Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Influenza viruses cause recurrent and widespread disease in humans. Vaccination is the most effective means of preventing influenza infection. The use of inactivated influenza virus vaccine (IIV) in humans has been the subject of numerous studies, with an emphasis on providing an effective vaccine with minimal reactogenicity. An alternative approach to influenza immunization using live attenuated virus administered by nasal spray has also been successful. This chapter reviews the research that led to the licensing of the live attenuated influenza vaccine (LAIV) in the United States, and the clinical experience with LAIV in several countries since licensure. In this chapter, inactivated influenza vaccines are generally referred to as IIV or in specific instances as trivalent or quadrivalent inactivated vaccine (TIV or QIV, respectively). More extensive background information on influenza can be found in Chapter 33 on inactivated influenza vaccines.
Influenza viruses are the most common cause of lower respiratory tract infections. The number of cases of influenza that occur varies depending on the susceptibility of the population to the virus and the infectiousness of the virus during an outbreak. In addition, influenza seasons vary in length and severity. In the United States, an estimated average of 36,000 deaths were associated with influenza epidemics between 1990 and 1999, and approximately 226,000 hospitalizations between 1979 and 2001 ; approximately 90% of the deaths attributed to influenza occurred among persons age 65 years or older. In 2010, the Centers for Disease Control and Prevention (CDC) released revised estimates for influenza-associated deaths from 1976 to 2007 that ranged from approximately 3000 to 49,000 per year. Indeed, WHO estimates that seasonal influenza can result in 290,000–650,000 deaths from respiratory causes every year around the world though this estimate does not take into account influenza-related mortality from other diseases such as cardiovascular disease. Among influenza viruses, influenza type A viruses are responsible for the greatest amount of morbidity and mortality, and the nonhuman reservoir of influenza A viruses creates the potential for global pandemics as was seen in 1918, 1957, 1968, and 2009. Although influenza B does not cause pandemics, it is responsible for regional epidemics that are generally less severe than influenza A epidemics.
Influenza incidence rates vary with the nature of the epidemic virus strains and the population affected. Rates calculated from surveillance of persons with upper respiratory illness suggest that influenza is responsible for roughly 10–20% of all respiratory illnesses per epidemic year, with rates for influenza A being somewhat higher than those for influenza B. Most studies have found infection rates in preschool- and school-age children to be much higher than in adults, particularly during influenza B epidemics. , Consequently, families with school-age or younger children suffer disproportionately from influenza. These findings support the ideas that influenza infects persons with the lowest prior immunity and that children are a common source of influenza in the community. Furthermore, it forms the rationale for influenza vaccine strategies that target children, to both directly protect them and indirectly protect the wider population including vulnerable groups, through reduction in influenza transmission.
Influenza viruses are spread from person to person primarily via coughs and sneezes of infected persons. The incubation period for influenza is 1–4 days, with an average of2 days. Adults and children are typically infectious from the day before symptoms appear, until approximately 5 days after illness onset. Children can be infectious for a longer period, and very young children can shed virus for up to 6 days before symptom onset. Severely immunocompromised persons can shed virus for weeks.
Uncomplicated influenza illness is characterized by the abrupt onset of systemic and respiratory signs and symptoms (e.g., fever, myalgia, headache, severe malaise, nonproductive cough, sore throat, and rhinitis). Respiratory illness caused by influenza is difficult to distinguish from illness caused by other respiratory pathogens including SARS-CoV-2 on the basis of symptoms alone. Sensitivity and predictive value of clinical definitions can vary, depending on the degree of cocirculation of other respiratory pathogens and the level of influenza activity.
Influenza illness typically resolves after a few days, although cough and malaise can persist for more than 2 weeks. Influenza can exacerbate underlying medical conditions (e.g., pulmonary or cardiac disease); lead to primary influenza viral pneumonia, or to secondary bacterial pneumonia; or occur as a coinfection with other viral or bacterial pathogens. Influenza infection is also associated with encephalopathy, transverse myelitis, Reye syndrome, myositis, myocarditis, and pericarditis.
Multiple mechanisms provide resistance to influenza in humans. Acquired immunity to influenza is mediated by serum antibodies, antibodies at mucosal surfaces, and influenza-specific T cells. Antibody alone is sufficient for protection, and T cells play a role in recovery. Innate immune responses, such as production of interferon or other antiviral factors by macrophages, may also contribute to resistance to influenza infection. Immunity after natural infection provides long-lived protection against influenza virus infection and disease in the absence of antigenic shift or drift. In 2009, when the swine-origin H1N1 pandemic influenza virus (H1N1pdm09) emerged, older adults who had prior exposure to antigenically related H1N1 influenza viruses were found to have cross-reactive antibodies to the pandemic virus, and disease was not as severe in this age group as in younger adults. The H1N1pdm09 strain has now replaced previously circulating seasonal H1N1 viruses, so in this chapter, H1N1 viruses isolated after 2009 are referred to as H1N1pdm09. Immunity acquired by natural infection can provide some protection against influenza strains that have undergone antigenic drift.
