Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Substantial material from Dr. Michael Frank’s chapter in Principles and Practice of Pediatric Infectious Diseases, fifth edition, has been used and he is therefore granted appropriate authorship. Dr. Frank was a giant in the field of complement and a beloved mentor and scientist who sadly passed away in the summer of 2019.
The collective term complement refers to a large group of plasma- and cell membrane−bound proteins that play a major role in first-line innate defense against a wide variety of infectious agents. The complement proteins are evolutionarily old, and some now interface with the adaptive immune system, co-evolving dependent functions. This chapter reviews the biochemistry and biology of complement and its role in host defense against infection and illustrates infectious complications in individuals with inherited or acquired deficiencies of complement proteins; neither aspect is covered exhaustively.
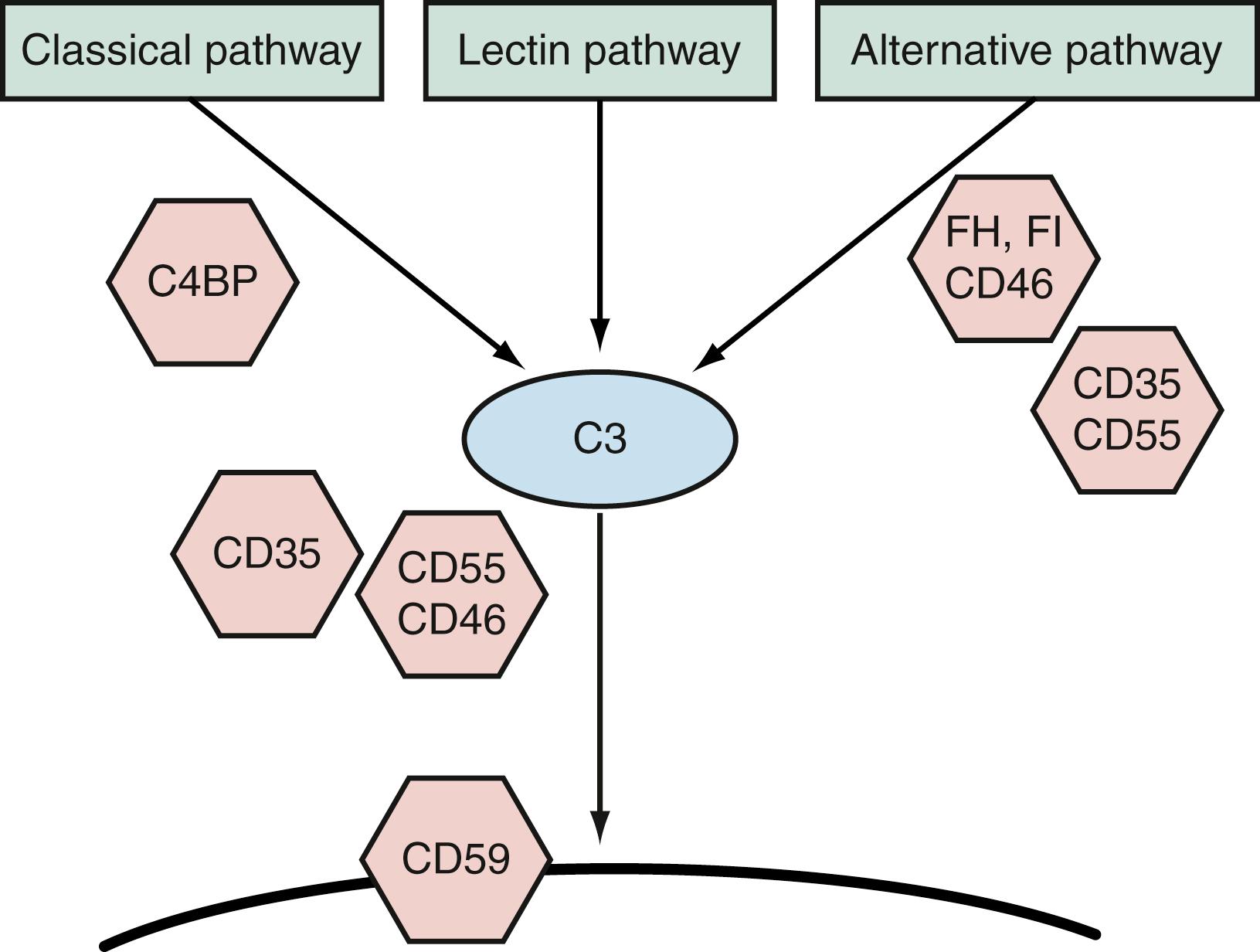
The basic biochemistry of the complement system was clarified decades ago. There are three activation arms that converge of C3. The terminal components, C5, C6, C7, C8, and C9 are most famously involved in pore formation, a cytolytic function against gram negative bacteria ( Fig. 103.1 ). Each activation arm is comprised of different proteins and is regulated distinctly. Thus, they will be discussed separately.

