Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pregnancy produces dramatic changes in systemic hemodynamics, leading to alterations in total circulating blood volume, cardiac output, and systemic vascular resistance. The kidney itself undergoes marked changes during gestation, including alterations in kidney size, glomerular hemodynamics, and tubular function. These adaptations are critical for favorable pregnancy outcomes. Although much of our knowledge of kidney anatomic and physiologic changes in human pregnancy is extrapolated from animal models and small studies in healthy pregnant women, understanding the adaptive changes that occur during pregnancy is crucial for differentiating healthy from complicated pregnancies.
Kidney size increases during pregnancy from a combination of increased kidney weight and dilatation of the urinary collecting system. In longitudinal studies of kidney size measured by ultrasound, kidney length increases by approximately 1 cm. Dilation of the collecting system is observed as early as the third month of gestation. The right renal pelvis is most often affected. Although traditionally believed to be the result of mechanical compression by the gravid uterus, dilatation occurs well before the uterus is large enough to cause obstruction, arguing for a hormonal contribution as well. Structural changes generally resolve by 12 weeks postpartum. Persistent hydronephrosis beyond 12 to 16 weeks postpartum suggests underlying mechanical obstruction that requires further investigation ( Box 47.1 ).
Increase in kidney size by approximately 1 cm
Dilation of the urinary collecting system; more prominent on the right
10- to 20-fold increase in aldosterone
8-fold increase in renin
4-fold increase in angiotensin
Resistance to pressor effect of angiotensin
Decreased set point for ADH release
Increased ANP release
Increased production of prostacyclin and nitric oxide
Increased cardiac output by 40%–50%
Increased plasma volume by 40%–50%
Drop in SBP by ≈9 mm and DBP by 17 mm Hg (prominent in second trimester)
Increase in GFR and RPF by 50% above normal
Decrease in BUN (to <13 mg/dL) and serum creatinine (to 0.4–0.5 mg/dL)
Increase in total body water by 6–8 L
Net retention of 900 mEq of sodium
Decrease in plasma osmolality by 10 mOsm/L
Decrease in serum sodium by 4–5 mEq/L
Mild respiratory alkalosis with compensatory metabolic acidosis (bicarb of 18–22 mEq/L)
Decrease in serum uric acid levels (to 2.5–4 mg/dL)
Glucosuria irrespective of blood glucose levels
Systemic adaptations to normal pregnancy begin soon after conception, with the development of a low-resistance placental circulation. Changes in maternal systemic vascular resistance and cardiac output can be detected as early as 6 weeks’ gestation. Pregnancy leads to systemic vasodilation, increased cardiac output, and plasma volume expansion. Despite the increase in blood volume and cardiac output, systemic blood pressure (BP) decreases over the first half of gestation and reaches a nadir between 18 and 24 weeks of gestation. The mechanisms leading to systemic vasodilation in healthy human pregnancy are not fully understood but likely reflect a balance between vasodilatory and vasoconstrictive mediators, such as the corpus luteum-derived hormone relaxin, nitric oxide (NO), and alterations of the renin-angiotensin-aldosterone system (RAAS). Systemic vasodilation results in venous pooling that triggers volume restorative responses, including increased RAAS activity and a lowered set point for antidiuretic hormone (ADH) release. These changes lead to progressive volume expansion throughout gestation. Despite increases in RAAS activity by 2- to 10-fold, healthy pregnant women are resistant to the vasopressor effects of angiotensin II and systemic BP declines.
Similar to the systemic hemodynamic changes seen in healthy pregnancies, renal vascular resistance falls, leading to increased renal plasma flow (RPF) and glomerular filtration rate (GFR). Existing studies in human pregnancy physiology are challenging to interpret because of variations in GFR measurement techniques. In general, GFR and RPF increase by approximately 40%. Increased GFR is noted as early as 4 weeks’ gestation, reaches peak level during the first half of pregnancy, and remains elevated until term ( Fig. 47.1 ).