Because of the complexity of the subject, it is beyond the scope of this chapter to address all aspects of influenza virology. For a comprehensive review of the biology of influenza viruses, the reader is directed to other texts.
Orthomyxoviruses have segmented, single-stranded, negative-sense RNA genomes. Influenza A and B viruses have eight RNA segments that encode at least 10 proteins ( Table 34.1 ): hemagglutinin (HA), neuraminidase (NA), three polymerase proteins (PA, PB1, and PB2), nucleoprotein (NP), nonstructural protein 1 (NS1), nuclear export protein (NEP), and matrix proteins (M1 and M2 in influenza A; M1 and BM2 in influenza B). Additional open reading frames in several gene segments encode proteins such as PB1-F2 and PA-X. The protective antibody response is directed at the two major surface glycoproteins, HA and NA. Influenza A viruses exhibit both antigenic drift and antigenic shift, , , whereas influenza B viruses show only antigenic drift. Antigenic drift is caused by point mutations in the HA and NA genes that are driven by immune pressure and the infidelity inherent in the replication of RNA genomes by an RNA-dependent RNA polymerase that lacks proof-reading function. Although not subject to antigenic change, other genes also undergo mutational drift. The rate of drift can vary among genes and viral subtypes. Antigenic shift, however, requires complete replacement of one or both of the surface glycoprotein genes and is a consequence of the segmented genome of influenza viruses. During mixed infections, gene segments from two parent viruses can reassort to generate a virus with a novel gene constellation. , New antigenic changes, occurring by either mechanism, allow the virus to overcome existing immunity in previously infected and/or vaccinated hosts.
| RNA | Gene Products | Functions |
|---|---|---|
| 1 | PB2 | Viral polymerase component, binds to cellular 5′ host premessenger RNA cap |
| 2 | PB1 | Viral polymerase component with RNA transcription and replication activities |
| PB1-F2 | Viral protein produced by overlapping reading frame with proapoptotic functions | |
| 3 | PA | Viral polymerase component with RNA and DNA endonuclease activity |
| PA-X | Viral protein produced by frameshift modulates host response to infection | |
| 4 | HA | Virion surface attachment and fusion glycoprotein; major antigenic target |
| 5 | NP | Major component of ribonucleoprotein and type-specific antigen |
| 6 | NA | Virion surface glycoprotein with sialic acid cleavage activity, major antigenic target |
| NB | Glycoprotein membrane ion channel found only in type B influenza | |
| 7 | M1 | Membrane matrix protein and type-specific antigen |
| M2 | Nonglycosylated membrane ion channel, found only in type A influenza | |
| 8 | NS1 | Viral nonstructural protein that modulates cellular host responses |
| NS2/NEP | Nonstructural protein involved in nuclear export of viral RNA |
The pathology caused by influenza virus has been reviewed extensively. , , Epithelial cells in the upper airway are most likely the primary site of infection and infection is usually restricted to the upper respiratory tract and trachea with bronchitis and tracheitis, although lung involvement may occur during severe influenza. Otitis media is common in children. Viremia has been reported only sporadically , amid numerous negative reports. Influenza B demonstrates pathology similar to that of type A and is common in children. The amount of virus present probably determines the extent and severity of symptoms. ,
The most precise diagnosis of influenza is obtained by reverse transcriptase–polymerase chain reaction (RT-PCR) or by virus isolation in embryonated eggs or in cell cultures of nasopharyngeal or tracheal aspirates. Serologic diagnosis can be made by demonstrating fourfold rises in hemagglutination-inhibiting (HAI) antibodies.
The segmented genome of orthomyxoviruses that permits genetic reassortment has been employed to generate vaccine strains containing the genes for the HA and NA of newly emergent wild-type (wt) viruses, while retaining other genes from attenuated strains though the incorporation of one or more internal protein genes from wild-type viruses that have been proposed and investigated in preclinical models to improve the efficacy of LAIV. , Attenuated master donor strains must be shown to not cause significant illness in humans and to reproducibly pass the property of attenuation on to reassortants through genes other than the HA and NA. Approaches to developing live attenuated vaccines for influenza include host-range mutants temperature-sensitive (ts) mutants, cold-adapted (ca) mutants, NS1 deletion mutants, M2 cytoplasmic tail mutants, replication-deficient viruses, genetic rearrangements (reviewed in ), and codon pair deoptimized influenza viruses, but this chapter will focus on the cold-adapted LAIV that is licensed for prevention of seasonal influenza in the United States and several other countries (see below).
