Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The formation and subsequent development of the heart is a complex process that involves multiple cell populations, a plethora of molecular regulatory mechanisms, and intrinsic, complicated spatiotemporal remodeling events. When all the developmental steps have been properly concluded, the fully septated four-chambered heart will beat a few billion times during the individual’s life span, thereby sustaining three interdependent blood circulations (systemic, pulmonary, and coronary). Although this chapter can only scratch the surface of all the aspects associated with the formation of the cardiovascular system, we believe that it provides relevant insights into the most important events in that system’s development.
Cardiovascular development about 3 weeks after ovulation (or at 3 weeks of development) a when, during the process of gastrulation, the three germ layers (ectoderm, mesoderm, and endoderm) become established. During this process, cells from the embryonic epiblast undergo epithelial-to-mesenchymal transformation (EMT) and migrate to the primitive streak, a visible groove that begins at the caudal end of the embryo and extends cranially. The mesenchymal cells enter the streak uncommitted as to their developmental potential but become committed to their mesodermal phenotype and migratory pathways after leaving the primitive streak. Once this process has been completed, the mesoderm occupies the space between the ectoderm and the endoderm. A part of the mesoderm is induced by the hypoblast and endoderm to enter the precardiac lineage. This involves molecular signaling by, for instance, the Wnt/β-catenin, fibroblast growth factor (FGF), bone morphogenetic protein (BMP), and activin/nodal signaling pathways. The mesoderm differentiates into the chorda and the paraxial, intermediate, and lateral plate mesoderm. Two precardial mesodermal cell population contributing to the formation of the primitive heart tube are located laterally in the embryo and are referred to as the heart-forming regions. As a result of the formation of the intraembryonic coelomic cavity, the lateral plate mesoderm splits into two layers: a splanchnic layer, located directly above the endoderm, and a somatic layer, found directly below the ectoderm. The region of splanchnic mesoderm expressing precardiac markers is now known as the first heart field (FHF) . Molecular markers used to identify the precardiac mesoderm include the transcription factors ISL1, NKX2.5, MEF2C, HAND1, HAND2, GATA4, and TBX5.
a For consistency with the embryology literature, weeks of development (postovulatory age) is used throughout this chapter, rather than postmenstrual or gestational age.
With folding of the embryo, the two heart-forming regions meet anterior to the developing head region, thereby forming a horseshoe-shaped structure. Continuation of the fusion process in an anteroposterior direction brings the left and right legs of the horseshoe together along the embryonic midline ( Fig. 45.1A–C ).

As the heart fields are fusing, a subset of the precardiac mesodermal cells undergo EMT. The mesenchymal cells differentiate into endocardial cells, which then form a network of tiny channels that remodel into a single endocardial channel with ongoing folding. Concomitantly, this endocardial tube becomes surrounded by a mantle of cardiomyocytes, which express characteristic sarcomeric proteins such as atrial and ventricular myosin heavy chain and cardiac troponin. Between the endocardial and myocardial layers an acellular, extracellular matrix (ECM)–rich substance known as the cardiac jelly is accumulating. Together, these three components form the linear primary heart tube.
The cardiomyocytes of the tubular heart do not proliferate. The heart tube grows and increases in length primarily by the addition of newly differentiated cardiomyocytes to both the anterior and posterior pole of the heart tube. The mesodermal cells that are added to the lengthening tube are referred to as the anterior or second heart field (AHF/SHF). Molecular markers for the SHF include the transcription factors TBX1, ISL1, FGF8, and FGF10. It is beyond the scope of this chapter to discuss in detail the importance of the FHF and SHF in the development of each single structure of the heart. Suffice it to note that numerous cell fate studies have established that, as far as the myocardial components are concerned, the FHF contributes mainly to the left ventricle and most of the myocardial tissues of the left and right atria. The SHF contributes mainly to the outflow tract (OFT), the right ventricle, the ventricular septum, and the dorsal mesenchymal protrusion (DMP) at the venous pole of the heart ( Fig. 45.2 ).
