Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter will:
Review the pathophysiology related to fluid status and the four phases of fluid resuscitation.
Review clinical parameters available to assess fluid volume, fluid responsiveness, and fluid overload.
Discuss practical issues regarding fluid administration and removal in hospitalized and critically ill patients.
Fluid administration is one of the most frequently used therapies provided in hospitalized patients. The most common reasons for fluid administration include hypotension and shock, sepsis, hypovolemia, replacement of fluid losses, and oliguria. Prompt resuscitation of patients with hypoperfusion with intravenous fluids was shown to improve outcomes more than a decade ago. However, studies in heterogeneous populations of critically ill and those undergoing surgery have shown that only about half of hemodynamically unstable patients respond to fluid administration. In severe sepsis or septic shock, the proportion may be even lower, and recently has been estimated to be less than 40%. These findings may challenge the notion that fluid administration is a “cornerstone in the treatment of resuscitation.” When a condition of hypoperfusion has been identified, clinicians must decide whether fluids may improve tissue perfusion by enhancing stroke volume or if other approaches are required. However, fluid balance assessment and management are challenging. For many clinicians, the current approach to fluid resuscitation focuses mainly on cardiac output (CO) and blood pressure. Other clinical and laboratory parameters also can facilitate fluid balance assessment and improve decisions regarding fluid administration and/or removal. Recent studies have evaluated the role of different strategies for fluid resuscitation and have not demonstrated any survival benefit. In addition, excessive fluid administration has been associated with worse cardiopulmonary and kidney outcomes, delayed wound healing, and decreased survival. Consequently, even if an initial fluid resuscitation is required, approaches aiming for neutral and negative fluid balance or “dry” patients have been proposed over the remaining course of the hospital stay. Neutral or negative fluid balance may be obtained by conservative fluid administration, diuretics, and/or renal replacement therapy.
We review important physiologic aspects related to fluid status, the four phases of fluid resuscitation, as well as assessment of fluid volume, fluid responsiveness, and fluid overload. We also summarize the pathogenesis of fluid overload and its association with adverse outcomes and comment on practical issues regarding fluid administration, removal, and monitoring in hospitalized patients.
The desired goal of fluid resuscitation is to improve organ perfusion. To better understand how fluid resuscitation can improve organ perfusion, we have summarized its most important underlying principles. According to the Frank-Starling principle, fluid administration will increase left ventricular stroke volume (SV) only if the bolus increases the mean circulatory filling pressure (MCFP) more than the central venous pressure (CVP), and if both ventricles are on the “ascending limb” of the Frank-Starling curve. The venous system is divided into the “unstressed” and “stressed” volumes. The “unstressed volume” is the intravascular volume filling the venous system until intravascular pressure starts increasing, whereas the “stressed volume” is the volume that stretches the veins and causes intravascular pressure to increase. The MCFP is the pressure distending the vasculature (normal values, 8–10 mm Hg). The stressed venous system is the major determinant of the MCFP, which will strongly influence the venous return.
Organ blood flow corresponds to the difference between the mean arterial pressure (MAP) and the CVP. If the MAP is within an organ's autoregulatory range, then the CVP becomes the major determinant of capillary blood flow. Because the venous system has a large capacitance, an increase in blood volume only minimally changes the MCFP. However, as the distending volume increases, the diastolic compliance of the heart decreases. Therefore, with large fluid administration, the cardiac filling pressures, more importantly the CVP, increase faster than the MCFP, decreasing the gradient for venous return, and therefore blood flow. The kidney is affected particularly by high venous pressure, which increases renal subcapsular pressure and reduces renal blood flow and glomerular filtration rate. Studies in patients with chronic congestive heart failure have shown that intrarenal venous flow (IRVF) pattern seemed to correlate with renal congestion.
Total body water (TBW) represents approximately 60% of body weight and traditionally has been divided into the intracellular (40%) and extracellular spaces (20%). In the extracellular fluid, the water is distributed in different parts: 75% in the interstitium, 20% in the plasma, and 5% acting as transcellular fluid. For decades, the so-called “third space” was considered as another extravascular compartment. Therefore fluid administration was optimized to replace this “loss” in critically ill and patients undergoing major surgery, in addition to deficits because of insensible perspiration and fasting. The “third space” most probably accumulates in the interstitium because of the destruction of an integral structure of the vascular wall, the endothelial glycocalyx. The endothelial glycocalyx is a network of membrane-bound glycoproteins and proteoglycans acting as a gel-like fringe covering the intravascular side of every healthy vessel ( Table 134.1 ). The glycocalyx retains plasma proteins from moving across the endothelium, prevents leucocyte and platelet aggregation, and limits tissue edema. The glycocalyx contains many antioxidative molecules, such as superoxide dismutase, and contains many receptors that influence cellular activation with neutrophils and macrophages in response to injury. In sepsis and multiorgan failure, glycocalyx shedding increases vascular permeability and contributes to capillary leak and can cause an activation of immune cascades. The type of fluids (albumin, synthetic colloids, or crystalloids) and amount of fluid resuscitation also may have an effect on the integrity of the glycocalyx barrier. An intact endothelial glycocalyx is therefore required for the vascular barrier to function properly.