The ca A/Ann Arbor/6/60 (H2N2) (A/AA ca) and ca B/Ann Arbor/1/66 (B/AA ca) viruses that are the backbones of the LAIV licensed in the United States were developed at the University of Michigan. , The A/AA ca virus was derived in primary chick kidney (PCK) cells from a virus originally isolated from an ill child. This virus was grown at successively lower temperatures in PCK cells followed by seven serial plaque-to-plaque purifications. This virus had the following three properties: ts, which implies that the vaccine virus replication was reduced at the higher temperature (39°C); ca, which implies that the virus grew well at a reduced temperature (25°C) compared with permissive temperature (33°C); and attenuation (att), which implies attenuated behavior in ferrets with reduced clinical signs of temperature elevation and/or weight loss and absence of detectable virus in the lungs. The B/AA ca vaccine donor strain was developed in the same manner, although it took fewer intermediate passages to achieve the final ca variant for type B virus than for type A. The wt B/AA/1/66 influenza virus was restricted for growth at 36°C at the time of isolation, and therefore the ts phenotype is defined by a reduction in replication titer at 37°C compared with the achievable titer at 25°C or 33°C. , , Changes in the nucleotide sequence for all eight gene segments of the attenuated A/AA ca virus, compared with the nucleotide sequence of its wt counterpart, have been identified. Four major loci at base positions 1195 and 1766 of the PB1 gene, 821 of the PB2 gene, and 146 of the NP gene were identified as determinants of the ts phenotype by reverse genetics. Position 2005 of the PB1 gene was also found to contribute to the ts phenotype. , The ts, att, and ca phenotypes of the B/AA ca donor strain are also conferred by multiple genes: PA and NP for ts; PA, NP, and M for att; and PA, NP, and PB2 for ca. The concept underlying the use of ts viruses as live attenuated respiratory virus vaccines is that the vaccine virus will replicate in the cooler upper respiratory tract and induce immunity but will be restricted in replication and will therefore be attenuated in the warmer, core body temperature of the lower respiratory tract.
The reassortant vaccine viruses included in LAIV were previously generated by classical reassortment, achieved by coinfection with vaccine donor viruses and wt influenza viruses, but they are now derived using plasmid-based reverse genetics as discussed later ( Fig. 34.1 ). The resulting recombinant reassortant virus contains six internal gene segments of the vaccine strain combined with the two antigen-encoding gene segments, HA and NA, and it is referred to as a 6 : 2 reassortant. Numerous reassortants have been made using the A/AA ca virus and B/AA ca donor viruses, and, in all cases, attenuation, antigenicity, and genetic stability have remained unaltered and consistent. , , Influenza A and B ca donor strains were also developed for a LAIV in Russia. The current Russian influenza A donor strain, A/Leningrad/134/47/57, contains changes in at least one of the polymerase genes as well as in the HA, NA, NP, and M genes. ,
![Fig. 34.1, (A) The eight-plasmid reverse-genetics system to generate recombinant live attenuated influenza vaccines. Six plasmids encoding the internal protein genes of the attenuated donor virus (three polymerase proteins [PA, PB1, and PB2], nucleoprotein [NP], nonstructural protein [NS], and matrix protein [M]) are mixed with two plasmids encoding the hemagglutinin (HA) and neuraminidase (NA) genes of the circulating influenza virus. Qualified cells are transfected with the plasmids, and the attenuated reassortant virus is isolated. (B) Generation of live attenuated influenza vaccine viruses with the six internal protein genes from the attenuated donor viruses (the five major attenuating mutations for the A/AA cold-adapted [ca] donor virus are indicated by asterisks), and the HA and NA genes from the circulating influenza virus by classical reassortment. The 6 : 2 reassortants generated by this method are selected in the presence of antiserum specific for the HA and NA of the attenuated donor virus. (Modified from 315 ) Fig. 34.1, (A) The eight-plasmid reverse-genetics system to generate recombinant live attenuated influenza vaccines. Six plasmids encoding the internal protein genes of the attenuated donor virus (three polymerase proteins [PA, PB1, and PB2], nucleoprotein [NP], nonstructural protein [NS], and matrix protein [M]) are mixed with two plasmids encoding the hemagglutinin (HA) and neuraminidase (NA) genes of the circulating influenza virus. Qualified cells are transfected with the plasmids, and the attenuated reassortant virus is isolated. (B) Generation of live attenuated influenza vaccine viruses with the six internal protein genes from the attenuated donor viruses (the five major attenuating mutations for the A/AA cold-adapted [ca] donor virus are indicated by asterisks), and the HA and NA genes from the circulating influenza virus by classical reassortment. The 6 : 2 reassortants generated by this method are selected in the presence of antiserum specific for the HA and NA of the attenuated donor virus. (Modified from 315 )](https://storage.googleapis.com/dl.dentistrykey.com/clinical/InfluenzaVaccineLive/0_3s20B9780323790581000347.jpg)
