Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Improving truncal stability is ranked as one of the highest priorities for functional improvement by individuals with paraplegia and tetraplegia alike ( ), and within 10 years of thoraco-lumbar-level spinal cord injuries, individuals would prefer to walk again over eliminating pain or improving other bodily functions ( ). Similarly, improving walking and upper body/trunk strength are identified as very important to more than half of individuals with paraplegia, with walking as the first or second choice in nearly 60% of the population ( ). Surgically implanted motor system neuroprostheses can address these high priority issues and provide options for seated balance and upright, bipedal mobility to individuals with paralysis resulting from central nervous system (CNS) trauma, disease or dysfunction such as spinal cord injury (SCI), stroke, or multiple sclerosis (MS).
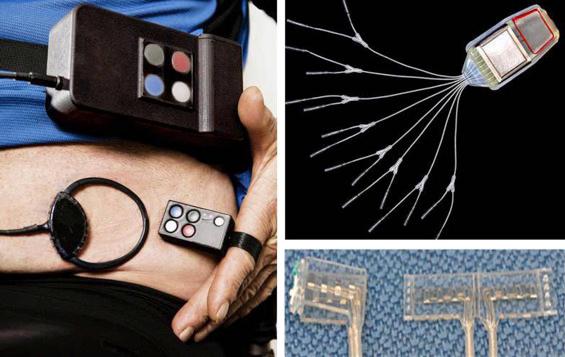
Surgically implanted neuroprostheses for exercise, standing, and transfers consisting of multichannel implanted pulse generators and stimulating electrodes ( Fig. 97.1 ) have been well accepted by individuals with low-cervical and thoracic-level injuries ( ); deployed clinically in multicenter trials ( ), they enabled recipients to release one hand from a support device to reach objects overhead, and allowed their users to stand unassisted an average of 10 min. Such systems exhibit good reliability with robust operation and functional outcomes, and continued patterns of usage on follow-up averaging six years postimplantation ( ). Implanted neuroprostheses can also allow individuals with motor complete mid- to low-thoracic injuries to step household distances independently in the vicinity of the wheelchair ( ), and enable individuals with incomplete SCI to walk faster and farther than can be maximally achieved by volitional motion alone ( ). Triggering stepping motions with physical sensors such as accelerometers placed on the body (shoes or belt), walker, or crutch tips can be customized for a given individual and automate progress through the stimulated gait cycle ( ). Furthermore, monitoring the voluntary activities of intact or partially paralyzed muscles can make walking more intuitive by integrating the actions of the neuroprosthesis with the remaining volitional substrate, allowing natural variations in step length and walking speed ( ).
The simplest orthotic intervention for ankle dorsi/plantarflexion weakness is an ankle-foot orthosis (AFO), which provides significantly beneficial effects on balance and walking ability in individuals with poststroke hemiplegia ( ) by passively improving toe clearance during swing and passively resisting dorsiflexion to increase stability and retard tibial advancement during stance ( ). Peroneal nerve stimulation elicits active contractions of the dorsiflexor muscles and is an alternative to the AFO to correct foot drop. Many commercial devices employing surface stimulation have been introduced, most notably the Odstock Foot Drop Stimulator (Odstock Medical Ltd, Salisbury, UK), WalkAide (Innovative Neurotronics, Austin, TX), and NESS L300 (Bioness Inc., Valencia, CA). Peroneal nerve stimulation is equally effective as an AFO at significantly improving walking after gait training, with no significant differences observed between the two interventions ( ). However, long term use of stimulation may improve walking endurance and functional ambulation ( ), and significantly more users prefer peroneal stimulation over AFOs ( ). In addition, peroneal nerve stimulation appears to be superior to AFOs for avoiding obstacles normally encountered during community ambulation in users with relatively low leg muscle strength ( ). Two fully implanted stimulators for foot drop were introduced in Europe, the ActiGait (Ottobock, Vienna, Austria) and STIMuSTEP (Finetech Medical Ltd., Hertfordshire, UK) systems. These devices are designed to balance dorsiflexion and in/eversion by delivering multiple independent channels of stimulation via a single epineural cuff, through electrodes inserted subepineurally to the deep and superficial branches of the peroneal nerve. Both systems have been shown to improve walking speed and distance, are well accepted by stroke survivors ( ), and have been used in patients with MS, traumatic brain injury, and incomplete SCI ( ).