| TERM | DEFINITION |
|---|---|
| Dynamic arterial elastance (Eadyn) | Pulse pressure variation (PPV) to stroke volume variation (SVV) ratio |
| Endothelial glycocalyx | Network of membrane-bound glycoproteins and proteoglycans covering the intravascular side of every healthy vessel to retain plasma proteins from moving across the endothelium |
| Fluid bolus | Infusion of 500 mL over a maximum of 15 min without close monitoring |
| Fluid challenge | Administration of 250 mL or 3 mL/kg over 5–10 min with rapid stroke volume (SV) reassessment |
| Fluid challenge responder | Some studies have defined a fluid responder as having an increase of 10%–15% of SV or cardiac output (CO) after fluid challenge |
| Fluid overload | Some studies define this term as a percentage of cumulative fluid balance (CFB) over initial body weight above 10% |
| Mini-fluid challenge | The administration of 100 mL over 1 min with assessment of changes in SV or CO |
| Passive leg raising (PLR) maneuver | The lower limbs are elevated at 45 degrees from the 45-degree semirecumbent position, transferring approximately 300 mL of blood from the limbs and abdomen to the thorax to simulate a fluid bolus |
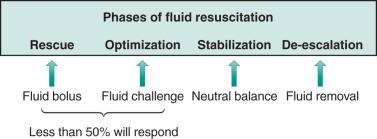
| Phases of fluid resuscitation (see Table 134.2 ) | Phases to characterize the role and timing of fluid administration or removal during hospital stay according to the clinical situation, including (1) rescue, (2) optimization, (3) stabilization, and (4) de-escalation |
As previously mentioned, most hospitalized patients will receive intravenous fluids. To characterize the role and timing of fluid administration or removal during hospital stay, four clinical phases of fluid therapy have been conceptualized ( Fig. 134.1 ), including (1) rescue, (2) optimization, (3) stabilization, and (4) deescalation (ROS-D). Rescue, or resuscitation, implies the “administration of fluid for immediate management of life-threatening conditions associated with impaired tissue perfusion.” Optimization or titration refers to the “adjustment of fluid type, rate and amount based upon context to achieve optimization of tissue perfusion.” Stabilization aims for achieving a neutral or slightly negative fluid balance to favor organ support, whereas de-escalation is defined by the “minimization of fluid administration; mobilization of extra fluid to optimize fluid balance.” The characteristics of each phase have been summarized in Table 134.2 .
| RESCUE | OPTIMIZATION | STABILIZATION | DE-ESCALATION | |
|---|---|---|---|---|
| Principles | Lifesaving | Organ rescue | Organ support | Organ recovery |
| Goals | Correct shock | Optimize and maintain tissue perfusion | Aim for zero or negative fluid balance | Mobilize fluid accumulated |
| Time (usual) | Minutes | Hours | Days to weeks | Days to weeks |
| Phenotype | Severe shock | Unstable | Stable | Recovering |
| Fluid therapy | Rapid boluses | Titrate fluid infusion/conservative use of fluid challenges | Minimal maintenance infusion only if oral intake inadequate | Oral intake if possible/avoid unnecessary IV fluids |
| Typical clinical scenario | Septic shock Major trauma |
Intraoperative GDT Burns DKA |
NPO postoperative patient “Drip and suck” management of pancreatitis |
Patient on full enteral feed in recovery phase of critical illness Recovering ATN |
| Amount | See appropriate guidelines (surviving sepsis campaign, prehospital resuscitation, trauma, burns…) | |||
Fluid boluses are used during the rescue phase, whereas fluid challenges are administered during the optimization phase. A fluid bolus typically includes the infusion of 500 mL over a maximum of 15 minutes without close monitoring, and a fluid challenge involves the administration of 250 mL or 3 mL/kg over 5 to 10 min with SV reassessment. A fluid challenge allows optimization of tissue perfusion and tests the effects of a more modest volume given more slowly to prevent inadvertent fluid overload. Some studies have defined fluid responders as an increase of 10% to 15% of SV or CO after fluid challenge. The concept of mini-fluid challenge also has been proposed and defined as the administration of 100 mL over 1 minute with assessment of changes in SV or CO. This test had a sensitivity and specificity of 95% and 78%, respectively, with an area under the receiver operating characterizing curve (AUROC) of 0.92 to predict fluid responsiveness.
The ability of crystalloids to expand the intravascular volume is limited. In healthy volunteers, only 15% of a crystalloid bolus remained in the intravascular space after 3 hours. In septic patients, less than 5% of a crystalloid bolus remains in the intravascular space after an hour. In the stabilization phase, fluid therapy is used for ongoing maintenance to replace normal fluid losses (i.e., renal, gastrointestinal, insensible) or ongoing losses because of unresolved pathology. Patients may experience stabilization and de-escalation and then move back to the rescue or optimization phases because of a new clinical event, and many hospitalized patients will not require fluid boluses.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here