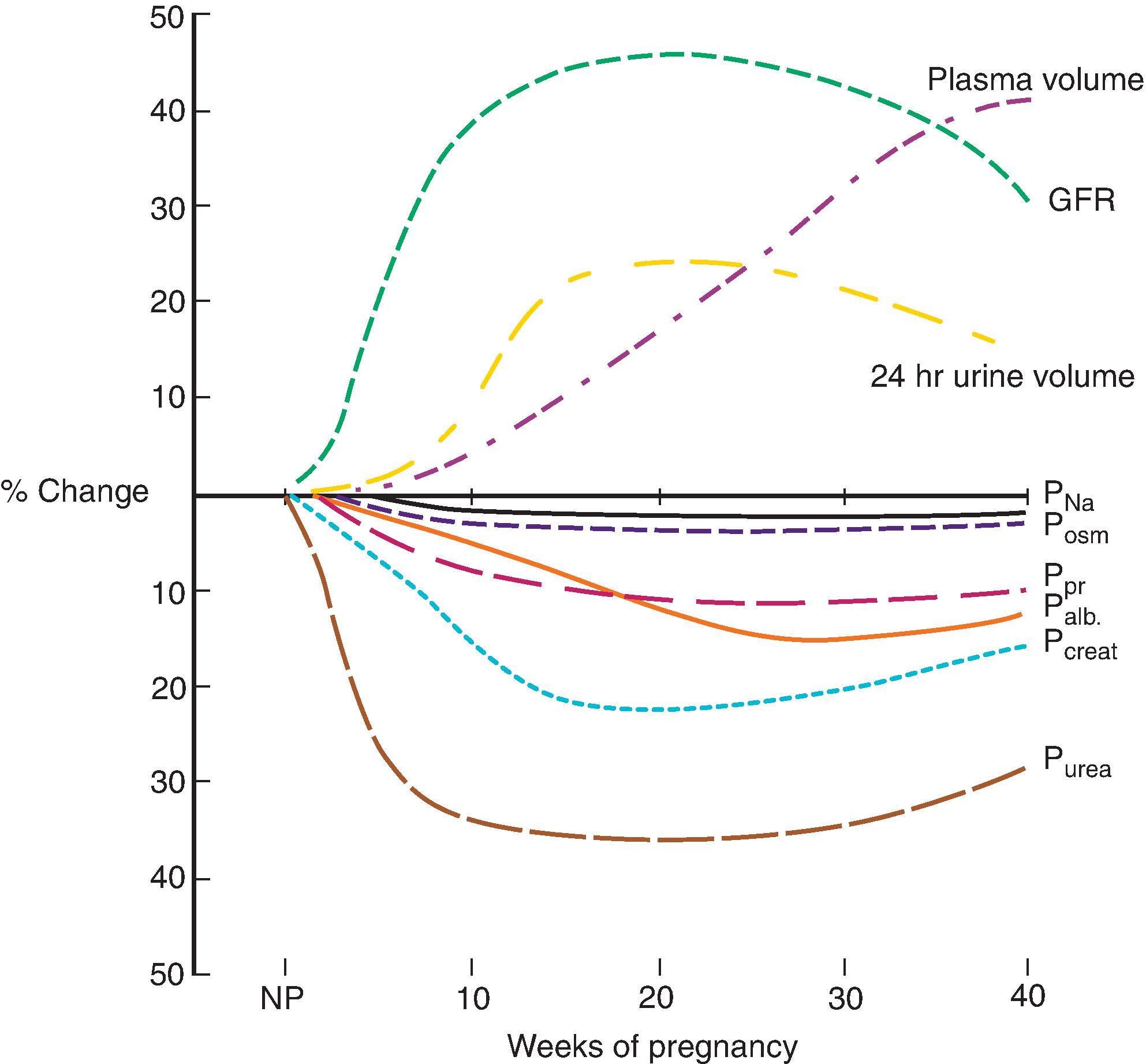
Total body water increases during pregnancy by 6 to 8 L, of which 4 to 6 L are extracellular. Changes in central osmostat regulation result in lower plasma osmolality (10 mOsm/L below normal), represented by a decrease in serum sodium by 4 to 5 mEq/L. Despite decreased plasma sodium concentrations, healthy pregnant women are in positive sodium balance, with a net gain of 3 to 4 mEq/day. Although normal pregnancy results in increased basal metabolic rate and acid generation, plasma pH is more alkaline because of a respiratory alkalosis mediated by elevated progesterone levels. This is accompanied by an appropriate renal metabolic adaptation with reduced serum HCO 3 levels (18 to 22 mmol/L).