The influenza virus genome comprises eight negative-sense virion (v) RNA segments that cannot function as messenger RNAs (mRNAs) to produce proteins; to initiate replication of the viral genome, viral polymerase proteins carried in with the virion must transcribe and produce mRNAs from each viral gene segment. Several systems have been developed that allow recombinant influenza viruses to be produced entirely from cloned plasmid DNAs. Hoffman and colleagues further refined these techniques and developed an eight-plasmid system. In this method, each viral complementary (c) DNA is inserted into a dual-promoter bidirectional plasmid where the viral gene is flanked by RNA polymerase I (pol I) and RNA polymerase II (pol II) promoters. Thus, eight negative-sense vRNAs and positive-sense mRNAs are generated from the eight plasmids.
This system is now used routinely to generate the seed virus for the AA ca seasonal LAIV. The HA and NA gene segments of the predicted epidemic strain are cotransfected into a cell with plasmid DNAs encoding the other six gene segments of the vaccine donor strain such as A/AA ca (see Fig. 34.1A ). The resulting recombinant virus contains six internal gene segments of the vaccine strain, combined with the two gene segments encoding the surface glycoproteins, HA and NA. Another application of reverse genetics is exemplified by the construction of live attenuated pandemic vaccine viruses by plasmid rescue. , Virulence determinants, such as the polybasic cleavage signal between the HA1 and HA2 subunits of the H5 HA, can be modified or removed by recombinant DNA techniques. Plasmid rescue techniques have advanced basic research on influenza virus as well as vaccine development.
LAIVs based on the A/AA ca and B/AA ca master donor strains were first licensed in the United States in 2003 77 as a trivalent vaccine. The need for a quadrivalent seasonal influenza vaccine to provide coverage against the two lineages of influenza B that circulate in the human population was a matter of debate for several years. The influenza B epidemic strain was difficult to predict, and there was often a mismatch between the vaccine strain and the circulating influenza B virus. For example, between 2001 and 2010, the influenza B component of the vaccine was of the correct lineage in five out of the 10 influenza seasons. Studies by the CDC determined that production of quadrivalent seasonal influenza vaccines would be possible, and would have added benefit in protection against influenza B disease. The clinical trials conducted with the trivalent formulation of LAIV also supported licensure of a quadrivalent LAIV (Q/LAIV), because both vaccines were manufactured using the same process, the dose of each of the component strains is the same, and both formulations are administered by the same route, in the same diluent. In addition to the clinical experience with trivalent LAIV, bridging noninferiority clinical trials were conducted in support of licensure of Q/LAIV. , Q/LAIV had a similar safety profile to the trivalent LAIV in adults and children and was shown to be noninferior to trivalent LAIV in terms of immunogenicity (see “Noninferiority Studies to Support Licensure of Quadrivalent LAIV” below). In early 2012, Q/LAIV was approved by the U.S. Food and Drug Administration (FDA). Q/LAIV, marketed in the United States as FluMist Quadrivalent, was available for the first time in the 2013–2014 influenza season, and contains two influenza B components, representative of both the B/Victoria and B/Yamagata lineages. Q/LAIV contains 10 6.5 –10 7.5 focus forming units (FFU) of the four component viruses and is administered in the same manner and volume as the trivalent LAIV. Q/LAIV has now replaced trivalent LAIV. Q/LAIV is manufactured and marketed by MedImmune in the United States. It is also licensed in Canada, the United Kingdom, Austria, Finland, Germany, Norway, Spain, Sweden, and Israel. In Canada, Q/LAIV is marketed as FluMist Quadrivalent; elsewhere it is marketed under the trade name Fluenz Tetra.