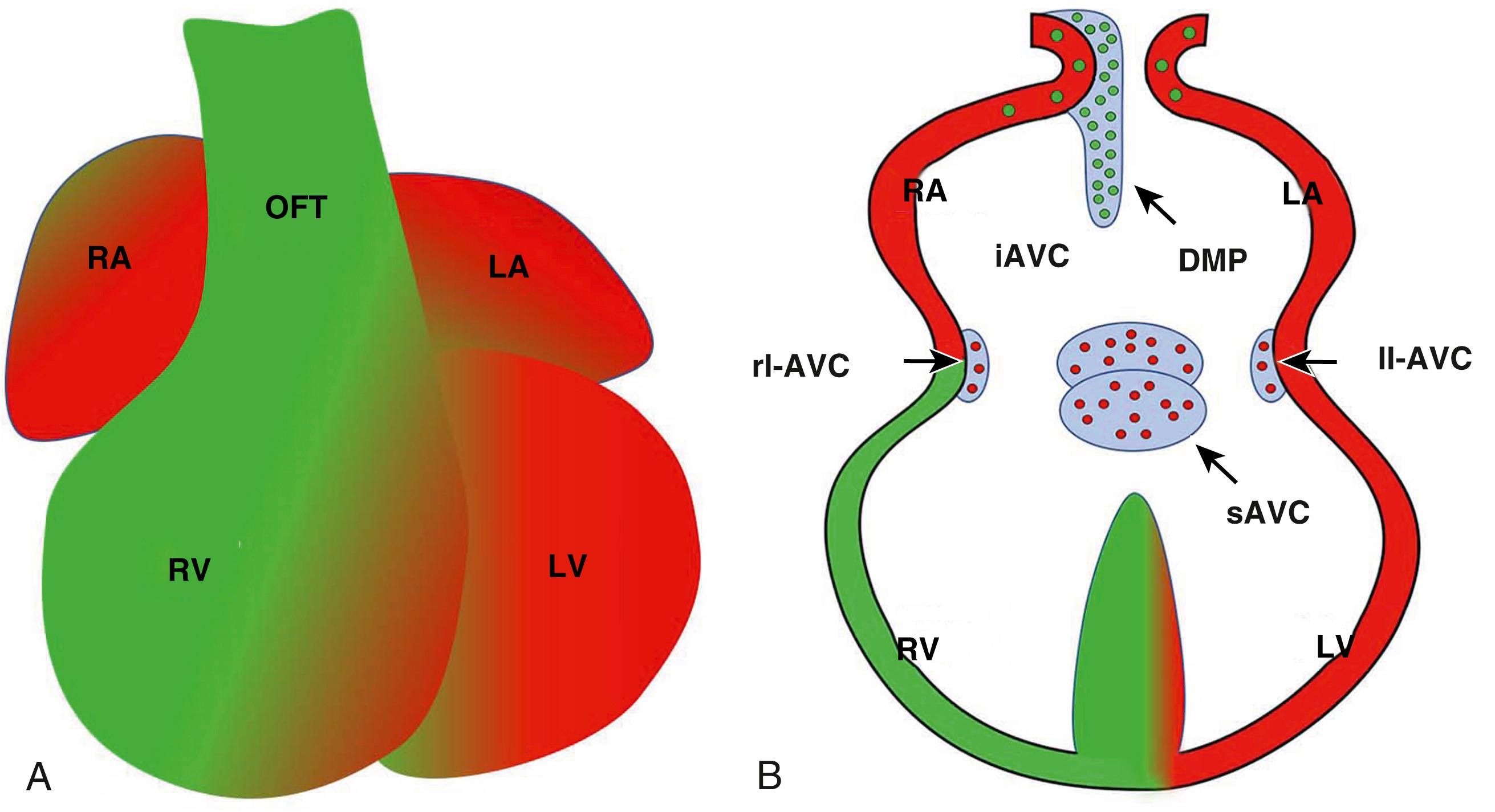
After its initial formation, the tubular heart is initially tethered over its entire length to the rest of the embryo by the dorsal mesocardium. As the heart tube starts to lengthen and loop, the dorsal mesocardium disintegrates in its center portion, leaving the heart attached to the rest of the embryo by its arterial and venous poles. This partial disintegration of the dorsal mesocardium is crucial, as it enables the process of cardiac looping during lengthening of the tube ( Fig. 45.3 ).