The classical pathway is so termed because it was the first pathway of activation, studied more than 100 years ago. Activation of the classical pathway usually is initiated by antigen-antibody complexes. Antibodies of the appropriate class (immunoglobulin M [IgM] or IgG subclasses IgG 1 , IgG 2 , IgG 3 ) combine with microbial or other antigens to form an immune complex, which then binds and activates the first component of complement (C1). Only one molecule of pentameric IgM, deposited on a surface such as a cell membrane, is needed for activation of C1. In contrast, two IgG molecules, side by side, are necessary for C1 to bind and cause activation. The requirement for an IgG doublet greatly reduces the efficiency of IgG compared with IgM in activating C1 and the classical pathway because thousands of IgG molecules may need to be deposited on a microbial surface in order for two to be aligned in close proximity.
C1 is a macromolecular complex composed of three biochemically distinct subcomponents, designated as C1q, C1r, and C1s. C1q binds to the Fc fragment of immunoglobulin in the immune complex. Binding is followed by cleavage of the bound C1r and then cleavage of C1s. Activated C1s then cleaves both the fourth component of complement (C4) and the second component (C2), each into smaller and larger products. The larger fragment of C2 (C2a) remains complexed with the larger fragment of C4 (C4b) to form a bimolecular enzyme, C4b2a, which is responsible for activating C3 and initiating the assembly of the terminal components.
The classical pathway usually is activated by immune complexes; however some enveloped RNA viruses, some Mycoplasma spp., and certain strains and species of both gram-negative and gram-positive bacteria bind C1q directly and activate the classical pathway. Thus, under some circumstances, the classical pathway can also be activated in an antibody-independent fashion and functions in “natural” immunity.
The alternative pathway provides an evolutionarily ancient and primitive innate immune defense mechanism. Activation commonly begins with the C3 molecule itself. Native C3 contains an internal thiol ester in its α chain that, under normal physiologic conditions, undergoes continuous low-grade hydrolysis to create a “C3b-like” molecule, C3(H 2 O). C3(H 2 O) can bind a circulating alternative pathway protein, factor B, which allows factor B to be cleaved by a serine protease, factor D, which is in turn activated by MASP3. Two cleavage products of factor B are generated: the larger Bb and smaller Ba. The association of the hydrolyzed C3 with Bb creates a new C3-cleaving enzyme, C3(H 2 O)Bb (termed the priming C3-convertase ), which is responsible for a continuous, low-grade cleavage of C3 and, hence, the generation of further nascent C3b. If nascent C3b binds covalently to a suitable surface, such as a bacterium, it can form a reversible complex with native factor B. This complex is cleaved by factor D to create a highly efficient C3-cleaving enzyme, C3bBb, termed the amplification C3-convertase . These convertases of the alternative pathway are not stable and, like the C3 convertase of the classical pathway, decay over time. Decay in the classical pathway is associated with release of C2, and in the alternative pathway it is associated with release of factor B of the convertase. Another circulating protein, properdin, stabilizes the alternative pathway convertase. Antibody is not required for the activation of the alternative pathway; however, antibody enhances activation. Thus, the alternative pathway can participate in both innate and “acquired” antibody-mediated host defense.
Because C3b is both the product of the alternative pathway C3-cleaving enzyme and forms part of it, the activation of C3 through the alternative pathway creates a positive-feedback amplification loop. Moreover, activation of the classical pathway, by creating nascent C3b, amplifies activation of the alternative pathway.