LAIV is sprayed into the nose using a simple syringe-like device that delivers a 0.1-mL volume of a large-particle aerosol into each nostril for a total volume of 0.2 mL ( Fig. 34.2 ). Vaccination of children requires minimal cooperation. The device is easy to use, and the vaccine is readily accepted and preferable to many over a parenteral injection by needle and syringe. Studies demonstrated self-administration of LAIV was successful, and may be an option for situations where mass immunization is necessary. ,
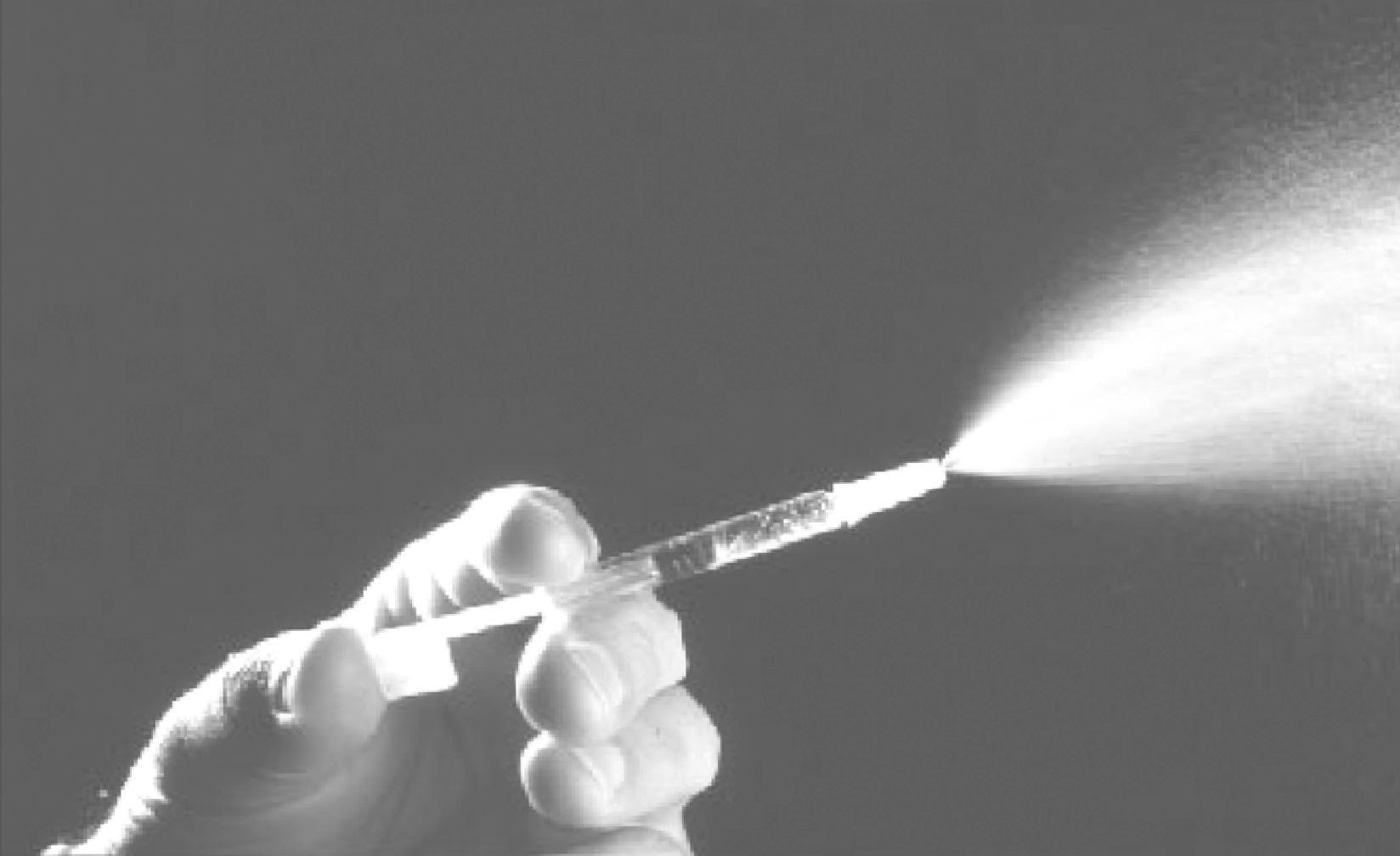
The component influenza virus strains in LAIV are updated each year as recommended by the WHO and national health authorities. Monovalent bulk vaccine is produced for each reassortant 6:2 vaccine strain in specific pathogen-free hen’s eggs. The allantoic fluids are clarified by centrifugation and stabilized by the addition of stabilizing buffer: each 0.2-mL dose contains 0.188 mg/dose monosodium glutamate, 2.00 mg/dose hydrolyzed porcine gelatin, 2.42 mg/dose arginine, 13.68 mg/dose sucrose, 2.26 mg/dose dibasic potassium phosphate, and 0.96 mg/dose monobasic potassium phosphate. Each dose contains residual amounts of ovalbumin (<0.24 µg/dose) and may also contain residual amounts of gentamicin sulfate (<0.015 µg/mL) and ethylenediaminetetraacetic acid (EDTA) (<0.37 µg/dose). It is free of preservatives and is produced in specific pathogen-free hen’s eggs. Monovalent bulk preparations of each of the four strains in the formulation are combined along with the stabilizing buffer to achieve a titer of 106.5–107.5 FFU/dose and filled into the sprayer device. FluMist Quadrivalent is a refrigerator-stable (i.e., 2–8°C) formulation of Q/LAIV. The shelf life of Q/LAIV is 18 weeks.