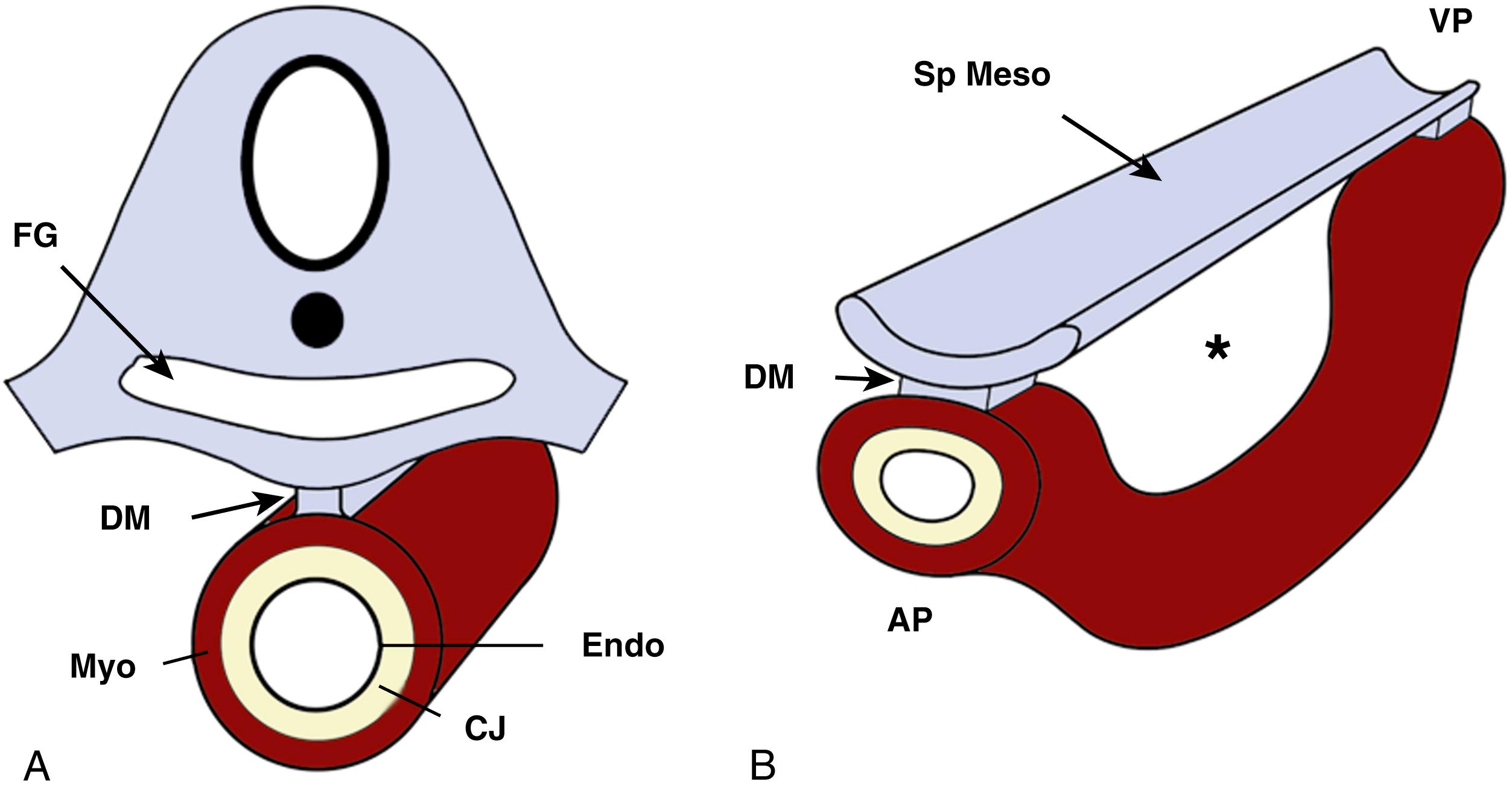
Cardiac looping is a somewhat ill-defined term that refers in the most general sense to the bending of the heart tube before septation. , The initial bend results in a C-shaped structure, with the outer loop of the C positioned to the right side of the embryo (see Fig. 45.1D and E ). Within the C-shaped heart, inner and outer curvatures can be distinguished. As the heart continues to remodel, the myocardium of the outer curvature differentiates and starts to proliferate. From this myocardium, the primitive left and right ventricles emerge—a process that has been described as ballooning . It is noteworthy that each ventricle has its own molecular identity. Much of the patterning of the primitive and ventricular myocardial molecular identities is linked to the expression of T-box gene family members such as TBX1, TBX2, TBX3, TBX5, and TBX20 . At the junction between the developing ventricles and atria, the atrioventricular (AV) myocardium maintains its “primitive” molecular characteristics. In addition, the right AV junctional myocardium has additional molecular features that are associated with the development of the AV conduction system. As the heart continues to grow, its shape changes significantly and becomes more complex. Once it has assumed an S shape (see Fig. 45.1E ), the respective compartments of the heart more or less assume their final position in relation to each other, even though all compartments are essentially still connected in series as one long tube. It is at this stage that the atria start to appear at the venous pole.