Multichannel surface stimulation can address proximal muscle weakness at the knee and hip to achieve higher functional outcomes in individuals with hemiplegia than peroneal stimulators alone ( ), and restore or improve standing and walking in individuals with complete or incomplete paraplegia. Although walking speeds and distances were limited, a multichannel surface stimulation system (Parastep, Signetics, Inc.) received FDA approval for short distance ambulation after thoracic-level SCI through appropriately timed activation of the hip and knee extensors and knee/hip flexors via a withdrawal reflex. After training 32 sessions, users could walk at least 10 m with few achieving distances as far as one mile. Benefits included increased blood flow and work capacity, reduced spasticity, and other physiological benefits ( ). Their relatively high average oxygen consumption (approaching seven metabolic equivalents) and cardiovascular stress make such systems good options for maintaining physical fitness, rather than mobility ( ). Although used by more than 400 individuals worldwide, Parastep is no longer commercially available.
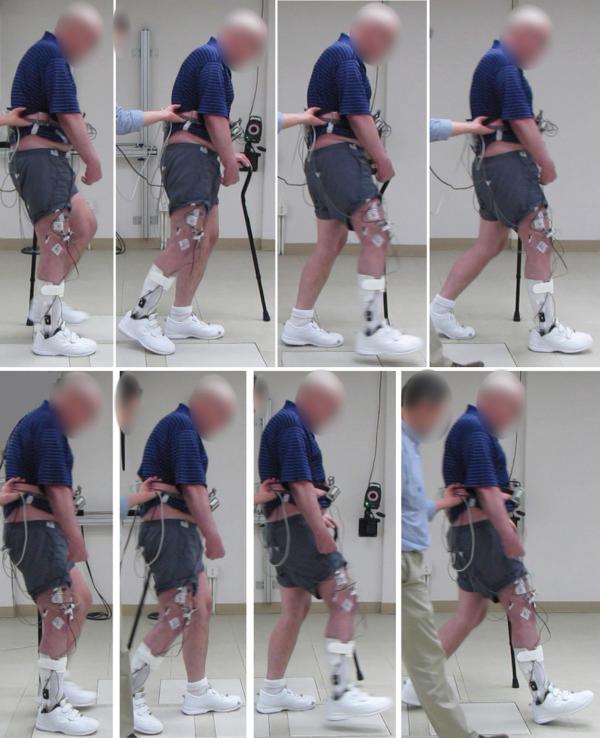
Neuroprostheses can be tailored to the specific needs of individuals with incomplete SCI to address the most critical gait deficits while allowing maximal utilization of remaining volitional lower extremity function. Application of surface stimulation may be most effective in the early phases of gait rehabilitation because its flexibility in electrode placement ( ), and adjustability as voluntary and stimulated strength improve ( ). Volitional walking speed improves when stimulation is integrated into gait training (a neurotherapeutic effect), and interactive stimulation during walking provides additional gains in gait speed and distance (a neuroprosthetic effect), particularly in slow walkers ( ). Stimulating the plantarflexors, in addition to the knee and hip extensors and flexion withdrawal reflex, can also increase walking speed and stride length by providing an active push-off and improving knee flexion during swing ( ). Limitations due to habituation of the flexion withdrawal reflex ( ) and the inability to access the deep hip flexors (Iliopsoas) from the surface can be obviated with neuroprostheses employing implanted electrodes ( ). The neural structures that control all major muscles of the lower extremities and trunk can be accessed with intramuscular ( ) or peripheral nerve cuff electrodes ( ) to achieve strong, isolated, and repeatable contractions of the target muscles. Customized implanted systems demonstrate better and more consistent therapeutic and neuroprosthetic benefits than surface systems ( ) and can facilitate taking a step even in individuals with excessive extensor tone ( ). Similarly, as illustrated in Fig. 97.2 , multijoint control with implanted systems can provide significant neuroprosthetic improvement in walking ability in individuals with stroke ( ) or MS ( ).