LAIV strains are genetically and phenotypically stable during the vaccine manufacturing process and after replication in humans.
The low-fidelity RNA-dependent RNA polymerase of influenza viruses results in an estimated error rate of 10 −4 –10 −5 misincorporations per nucleotide position per genome replication. Because the manufacturing process of LAIV requires sequential amplification of virus seeds in embryonated hen’s eggs, it is possible that mutations that alter the biological properties of the vaccine virus could accumulate, possibly resulting in loss of attenuation or change in antigenicity. Analysis of the genetic sequence of monovalent ca LAIV revealed a high level of genetic homogeneity in vaccine viruses at several stages of the manufacturing process. A total of six nucleotide changes resulting in amino acid substitutions were identified in the internal protein genes of four influenza A and five influenza B vaccine viruses compared with their respective ca donor viruses, and these changes were generally found to occur in the manufacturing steps upstream of amplification in eggs—that is, during reassortment and clonal isolation. None of the changes occurred at positions that confer the ts and att phenotypes of the cold-adapted viruses. , A total of 14 nucleotide changes were observed in the HA and NA genes of four influenza A and five influenza B vaccine viruses compared with their respective wt parent viruses (one to two changes per virus for six of the viruses), but the antigenic properties of the HA were not affected.
During the early evaluation of LAIV, the stability of the ts and ca phenotypes were monitored as surrogates for genetic stability. As sequencing technology advanced and the genetic bases for the phenotypes of the LAIV donor virus were determined, genetic stability and phenotypic stability were evaluated in virus isolates from vaccine recipients. The ts and ca phenotypes of several monovalent AA ca-based LAIV were found to be stable after replication in humans. In one study, an AA ca reassortant virus was recovered that had partially lost its ts phenotype after replication in children, but the virus retained the att phenotype in ferrets.
Genetic stability of the trivalent ca influenza virus vaccine, containing ca influenza A H1N1, H3N2, and ca influenza B reassortant viruses, was reported by Cha and colleagues. Virus isolates from nasal and throat swab specimens obtained from 17 children after vaccination were subtyped by multiplex RT-PCR and genotyped. All viruses detected during the 2-week postvaccination period were vaccine viruses, and all maintained the 6 : 2 reassortant genotype, as well as the ca and ts phenotypes.
Two studies of the genetic and phenotypic stability of trivalent LAIV in children were undertaken in a daycare setting. In one study of children younger than 3 years, it was found that the ts and ca phenotypes were preserved in all recovered viruses tested ( n = 135). One placebo recipient in this study became infected with an influenza B virus that was a component of the LAIV. This virus retained the ts, ca, and att phenotypes of the vaccine strain and had the same genetic sequence as the influenza B vaccine virus isolated from a vaccine recipient in the same playgroup. In another study, Buonagurio and colleagues examined 56 independent isolates obtained from children after vaccination. Between one and seven nucleotide changes per virus were observed in 80% of isolates compared with the vaccine viruses, but none resulted in changes in the ts, ca, and att phenotypes.
In the United States, LAIV is approved for use in healthy adults and children from 2 to 49 years of age including those with a history of egg allergy. Persons with a history of severe egg allergy (including angioedema and respiratory distress) should receive their vaccine in a medical setting under supervision of a healthcare provider. Similar recommendations are in place in the United Kingdom, where it is advised that children with an egg allergy—including those with previous anaphylaxis to egg—can be safely vaccinated with LAIV anywhere. The exception is children who have been previously admitted to intensive care following severe egg anaphylaxis. This group should receive the vaccine in a hospital setting. In the United States, LAIV is contraindicated in children 2–4 years of age who have received a diagnosis of asthma; and precaution is required for those older than 4 years of age. In the U.K. JCVI advice (2019) is that children on inhaled corticosteroids can be safely given LAIV, but that it is not recommended for those experiencing an acute exacerbation of symptoms. For children on regular oral steroids or requiring intensive care for acute asthma, data are limited, so LAIV should be given on the advice of a specialist. If they are unable to receive LAIV, they should be prescribed a suitable inactivated vaccine because they are at elevated risk of severe disease.