The lectin pathway is a third C3 activation pathway and, like the alternative pathway, is evolutionarily ancient. Mannose-binding lectin (MBL) is a member of the collectin family of proteins and is capable of binding to a number of sugars on the surface of a variety of micro-organisms, including human immunodeficiency virus (HIV), Haemophilus influenzae, Neisseria meningitidis, streptococci, and many others. In the circulation, MBL is associated with 2 serine proteases, MBL-associated serine proteases 1 and 2 (MASP1 and MASP2). MASP2, when activated, cleaves both C4 and C2 to create C4b2a, identical to the classical pathway C3 convertase. The ficolins have similar structure and can also activate MASP1 but bind to acetylated sugars as targets.
All three activation arms converge on a critical molecular, namely, C3. C3 is comprised of α and β chains. Hundreds of C3 molecules can be cleaved by each C3 convertase molecule, allowing for amplification of the response. Cleavage of C3 releases a small peptide (C3a) from the α chain into the fluid phase, where it acts as an anaphylatoxin. C3a induces histamine release from mast cells and basophils, generating an anaphylaxis-like response. The larger C3 fragment, C3b, binds to the microbial cell surface, where it acts as an opsonin. The target-bound C3b can be degraded by circulating complement control proteins, factor H and factor I (see below). This central regulatory mechanism relies on factor H binding to host cells to protect them from damage. If C3b is not degraded, it joins an alternative pathway C3 convertase to continue the complement cascade. C3b itself is recognized by a specific receptor present on phagocytes and B lymphocytes, CD35 (sometimes termed complement receptor 1 [CR1]). Binding of microbes to CD35 on phagocytes (via C3b) markedly enhances phagocytosis. The first step in C3b degradation leads to the decay fragment iC3b, which is recognized by the opsonization receptors CD11b/CD18 and CD11c/CD18 (two of the B2 integrins, also termed CR3 and CR4). These receptors are deficient or abnormal in leukocyte adhesion deficiency (LAD). The cleavage fragment C3d is recognized by CD21 (CR2), a receptor present on B lymphocytes and follicular dendritic cells. On B cells, it provides a co-stimulatory signal. On follicular dendritic cells, it has a key role in antigen processing and immune response.
After C3 convertases are formed and bind C3, ultimately bound to the microbe, the convertase can then bind C5 and function as a C5 convertase. Activation of C5 by either the alternative or classical pathway by C5 convertases results in cleavage of the α chain of the C5 molecule to create a low-molecular-weight product, C5a, and a high-molecular-weight product, C5b, which remains bound to the complex. The smaller cleavage product, C5a, is released into the fluid phase, where it, like C3a, acts as an anaphylatoxin. In addition, C5a possesses potent chemotactic activity for neutrophils and monocytes and can activate a series of cells with C5a receptors (CD88). C5a facilitates phagocytosis by neutrophils and is the major endogenous chemotactic factor for neutrophils. Nascent C5b combines with native C6 to initiate formation of the membrane attack complex, a multimolecular assembly of C5b, C6, C7, C8, and C9, which is capable of cytolytic and bactericidal activity. Among these terminal components, it is the C8 protein that breaches the membrane, and C9 serves to stabilize a pore structure.
Uncontrolled complement activation can result in widespread immune-mediated damage to the host, and each step in the cascade is under complex control. Each enzyme undergoes spontaneous decay under physiologic conditions. In addition, a number of control proteins inhibit the classical pathway (i.e., C1 esterase inhibitor, C4-binding protein, factor I, and decay-accelerating factor, CD55) and the alternative pathway (i.e., factors H and I and membrane cofactor protein, CD46) ( Fig. 103.2 ). The membrane attack complex, composed of C5b through C9, also is regulated by control proteins (i.e., CD59). Thus, the activation of complement proceeds in a highly controlled process and is limited to the immediate vicinity of the initiating substance (e.g., a microbial surface). Infectious agents have evolved proteins that control or prevent complement activation or binding or specifically bind complement control proteins from serum, allowing them to evade host defense. Well-studied examples involve staphylococci, gonococci, and vaccinia virus. This subversion of complement by pathogens is a common trait and additional examples are shown in Fig. 103.3 .