Left-right (L/R) axis determination is a critical element in vertebrate body design. As is the case with most internal organs (e.g., liver, lungs, stomach), the cardiovascular system shows distinct L/R asymmetry. For instance, the pulmonary veins (PuVs) enter the left atrium, whereas the systemic veins enter the right atrium. The absence of correct establishment of the L/R axis typically results in complex abnormalities, as seen in patients with heterotaxy syndromes. The hearts of patients in which L/R laterality has been developmentally perturbed typically show severe abnormalities in atrial, AV, and OFT morphology. The ventricular chambers are typically less severely affected. ,
Laterality becomes established very early in embryonic development. Studies to determine the underlying mechanisms have historically been conducted on animal models. Experiments in mouse embryos have shown that the oriented motion of cilia on cells in the Hensen node, located at the cranial tip of the primitive streak, is critical in determining the L/R axis. FGF is an important factor in this process, as it plays a role in ciliary growth. The ciliary activity leads to asymmetric calcium transients and asymmetric expression of laterality genes, including nodal, sonic hedgehog, lefty, and the homeodomain transcription factor PITX2C. , As is true in general for developmental processes, most of the molecules involved in determining L/R signaling play a role across different species. Interestingly, in an intriguing twist on that theme, some of the molecules required for left sidedness in mice are determinants of right sidedness in birds. Many of the genes that have been found to control L/R determination in animal models are candidate genes involved in the pathogenesis of heterotaxy syndromes.
The anteroposterior (craniocaudal) body axis is also established during gastrulation. Retinoid signaling pathways are critical to normal anteroposterior axial patterning in general and for cardiac development in particular. , Furthermore, retinoids are also implicated in the regulation of SHF differentiation. Failure of formation of atrial chambers and the systemic venous connections with the heart is observed in conditions of retinoid deficiency. Excess retinoids create cardiac malformations, often involving the OFT, and result in the ventricular expression of several genes that are normally largely restricted to the atria at the equivalent stages of normal development. ,