Numerous studies have been performed before and after licensure to evaluate the safety of LAIV in both children and adults. These studies assessed the risk of fever, runny nose and congestion, cough, bronchitis, headache, fatigue, vomiting, and serious adverse events (SAEs) that resulted in hospitalization. LAIV is generally well tolerated in adults, and only runny nose and congestion have been observed at a greater frequency in LAIV recipients than in trivalent inactivated influenza vaccine (TIV) or placebo recipients. Children had a significant increase in runny nose, congestion, and fever after one dose of LAIV, but these symptoms did not occur as often following the second dose. Some studies also reported an increased rate of SAEs such as wheezing, upper respiratory tract infections, and increased hospitalization, that are likely to have resulted from vaccination, in children who received LAIV compared with placebo. , , Although the rates of some of these events were not statistically different between vaccinated children and placebo recipients, a post hoc analysis of one study determined that the reported SAEs occurred exclusively in children younger than 24 months of age. The vaccine is known to be safe in 2–17-year-old children, and is not licensed for use in children younger than2 years of age.
Postlicensure analyses of all adverse events (AEs) reported to the U.S. Vaccine Adverse Event Reporting System (VAERS) during the 2003–2004 and the 2004–2005 influenza seasons and for the seasons from 2005 to 2013 have been reported for trivalent LAIV. In the first two postlicensure seasons, approximately 2,500,000 persons received trivalent LAIV, and VAERS received 460 AE reports. No fatalities were reported. There were seven reports of possible anaphylaxis, two reports of Guillain–Barré syndrome (GBS), one report of Bell’s palsy, and eight reports of asthma exacerbation among persons with a prior history of asthma. Events in persons for whom the vaccine was not indicated accounted for 73 reports (16%). Between 2005 and 2013, approximately 50 million doses of trivalent LAIV were distributed for use in adults and children. Separate reviews of reports to VAERS were conducted for adults and children. Limited safety data are available, particularly for adults. Over eight influenza seasons, 1207 events were reported to VAERS for adults – in the approved age range of 18–49 years, of which 8.9% were serious. Nine deaths were reported. Deaths and nonfatal SAEs were only reported in the U.S. Department of Defense population, which includes military personnel and Department of Defense beneficiaries treated at military clinics or hospitals. The most common categories of reported serious nonfatal events were neurological (39%), cardiovascular (14%), or other noninfectious conditions (19%). A higher rate of GBS and cardiovascular events were reported in the Department of Defense versus the civilian population. This finding is the subject of further investigation. The most commonly reported AEs were not serious in nature: headache, fever, and nausea. There were 207 reports of the administration of expired vaccine, 155 reports of administration of vaccine to adults 50 years of age and older, and some reports of inadvertent administration to pregnant women, none of which resulted in a SAE. Over the seven influenza seasons between 2005 and 2012, there were 2619 AE reports to VAERS associated with administration of trivalent LAIV in children, of which 7.5% were serious, and there were five deaths. Four of the five deaths were in children with chronic underlying conditions. An increase in reported events was observed from 2005 to 2011, mainly attributable to an increase in reports of nonserious AEs. SAE reports for 2–4-year-old children peaked in the 2007–2008 influenza season at 25% (15 of 60 vaccinees); this was the first influenza season following approval for use of LAIV in this age group. The two most frequently reported categories of nonfatal SAEs were neurologic (29.2%) and respiratory events (22.4%). The most frequent neurologic diagnoses were seizures and GBS, and the most frequent respiratory diagnoses were pneumonia and asthma or reactive airway disease. The most commonly reported vaccine administration error was administration of expired vaccine, which was not associated with AEs. For both pediatric and adult populations, the reviews of reports to VAERS for trivalent LAIV were reassuringly consistent with prelicensure safety analyses and postlicensure safety data from the first two influenza seasons after FDA approval. Because VAERS is a passive surveillance system it is not possible to assess the reports for causal associations between vaccines and AEs. ,