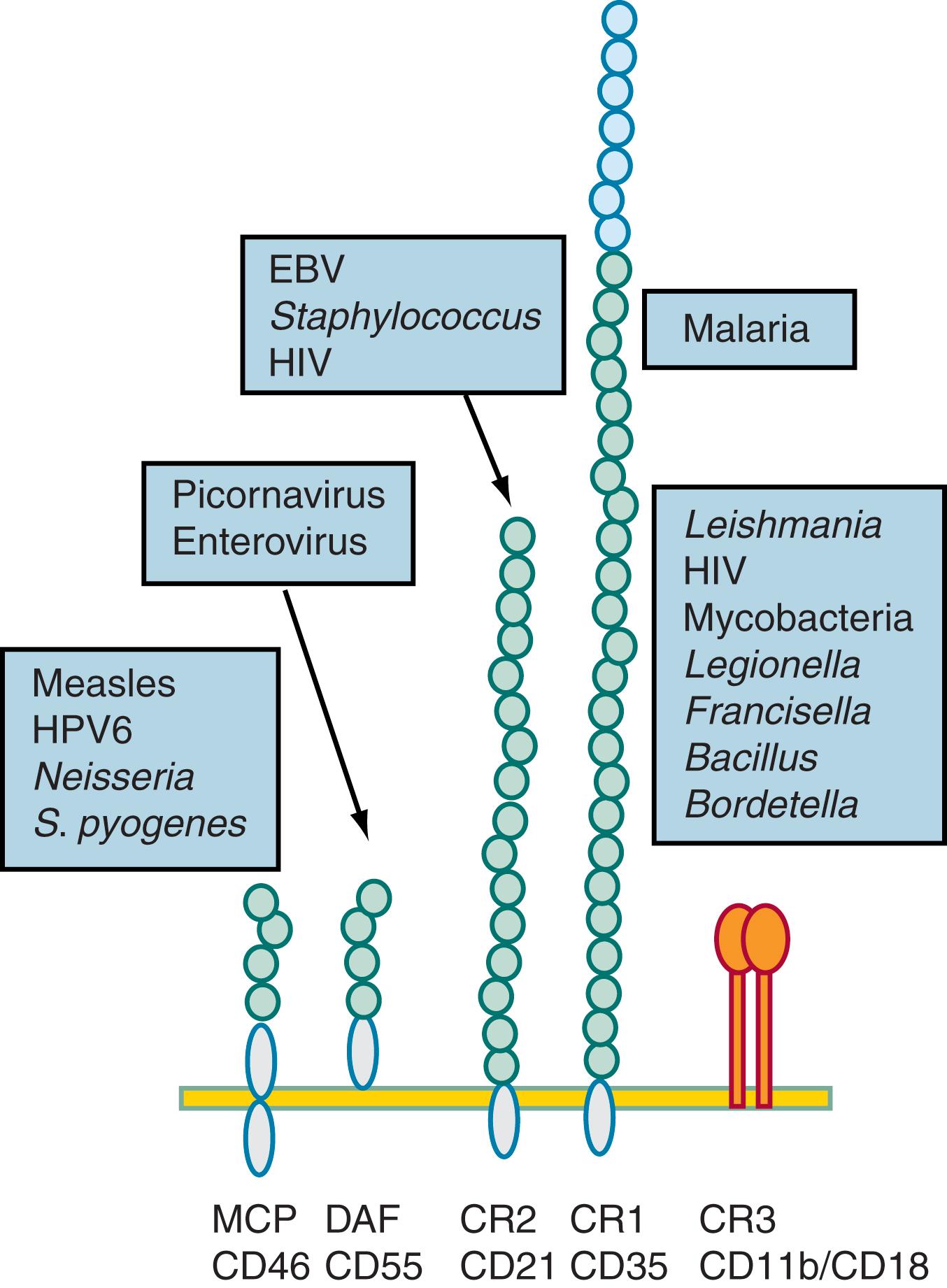
The complement system is critical for human host defense against a surprisingly narrow range of infections. Although complement is produced primarily in the liver, some complement components are produced by hematopoietic cells and other tissues. Complement has a unique role in some of these tissues such as its role as a B-lymphocyte co-stimulatory molecule and regulator of T-lymphocyte survival and function. The classical pathway components are also responsible for clearance of apoptotic cells and contribute to cholesterol clearance. , Indeed, patients with deficiencies of the early classical pathway components are at high risk of systemic lupus erythematosus (SLE) and early death from myocardial infarction. The narrow range of infectious susceptibilities in humans is at odds with data from animal model systems in which pharmacologically depleted of complement or genetically deficient animals are more susceptible to infection with H. influenzae , Streptococcus pneumoniae , S taphylococcus spp., Sindbis virus, influenza A virus, meningococcus, West Nile virus, and Candida spp., to name just a few.
