Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The use of Focused Assessment With Sonography in Trauma (FAST) in management of the trauma patient has become increasingly widespread over the past three decades. Serial improvements in ultrasound technology, its portability, and the quality of the images have facilitated rapid and reliable diagnostic images during the initial resuscitation in the emergency room. Confirmation of the acceptance, and importance, of its use was highlighted by the American College of Surgeons’ incorporation of FAST into the Advanced Trauma Life Support curriculum in 1997. Presently, FAST is performed worldwide by multidisciplinary teams, including trauma surgeons, emergency medicine physicians, and prehospital providers. There are several guidelines and evidence-based reviews about FAST in different settings, including operational and tactical scenarios in war zones. Extensions of the classic technique have been promoted; wireless devices have been developed, and they are constantly improving. The aim of this chapter is to describe the techniques, indications, pitfalls, controversies, evolution, and current status of the different applicability scenarios that this diagnostic modality has for the trauma patient.
In 1917, the first piezoelectric generator was used in the creation of ultrasound. The crystals of the generator also served as a receiver, generating electrical signals upon detection of the reflected mechanical vibrations or sound waves. During World War II the vast potential of ultrasound technology began to be recognized with its use in sonar (sound navigation and ranging) systems that enabled the detection of submarines and subsequently saved countless lives. In 1959, the use of the Doppler effect during ultrasound examination enabled the flow of peripheral arteries to be detected; however, it was the introduction of the gray scale, in 1971, that marked the beginning of the widespread acceptance of ultrasound as a diagnostic tool.
In the 1980s, multiple publications regarding ultrasound in trauma appeared in German literature. Subsequently, this diagnostic technique spread to many countries in Europe and was finally accepted in other continents. In 1992, a prospective study of 163 patients with blunt trauma was published by a research group headed by Tso and colleagues. These patients had undergone ultrasound by trauma fellows (postgraduate year 6) within the first hour in the emergency department. The study was conducted over an 8-month period, and the trauma fellows received at least 1 hour of theoretical training and 1 hour of practical training focused on identifying free fluid in the peritoneal cavity or organ damage. The findings were compared with the results of diagnostic peritoneal lavage and computed tomography (CT) scan. The images acquired were independently interpreted by the radiologists who knew neither the results of the other diagnostic modalities nor the interpretation given by the trauma fellows. In this first major study, a sensitivity of 91% for ultrasound’s capacity to detect hemoperitoneum was reported. For detection of intra-abdominal lesions, ultrasound had a sensitivity of 69%, specificity of 99%, and accuracy of 96%, resulting in an LR+ of 69 and an LR− of 0.31. At that time, it was noted that it was a noninvasive tool, portable, and with significant accuracy to determine the need for further diagnostic or even surgical interventions, not so much to exclude the need for them if the result was negative. Subsequently, Rozycki et al. published a study of 476 patients in 1993 where 4 surgeons, 4 trauma fellows and 25 surgical residents of a level I trauma center managed to evaluate the diagnostic performance of ultrasound in the approach of the patient with thoracoabdominal trauma. The authors reported a sensitivity of 79% with a specificity of 95.6%, resulting in an LR+ of 17.96 and an LR− of 0.22. Thus, they concluded that surgeons can quickly learn to interpret this diagnostic technique and that ultrasound is a fast, sensitive, and specific tool to detect intra-abdominal free fluid and pericardial effusion. It should be noted, however, that the estimate of the LR− is not sufficient to guarantee the inexistence of false-negative results since the possibility of a real case of free fluid is extremely high (LR + greater than 10).
In 1995, after the publication of several case series demonstrating the successful use of ultrasound in the management of the trauma patient, the American College of Surgeons incorporated the FAST into its algorithm for the management of abdominal trauma. Initially, the technique was limited to the abdominal region and, in 1996, the term FAST (focused abdominal sonography for trauma) was described according to publications published by Rodriguez, Rozycki, and others. Later in the same year, the method was included in the Advanced Trauma Life Support training program, and the definition of FAST was changed to F ocused A ssessment With S onography for the T rauma patient. In 1997, when it was included into the Advanced Trauma Life Support course, FAST became acknowledged as a fundamental and valuable tool in trauma patient care.
