hypotonia are those that are not traditionally considered neurologic disorders, though may mimic neurologic disorders. Connective tissue disorders often produce a phenotype similar to those of neurologic causes of hypotonia in infancy and early childhood, with associated delay of developmental milestones. Connective tissue disorders are distinguished by joint hyperextensibility disproportionate to the extent of weakness and by the absence of other neurologic abnormalities or microcephaly (velocardiofacial syndrome, achondroplasia, Marfan syndrome, Ehlers-Danlos syndrome). In several congenital disorders, hypotonia is a regular feature as a result of a combination of abnormalities of neurologic, muscle, and connective tissue function, including Sotos syndrome, Prader-Willi syndrome, Angelman syndrome, Noonan syndrome, Rett syndrome, and Smith-Lemli-Opitz syndrome.
Any child with hypotonia and weakness should be evaluated for a systemic disorder. Laboratory evaluation, such as electrolyte measurements, renal function tests, thyroid function tests, and acid-base balance assessment, should be considered. Laboratory evaluation should also be considered for uncommon metabolic disorders in children with chronic hypotonia, especially those with other neurologic findings and those with recurrent bouts of lethargy, episodic severe hypotonia, vomiting, or acidosis. Appropriate metabolic screening tests include plasma and urine amino acid quantification, urine organic acid quantification, and measurements of blood ammonia, blood lactate, pyruvate, and GDF-15.
Genetic analysis should be performed for any hypotonic child who additionally demonstrates microcephaly, growth retardation, congenital malformations, dysmorphism, global developmental delay, or features of specific genetic disorders. If characteristic neurologic or dysmorphic features are present, specific disorders, such as Rett syndrome, Angelman syndrome, Prader-Willi syndrome, Noonan syndrome, Sotos syndrome, and fragile X syndrome, must be considered.
The child with trisomy 21 generally has recognizable features, including microcephaly, up-slanted palpebral fissures, epicanthal folds, flat nasal bridge, protuberant tongue, excess posterior nuchal skin, and single palmar creases (see Chapter 29 ). Hypotonia and associated weakness are nearly universal findings. As the child grows, the muscle strength generally improves, but the hypotonia persists. The diagnosis is established by genetic analysis showing trisomy 21.
Prader-Willi syndrome manifests in early infancy with marked hypotonia and virtually no other identifiable symptoms, though cryptorchidism in males and features of hypogonadism in both males and females may be apparent. As the child grows, the phenotypic features become more apparent, including microbrachycephaly, almond-shaped palpebrae, short stature, and small hands and feet. At 3–6 years of age, the child develops a disorder of appetite that results in ravenous food-seeking behaviors, impaired satiety, and eventual marked obesity if access to food is not regulated. Weakness associated with the disorder is most prominent in the neonate and older infant and gradually lessens, whereas the hypotonia persists.
DNA methylation analysis is the only technique that accounts for paternal deletion, maternal uniparental disomy, and imprinting defects and identifies >99% of affected patients. Up to 75% of affected children have a deletion of chromosome 15q11-q13 of paternal origin, and 20–25% have maternal disomy. Because the clinical findings are nonspecific during the early months, such testing should be performed in any neonate or infant with hypotonia of unknown cause.
Metabolic disorders that are associated with hypotonia include the following (see Table 35.1 ):
Aminoacidopathies, organic acidurias, urea cycle defects, fatty acid oxidation defects
Lowe syndrome
Peroxisomal disorders (Refsum syndrome, adrenoleukodystrophy, Zellweger syndrome)
Acyl coenzyme A dehydrogenase deficiencies
Storage disorders (mannosidosis, Krabbe disease, sialuria, mucolipidosis type IV, Tay-Sachs disease)
Neurologic disorders associated with hypotonia are often recognizable by unusual neurologic features that include the following:
Angelman syndrome : global developmental delay, wide-based ataxic gait with arms flexed at elbows and hands flapping (“happy puppet”), little or no language, inappropriate laughter, and seizures. A characteristic dysmorphic feature includes a wide mouth with increased space between teeth. DNA methylation analysis reveals that up to 80% have abnormal methylation in the maternally inherited 15q11.2-q13 locus, the same chromosome that is affected in Prader-Willi syndrome.
Rett syndrome : developmental regression characterized by partial or complete loss of previously attained fine motor abilities, language and gait dysfunction, autism, characteristic hand-wringing movements, mostly in females, though affected males present with severe neonatal-onset encephalopathy that is progressive and typically fatal in infancy or early toddlerhood. Genetic sequencing of the MECP2 gene demonstrates heterozygous pathogenic variants in females and a hemizygous pathogenic variant in liveborn males.
Congenital malformation syndromes are recognizable by their characteristic features:
Sotos syndrome : prominent forehead with dolichocephaly; macrosomia; down-slanted palpebrae; long, narrow face; intellectual delay; mild ventriculomegaly.
Noonan syndrome : short stature, down-slanted palpebrae, low-set posteriorly rotated ears, hypertelorism, congenital heart disease, pectus deformities, webbed/short neck, ptosis, intellectual disability in some. Diagnosis is based on clinical features combined with a comprehensive genetic panel or genomic sequencing demonstrating a disease-causing pathogenic variant.
Lowe syndrome : congenital cataracts, aminoaciduria, metabolic acidosis, hypotonia, global developmental delay, seizures in some, behavioral abnormalities (obsessive-compulsiveness or self-stimulation). Pathogenic variants in the OCRL gene are responsible for this syndrome.
Connective tissue disorders associated with hypotonia, and particularly with joint hyperextensibility, can also generally be recognized by their associated symptoms:
Stickler syndrome : Pierre Robin sequence (micrognathia, cleft palate, glossoptosis), flattened facies, myopia
Velocardiofacial syndrome : congenital heart disease, micrognathia, hypocalcemia, T-cell disorders, cleft palate
Achondroplasia : disproportionate short stature, risk of brainstem compression
Ehlers-Danlos syndrome : skin bruising and scarring, skin hyperelasticity, smooth skin, joint hypermobility
Marfan syndrome : tall stature; long, thin arms and fingers; ectopic lens; blue sclera; aortic dissection; mitral valve prolapse
Osteogenesis imperfecta : frequent fractures, either atraumatic or associated with minimal trauma
Although many of these disorders may result in delayed motor milestones due to the hypotonia or skeletal abnormalities, and a subsequent evaluation for presumed weakness, their underlying condition does not cause primary pathology affecting the neural axis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here