The protective effects of complement appear to be most important in the first hours following the onset of infection, when complement helps to limit the spread of micro-organisms from an initial focus of infection. Complement also plays a critical role in the clearance of pathogens from the bloodstream. Different proteins of the system participate in different aspects of host defense. For example, in vivo studies have shown that the chemotactic cell-activating activities associated with C5a are important in attracting neutrophils to the initial site of infection, whereas the opsonic activity of C3b is critical in the clearance of bacteria from the bloodstream.
Most of the genetically determined deficiencies of the complement system are inherited as autosomal recessive traits. However, C1 esterase inhibitor deficiency is inherited as an autosomal dominant trait, and properdin deficiency is inherited as an X-linked recessive trait ( Table 103.1 ). Some noninfectious phenotypes can be associated with gain-of-function mutations and be inherited in an autosomal dominant fashion. Gain-of-function mutations in C1R and C1S are associated with a connective tissue disorder. Gain-of-function mutations in FB and C3 are associated with atypical hemolytic uremic syndrome (HUS). Heterozygous individuals with loss-of-function mutations and half the normal level of complement proteins usually do not appear to have an increased incidence of infections.
| Deficiency | Inheritance | Major Clinical Manifestations |
|---|---|---|
| C1q | Autosomal recessive | SLE and infections with encapsulated organisms |
| C1r | Autosomal recessive | SLE and infections with encapsulated organisms |
| C1s | Autosomal recessive | SLE and infections with encapsulated organisms |
| C1r | Autosomal dominant GOF | Periodontal Ehlers Danlos |
| C1s | Autosomal dominant GOF | Periodontal Ehlers Danlos |
| C4 (Both Ca and C4B genes mutated) | Autosomal recessive | SLE and infections with encapsulated organisms |
| C2 | Autosomal recessive | SLE and infections with encapsulated organisms |
| C3 | Autosomal recessive | Infections with encapsulated organisms and renal disease |
| C3 | Autosomal dominant GOF | Atypical hemolytic uremic syndrome (atypical hemolytic uremic syndrome [aHUS]) |
| C5 | Autosomal recessive | Meningococcal septicemia and meningitis |
| C6 | Autosomal recessive | Meningococcal septicemia and meningitis |
| C7 | Autosomal recessive | Meningococcal septicemia and meningitis |
| C8 α—γ | Autosomal recessive | Meningococcal septicemia and meningitis |
| C8 β | Autosomal recessive | Meningococcal septicemia and meningitis |
| C9 | Autosomal recessive | Meningococcal septicemia and meningitis |
| Factor B | Autosomal recessive | Encapsulated organisms and Neisseria |
| Factor B | Autosomal dominant | aHUS |
| Factor I | Autosomal recessive | Infections with encapsulated organisms OR aHUS |
| Factor H | Autosomal recessive for infection and complex for aHUS | Infections with encapsulated organisms OR aHUS |
| MCP (CD46) | Autosomal recessive | aHUS |
| Factor D | Autosomal recessive | Systemic meningococcal infections |
| Properdin | X-linked recessive | Meningococcal septicemia and meningitis |
| CD59 | Autosomal recessive | Hemolytic anemia, thrombosis, neurologic |
| CD55 (DAF) | Autosomal recessive | Protein losing enteropathy, thrombosis |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here