Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ischemic stroke presents several opportunities for medical intervention, distinguished by their relative timing:
primary prevention of a stroke;
control of tissue damage in the acute ischemic stroke setting;
secondary prevention following a transient ischemic attack (TIA) or stroke.
Considering the high morbidity and mortality associated with stroke, primary and secondary prevention are paramount. Traditionally, antiplatelets are used in this third setting, to prevent recurrent ischemic stroke. However, antiplatelet agents are not the drug of choice across the board to prevent all types of ischemic stroke. For example, an elderly woman with cardioembolic stroke due to atrial fibrillation would be better served with an anticoagulant to minimize risk of future cerebral infarction, rather than an antiplatelet agent. The decision whether a physician should utilize antiplatelet agents for secondary stroke prevention depends on the pathogenesis or cause of the initial cerebral infarct.
The mechanisms of action of antiplatelet agents present a compelling overlap with the pathogenic mechanisms that define brain ischemia; therefore, antiplatelet agents theoretically could have a role in all three stages of the stroke timeline. We herein review antiplatelet agent mechanisms in the context of stroke pathology for which their use is appropriate, outline the current index of antiplatelet agents, and highlight the practical concerns of these medications in their clinical use.
The decision to use an antiplatelet agent versus an anticoagulant should be driven by the white-clot/red-clot paradigm . There are significant differences in the manner in which white thrombi and red thrombi form, and the treatments used to prevent each type of clot vary ( Table 166.1 ). Red erythrocyte–fibrin clots are treated with thrombolytic drugs and anticoagulants such as heparin, warfarin, and the newer novel oral anticoagulants. White platelet-fibrin clots do not contain red blood cells and are prevented better by antiplatelet agents. If a physician determines that their patient’s stroke was likely due to an increased risk of forming white thrombi, then antiplatelet medications can rationally be selected for future stroke prevention .
| White Clots | Red Clots |
|---|---|
| Composed mainly of platelets and fibrin | Composed mainly of red blood cells and fibrin |
| Form within arteries | Form in arteries or veins |
| Form almost exclusively in areas that have an abnormal endothelial surface | Do not require an abnormal vessel wall or tissue thromboplastin |
| Do not form on the surface of myocardial infarcts | Form on the surface of myocardial infarcts |
| Often form on damaged heart valves | Often form on damaged heart valves |
| Develop in fast-moving bloodstreams | Develop in areas of slow blood flow |
| Prevented with antiplatelet agents | Prevented with anticoagulants |
White thrombi in general are both platelet and thrombin-rich, typically forming in fast-moving arteries where the endothelium has been damaged. Endothelial damage can initiate an intravascular process that ultimately disrupts blood flow to the brain ( Fig. 166.1 ). The damage may occur with an apparent disruption of the endothelium, such as at the site of an atherosclerotic-ruptured plaque, as well as with more subtle irritations such as that seen with syphilitic vasculitis. The damaged barrier of endothelium invites platelet adhesion, a process mediated by GPIb/IX receptors on platelets and endothelial collagen-bound von Willebrand factor (vWF) multimers. The remainder of the process is a review of the normal cascade of platelet-mediated hemostasis .
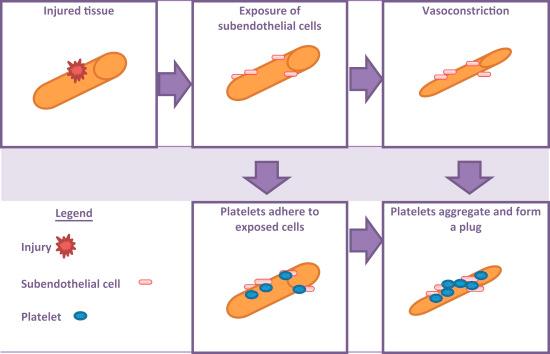
Platelets are locally activated by thrombin, thromboxane, and adenosine diphosphate (ADP). Thrombin serves as the main bridge between platelet activation and the coagulation cascade. Thromboxane activates the GIIb/IIIa receptors on platelets and initiates platelet aggregation. ADP binds to the P2Y 12 G-protein-coupled receptor that, in turn, increases the platelet cytosolic calcium (Ca 2+ ) level and induces platelet activation. Additionally, the P2Y 12 receptor, also bound by ADP, causes a morphological change in the platelet to a more spherical shape and the development of pseudopodia. Following the thromboxane-induced activation of the GIIb/IIIa platelet-bound receptors, these receptors are then capable of binding either endothelial vWF or fibrinogen. Fibrinogen is coupled with another GIIb/IIIa receptor on a neighboring platelet. This process allows for the stabilization and propagation of the clot, respectively .
Multiple factors work to inhibit this process, which, when in a healthy state, provide the delicate balance required for hemostasis. Prostacyclin is released by the endothelium and counteracts the interaction of ADP and the P2Y 12 receptor. When prostacyclin binds to a separate platelet-bound G-protein-coupled receptor, it decreases the cytosolic Ca 2+ and inhibits platelet activation. Prostacyclin has a synergistic antiaggregating effect with another vasodilator, nitric oxide (NO). NO is a gaseous signaling molecule synthesized in the vascular endothelium that crosses the cell membrane easily and prompts relaxation of vascular smooth muscle. The resultant vasodilation and increase in blood flow creates an unfavorable environment for thrombogenesis. NO also inhibits platelet adhesion by activating a molecular signaling pathway . Each antiplatelet agent inhibits at least one of the processes in this cascade to a greater or lesser degree. Although the effects of the different antiplatelet agents are similar, their mechanisms can differ.
What risk factors are associated with platelet-dependent thrombus formation? The cascade of white clot formation can be triggered by a variety of diseases and conditions. Atherosclerotic disease can cause direct arterial endothelial insult and subsequent platelet fibrin-rich thrombus formation, particularly in areas of ulceration. Hypercholesterolemia is a risk factor for the development of atherosclerotic disease and also directly enhances platelet thrombus formation on injured arteries. Infections such as syphilis, bacterial and fungal meningitis, tuberculosis, vasculitis, sickle cell disease, and HIV all lead to an increase in stroke risk secondary to endothelial damage. Lastly, hyperhomocysteinemia, a result of either a vitamin deficiency (vitamin B 6 , B 12 , or folate) or inherited enzymatic defects, can also cause direct harm to the endothelium .
Smoking is a significant risk factor for stroke, by way of multiple mechanisms. As smoking predisposes to hypertension and hyperlipidemia by indirectly increasing the risk of stroke. However, smoking directly escalates the risk of white-thrombus formation by causing toxin-mediated damage to the vascular endothelium as well as causing platelets to be in a hyperaggregable state . This phenomenon is also seen in diabetes mellitus, antiphospholipid antibody syndrome, and heparin-induced thrombocytopenia . Collagen vascular diseases, such as systemic lupus erythematosus and scleroderma, are associated with anticardiolipin antibodies, and thus potential for platelet-dependent thrombus formation. These antibodies bind to the vascular endothelium and inhibit the release of prostacyclin, creating a prothrombotic state .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here