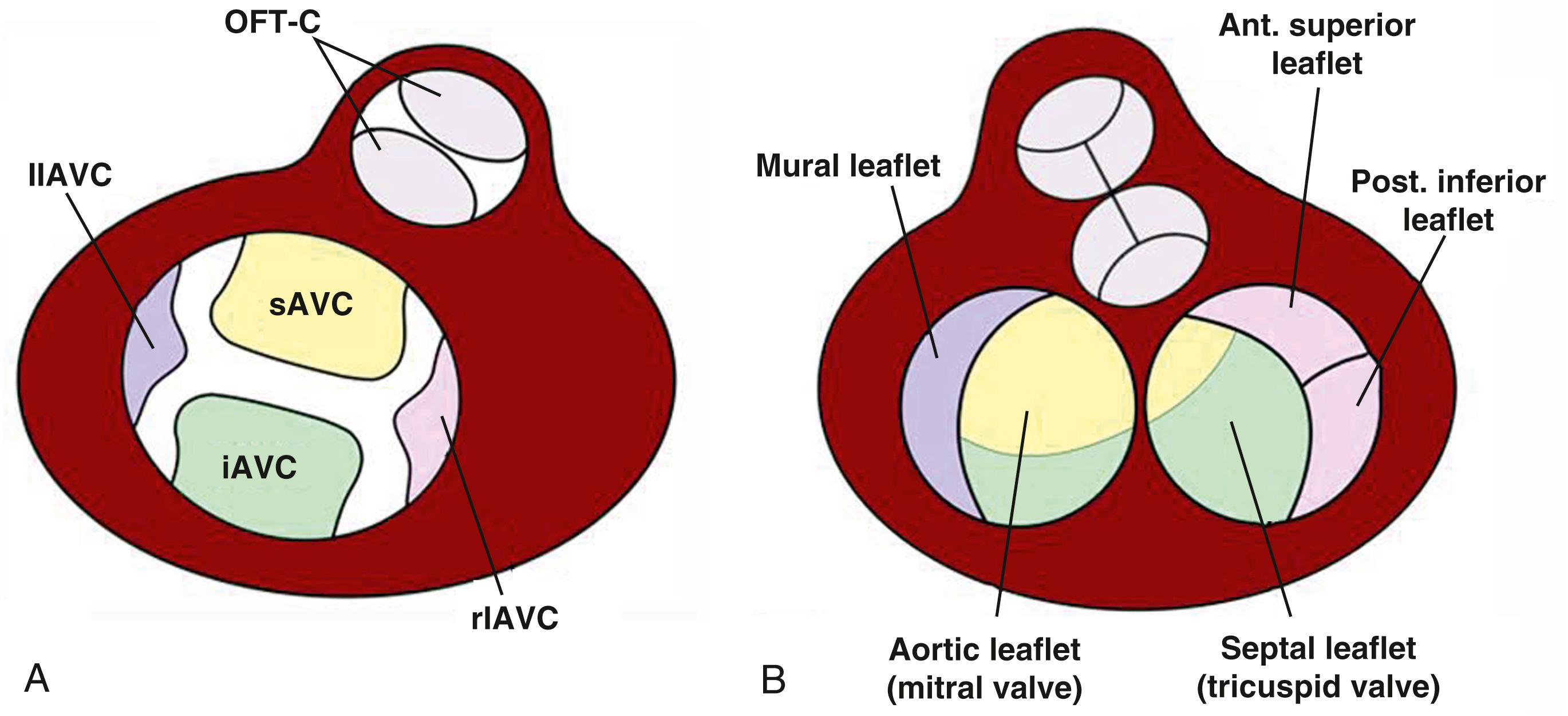
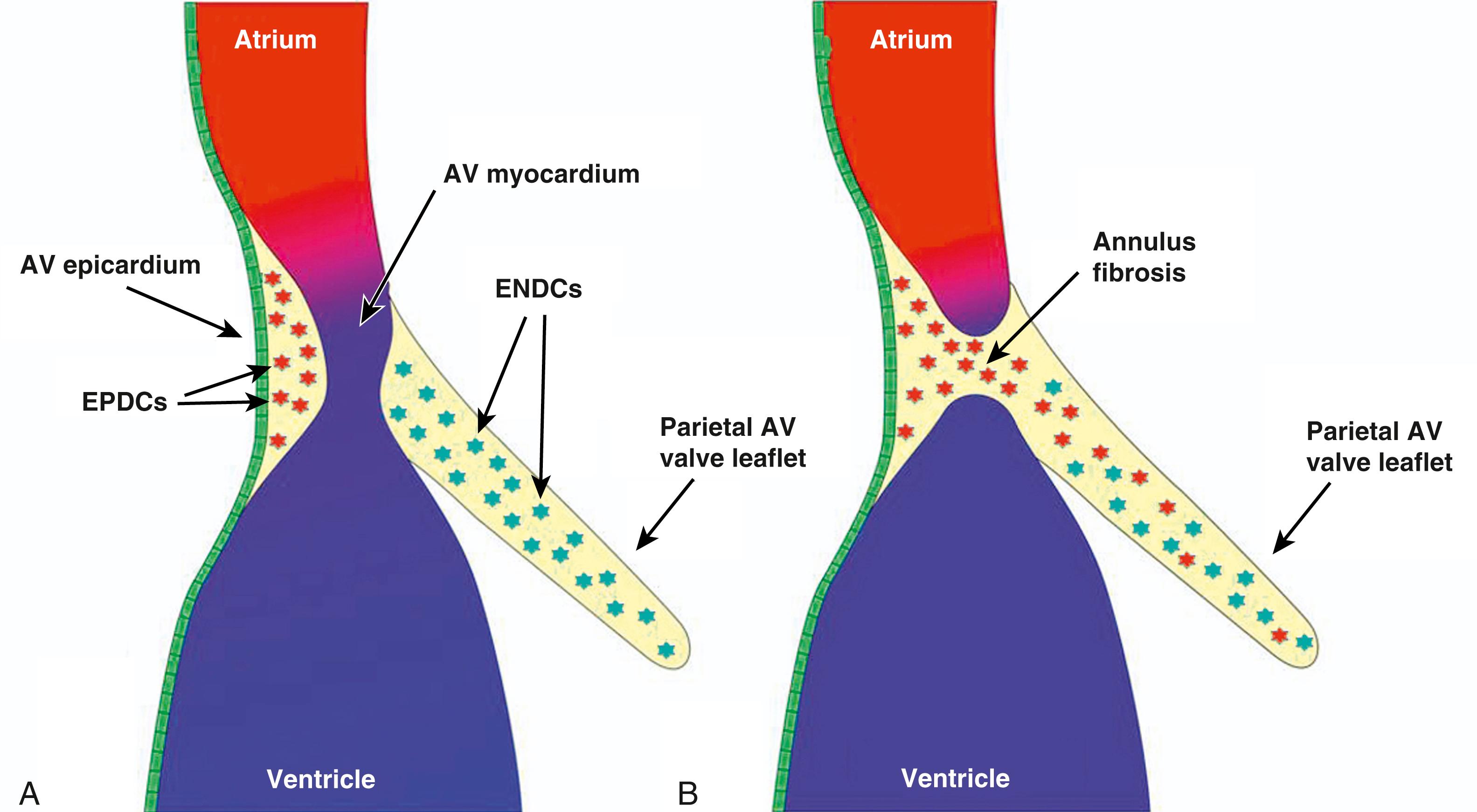
The formation of the AV valves is critically dependent on the proper development of their precursors, the AV cushions. During cardiac looping, the accumulation of cardiac jelly in the subendocardial space between the endocardium and myocardium at the AV junction leads to the formation of the two initially acellular ECM-rich major AV cushions. The inferior AV cushion (iAVC) is attached to the dorsal wall of the AV canal, whereas the superior AV cushion (sAVC) is attached to the ventral wall. After their initial development, the cushions become populated by cells derived from an endocardial-to-mesenchymal transformation (endMT) of the endocardial lining, a process in which cells delaminate from the endocardium and migrate as mesenchymal cells into the cardiac jelly. The process of EMT in the major AV cushions has been thoroughly studied, and much is known about the molecular mechanisms regulating this process. Key molecular players include TGFβ2, BMP2, and Notch. Later in development, two smaller cushions emerge on the wall of the AV canal between the major cushions. These are known as the right and left lateral AV cushions ( Fig. 45.4A ). Like the major AV cushions, they also initially become populated by endocardially derived cells (ENDCs), but studies have shown that after this initial migration of ENDCs into the cushions, significant numbers of epicardially derived cells (EPDCs) start to populate the lateral AV cushions ( Fig. 45.5 ). As the major cushions fuse, they separate the common AV canal into left and right AV canals. The mitral and tricuspid valves will develop in these orifices. Each AV cushion plays a specific role in valve formation (see Fig. 45.4B ). The fused major cushions contribute to the valve leaflets that