A similar analysis was conducted for Q/LAIV for the first influenza season following licensure, 2013–2014, when approximately 12.7 million doses of Q/LAIV were distributed in the United States. VAERS received 779 AE reports for individuals in the approved age range of 2–49 years, 95% of which were nonserious. In children, the most commonly reported AE was administration of expired vaccine (42%), followed by fever (13%) and cough (8%). In general, the safety profile of Q/LAIV was similar to that of trivalent LAIV. The most commonly reported AEs in adults were headache (18%), administration of expired vaccine (15%), and exposure during pregnancy (12%). One death, resulting from complications of a brain tumor, was reported in a child, and no deaths were reported for adults. Neurologic conditions were the most commonly reported nonfatal serious events in both children and adults. Two cases of GBS were reported in children, and none in adults; disproportional reporting of this AE compared to other vaccines was not observed. There were 100 reports of Q/LAIV being administered to individuals outside of the approved age range; 28 children younger than the age of 2 years and 72 adults older than the age of 49 years received Q/LAIV. Seventy-five percent of these children and 87.5% of the adults did not experience AEs. There were 49 reports where the age of the vaccinee was unknown. As was the case with the postlicensure VAERS reporting for the trivalent LAIV, the most commonly reported vaccine administration error was administration of expired vaccine. Administration of expired vaccine may be related to the change in LAIV formulation from frozen to refrigerator-stable in 2007 that resulted in a shorter shelf life of the vaccine, or to the relatively shorter shelf life for LAIV compared to IIV. This error would be expected to result in lack of protection afforded by the vaccine, rather than adverse reactions to it. The administration of expired vaccine, administration to individuals outside the approved age group, and administration to individuals in whom LAIV is contraindicated (e.g., pregnant women) underscore the need for continued education and training of healthcare workers. In summary, none of the postlicensure reviews of events reported to VAERS for trivalent or quadrivalent LAIV identified any unexpected serious risks when the vaccine was used as approved.
More recently, in the United Kingdom, in a phase 4 intervention study, 779 children 2–18 years of age with known egg allergy, of whom 445 had physician diagnosed asthma, were immunized with LAIV. Two hundred and seventy of the cohort reported previous anaphylaxis to egg. No systemic adverse reactions were reported in the cohort. Nine reported mild symptoms consistent with IgE-mediated allergic reaction. Sixty-two reported lower respiratory tract symptoms within 72 hours, though none required hospitalization. The authors concluded that there was a low risk of systemic allergic reactions and that the vaccine was well tolerated in those with well controlled asthma. Further safety studies were done in these groups in the United Kingdom to support the introduction of the pediatric influenza vaccine program, showing that LAIV appears to be also well tolerated in most children with asthma including in those whose disease is severe or poorly controlled in a follow-up study of 478 children 2–18 years of age including 122 with severe asthma.
LAIV strains replicate in the nasopharynx of the recipient and are shed in the respiratory secretions. Data in the published literature failed to detect transmission of vaccine viruses derived from the passaged ca master donor viruses in multiple age groups and settings. , , , In an evaluation of direct transmissibility in a daycare setting in children 8–36 months of age, 80% of the 98 FluMist recipients shed one or more vaccine strains, with a mean shedding duration of 7.6 days. One possible transmission event was observed among the 99 placebo recipients and was confirmed by phenotype and genotype analysis. The safety profile for this child after transmission was similar to that for the other children in the study who received vaccine or placebo; the child was not ill and did not experience an SAE. An open-label, single-arm, multicenter Phase II study reported by Mallory and colleagues demonstrated that at least one component of seasonal LAIV was shed by 79% of children between 6 and 59 months of age. In this study, virus shedding was most frequently seen in children 6–23 months of age, and it occurred mostly on postvaccination days 1–11, with shedding beyond day 11 detected almost exclusively in the younger age group. Peak titers of vaccine virus were detected on postvaccination day 2, and mean peak titers were generally less than 10 3 TCID 50 (median tissue culture infective dose)/mL.
In the year after initial FDA approval, the clinical use of LAIV was restricted at some institutions because of a theoretical concern that live attenuated virus might transmit from vaccinated hospital employees to unvaccinated patient contacts. After several years of experience, this concern proved to be unfounded, and person-to-person transmission of LAIV has not been a problem. Izurieta and colleagues summarized vaccine AE reporting regarding LAIV, and no instances of recognized transmission were identified. In the one case in which transmission was suspected, and in which viral isolates were obtained, it turned out that a wt influenza virus, not vaccine virus, was involved in the transmission. The Advisory Committee on Immunization Practices (ACIP) to the CDC modified its initial recommendation to reflect the practicalities of vaccine usage in hospitals. The only concern that remains now is a theoretical one. Employees who are vaccinated should not have contact within 7 days with immunosuppressed patients who are in reverse isolation (such as bone marrow transplant recipients). All other situations are considered acceptable for using LAIV.
Replication of LAIV in the upper respiratory tract induces both antibody and cellular immune responses. This section addresses the induction of these immune responses in healthy adults and children.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here