Approximately 90% of individuals with MS will eventually experience impaired mobility ( ). Despite the prevalence of gait dysfunction and the absence of meaningful interventions to correct it, application of neural stimulation in this population is only just beginning to be explored. Small studies using surface stimulation typically limited to the peroneal nerve have demonstrated improvements in walking speed, distance, and various measures of quality of life ( ). However, other studies of similar caliber have either shown that surface stimulation of the peroneal nerve is not better than exercise alone, or confirmed a significant neuroprosthetic effect on walking performance without carryover to voluntary function ( ). The absence of a meaningful therapeutic carryover suggests that activating the proximal lower extremity musculature is likely to be critical to improving ambulation in the community and during activities of daily living for patients with MS. This can be achieved with a multichannel, implantable system comparable to what has been utilized in patients with SCI. Case studies of interventions targeting multiple joints with implanted neuroprostheses suggest that activating the hip and knee flexors (sartorius, tensor fascia latae) in addition to the peroneal nerve can increase maximal walking distance eightfold over volitional function alone ( ), and produce stepping robust and consistent enough to reduce Expanded Disability Status Scale (EDSS) scores from 7.5 to 6.0, thus preserving independent mobility and delaying wheelchair dependence.
Unlike walking with partial paralysis from incomplete SCI, stroke, or MS that can be improved with a relatively small number of stimulus channels to activate the muscles responsible for focal gait deficits, complete paralysis often requires more than 16 stimulus channels to control all the muscles necessary to restore stable stepping in the absence of extensive external bracing ( ). Although the maximal walking speeds and distances reported for implanted lower-extremity neuroprostheses approach those typical for community ambulation, high metabolic energy consumption (up to four times normal) and reliance on the upper extremities for balance, support, and propulsion all limit stepping with these systems to the vicinity of the wheelchair or for exercise ( ). To address these issues, hybrid neuroprostheses consisting of neural stimulation and external bracing are being developed. Early hybrid systems combined alternating activation of the rectus femoris (hip flexion) and hamstring (hip extension) muscles via surface stimulation with a reciprocal gait orthoses (RGO) that coupled the hips so that extension of one joint via volitional truncal extension resulted in flexion of the contralateral joint ( ). With knees locked and ankles fixed at neutral, this simple hybrid neuroprosthesis reduced energy expenditure 15%–30% and increased maximal walking distance from 100 to 800 m compared to the RGO alone. Exercise with the system produced positive effects on bowel/bladder function, endurance, back/muscle pain, energy, weight, spasticity, skin problems, and range of motion ( ).
In spite of significantly improved posture and trunk stability, the locked knees and reciprocating mechanism limited both step length and walking speeds (0.05–0.37 m/s), and required excessive upper body effort compared to walking with stimulation alone ( ). This motivated development of dynamic hybrid systems with context-dependent hip and knee joints that could be locked for stability in stance, unlocked for free joint movement during swing, or coupled to simulate a reciprocator at the hips ( ). Movements in these systems are powered entirely by multichannel implanted stimulation coordinated with the joint mechanisms via microprocessor-based controllers acting on the external knee ( ) and hip ( ) mechanisms to progress smoothly through the phases of gait. This significantly decreased forward lean compared to neural stimulation alone, and reduced maximum upper extremity forces on the walker by 42% and 19% compared to walking only with a standard RGO or neural stimulation, respectively. Walking speed also increased by 15% as compared to an RGO ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here