In the nonpregnant state, kidneys efficiently reabsorb glucose and amino acids. In a small study of euglycemic women who displayed glycosuria, the maximal tubular reabsorption capacity for glucose was significantly decreased. The precise incidence of glycosuria in pregnancy is unclear, with extensive variability noted both between women and within individual women at different times during pregnancy. There does not appear to be a relationship between glycosuria and clinical diabetes, and the majority of women with glycosuria have normal glucose screening in pregnancy. Uric acid levels drop to 2.5 to 4 mg/dL from the combined effects of increased filtration and decreased tubular reabsorption. Uric acid levels nadir in the second trimester and gradually increase as pregnancy progresses toward term. High renal clearance of uric acid is believed to be necessary to clear the increased production that occurs with fetal growth.
Serum creatinine-based formulas are not accurate for estimating GFR in pregnancy. Both the Modification of Diet in Renal Disease (MDRD) and Chronic Kidney Disease Epidemiology (CKD-EPI) equations underestimate GFR measured by inulin clearance by approximately 40%. Creatinine clearance (CrCl) measured in a 24-hour urine collection remains one method to estimate GFR during pregnancy, although complete collection can be difficult because of urinary retention. Cystatin C levels have been studied in a variety of clinical settings; however, their use in pregnancy is not established, as cystatin C may be released by the placenta in response to ischemia. Gestational hyperfiltration and subsequent increased GFR result in decreased blood urea nitrogen (BUN) and serum creatinine levels. In a study of over 1 million pregnancies in Ontario, Canada, the mean baseline serum creatinine concentration was 0.68 mg/dL, declining by 4 weeks’ gestation, ultimately reaching a nadir of 0.53 mg/dL between 16 and 32 weeks’ gestation, and then rising to 0.72 mg/dL within a few weeks postpartum, with gradual return to mean prepregnancy concentrations by 18 weeks’ postpartum. Using the 95th percentile as a potential cutoff for abnormal serum creatinine, the authors suggest a mid-trimester creatinine of 0.7 to 0.8 mg/dL or higher is of concern in normal pregnancy and should prompt further investigation.
Routine prenatal care includes dipstick urine protein assessment at each prenatal visit. While inexpensive, the urine dipstick has high false-positive and false-negative rates. Twenty-four-hour urine protein excretion remains the gold standard for measurement of proteinuria in pregnancy, although, again, it can be difficult to obtain complete collections because of incomplete bladder emptying and urinary stasis. Assessment of the urine protein-to-creatinine ratio (UPCR) or albumin-to-creatinine ratio in spot urine specimens is probably the most practical way to follow protein excretion in pregnancy.
Urinary protein excretion remains below 200 mg/24 hr in normal pregnancy despite glomerular hyperfiltration. Most obstetric guidelines define significant protein excretion as greater than 300 mg in a 24-hour period; however, this cutoff is based on small studies. In one of the largest studies, the mean 24-hour protein excretion was near 100 mg, significantly lower than the established cutoff. As such, even low levels of proteinuria should not be attributed to gestational hyperfiltration and should prompt further evaluation.
Hypertension in pregnancy is defined as BP ≥140/90 mm Hg, measured on at least two separate occasions. Hypertensive disorders complicate up to 10% of pregnancies and are a major cause of maternal morbidity and mortality. Hypertensive disorders in pregnancy are classified into four categories: chronic hypertension, gestational hypertension, preeclampsia, and superimposed preeclampsia. Management of hypertension in pregnancy requires the clinician to balance the effects of treatment on both the mother and developing fetus.
The diagnosis of chronic hypertension is most often based on essential hypertension diagnosed before pregnancy or a BP greater than 140/90 mm Hg diagnosed before 20 weeks of gestation that does not resolve after delivery. The prevalence of chronic hypertension in pregnancy appears to be increasing because of higher pregnancy rates in women of advanced maternal age and higher rates of maternal obesity. Chronic hypertension is associated with increased risk for preeclampsia (25%), intrauterine growth restriction (IUGR; 17%), and perinatal mortality (4%), compared with the general population.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here