are attached to the ventricular septum, whereas the lateral AV cushions give rise to the leaflets that are attached to the left and right ventricular free wall. In the human, the tricuspid valve begins to form around the fifth week of development. The AV cushions are actively reconfiguring at this time. Despite the overall advanced stage of cardiac morphogenesis at this point, the leaflets are still very primitive in appearance and not freely mobile. The inferior leaflet is fully delaminated by the end of the 8th week of development, the anterior leaflet by the 11th week, and the septal leaflet in the 12th week. The commissure separating the anterior and septal leaflets is not complete until the septal leaflet is fully delaminated. Proper development of the mitral valve is dependent on the fusion of the inferior and sAVC and the formation of the left lateral cushion, which is the precursor to the posterior or mural leaflet; it is visible by the 7th week of development. At about this time, initial delamination of the mitral valve structures becomes detectable and continues until the 10th week. Between the 10th and 14th weeks of development, myocardial elements of the leaflets are eliminated by apoptosis. Furthermore, to support the function of the mitral valve leaflets, two mitral papillary muscles evolve at approximately 5 weeks of development from an enlarged trabecular complex. With the formation of a free motile valve leaflet, the papillary muscles also achieve their adult appearance, with chordae tendineae connecting the valve leaflets with the tip of the papillary muscle. Molecularly, a pathway linking FGF4 signaling to the expression of scleraxis and transcriptional activation of tenascin has been described in chick limb tendon and is proposed to be active in the normal formation of chordae tendineae. With subsequent development, the valve leaflets further mature and become organized in three layers: atrialis, spongiosa, and fibrosa. Each layer comprises specific ECM compositions and valve interstitial cells that are essential for proper function of the leaflets.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here