Finally, around 1999, Scalea et al. published the results of the international consensus conference on FAST. This conference dealt with important issues such as the definition and the consequent acronym with which this diagnostic modality would be identified. Finally, they decided to modify focused assessment sonography for trauma, modifying the word “abdominal” for “assessment,” considering the possibility of a greater potential of this tool for a holistic approach of the traumatized patient, leaving open the possibility of its usefulness to evaluate other body regions different from the abdomen. Likewise, and among other considerations, they mention that the evaluation of the parenchymal injuries of abdominal organs by means of FAST is still subject to debate, and they state the need of new studies to clarify the usefulness of ultrasound in this particular item. Last but not least, they gave the definition of a positive FAST or a negative FAST, according to the presence or absence of free fluid respectively in both the abdominal and pericardial cavity.
The technique initially was widespread between surgeons and surgical residents. But actually, other specialties involved in trauma care also participate in the training process, including acute care physicians (emergency medicine and critical care physicians) and prehospital providers. The aim of the extended version of the classical FAST examination (E-FAST) training program is simply to improve the ability to accurately diagnose the presence of free fluid (hemoperitoneum) and air (pneumothorax). FAST is a rapid, accurate, noninvasive, and easily repeated method of detecting free fluid that can be performed during the primary survey and also in the unstable patient during the initial resuscitation. Guidelines for appropriated FAST use have been developed in several consensus, including the following recommendations:
Evaluation with ultrasound must be available to be performed 24 hours a day at the bedside in the resuscitation room.
The acute care physicians (emergency medicine and critical care physicians) and trauma surgeons performing E-FAST must be appropriately trained in accurately performing and interpreting the scan.
Each hospital must train and evaluate its physicians to ensure effective use of the resource, with use of different specialists (including radiologists and echocardiographers or well-trained physicians in ultrasound) to help candidates improve their interpretation and decision making.
Training in E-FAST must form part of the curriculum for surgical, emergency medicine, and critical care medicine residents ( Fig. 1 ).
The value of E-FAST in decision-making during the management of the trauma patient is operator dependent; therefore, effective teaching and continued training of doctors in the accurate performance and interpretation of the technique are priorities.
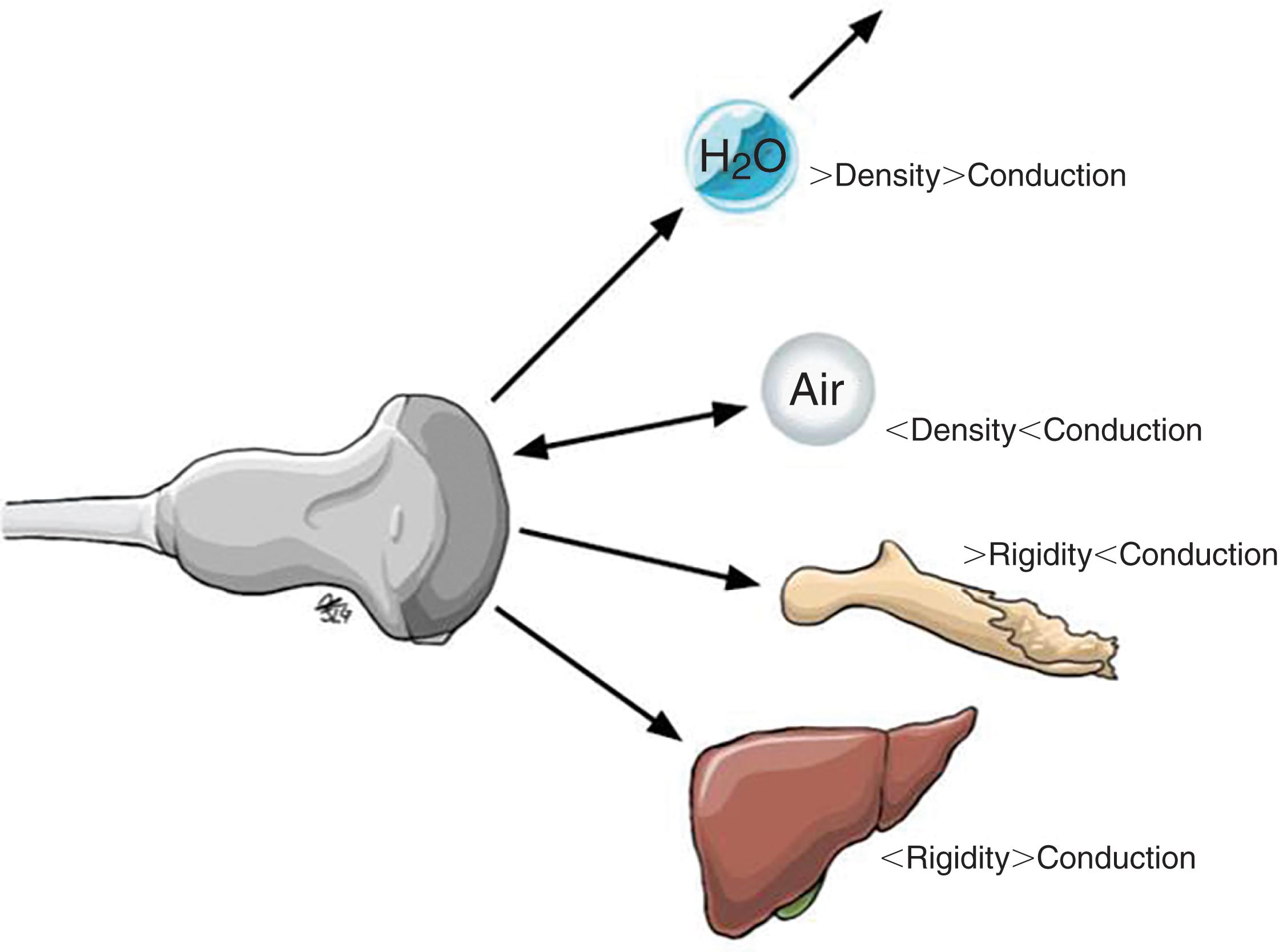
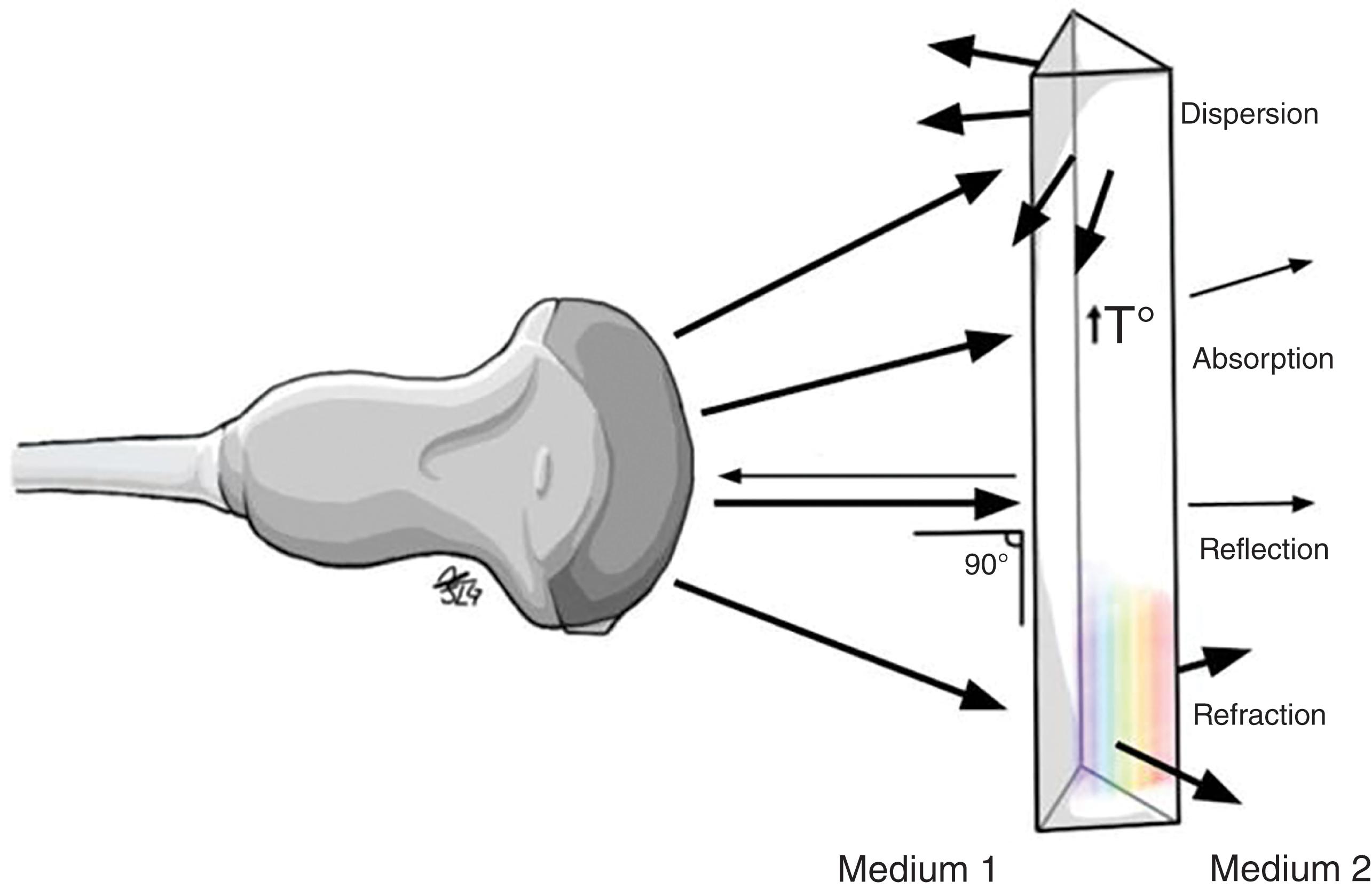
Ultrasonography may be defined as the imaging of deep body structures by recording the echoes of pulses of ultrasonic waves directed into the tissues and reflected by tissue planes where there is a change in density. Audible sound waves have a frequency range between 15 Hz and 20 kHz, infrasound wave frequency is below 15 Hz, and ultrasound waves used in medical imaging range from 1 MHz to 60 MHz. The ultrasound waves used in medical imaging are longitudinal waves that may pass through liquid and soft tissues but are not readily transmitted in the absence of these substances; hence, air-filled structures such as the lungs are poorly visualized by ultrasound since its capacity to allow ultrasound transmission is very low. The same happens with bone tissue, which due to its high rigidity and impedance does not allow ultrasound to be transmitted beyond the point where ultrasound makes contact with it. Once an ultrasound wave encounters a material with a different density or acoustic impedance ( Fig. 2 ), a fraction of that sound wave is reflected and detected by the transducer ( Fig. 3 ). The greater the density of the tissue or structure, the greater the reflection or echo, and therefore higher-density tissues appear as brighter images upon the display. The lower the tissue density, the lower the echoes will be, and therefore it will be shown on the screen as an “echo-free” or anechoic area (in the darkest spectrum of the gray scale) (see Figs. 9 and 10 ).
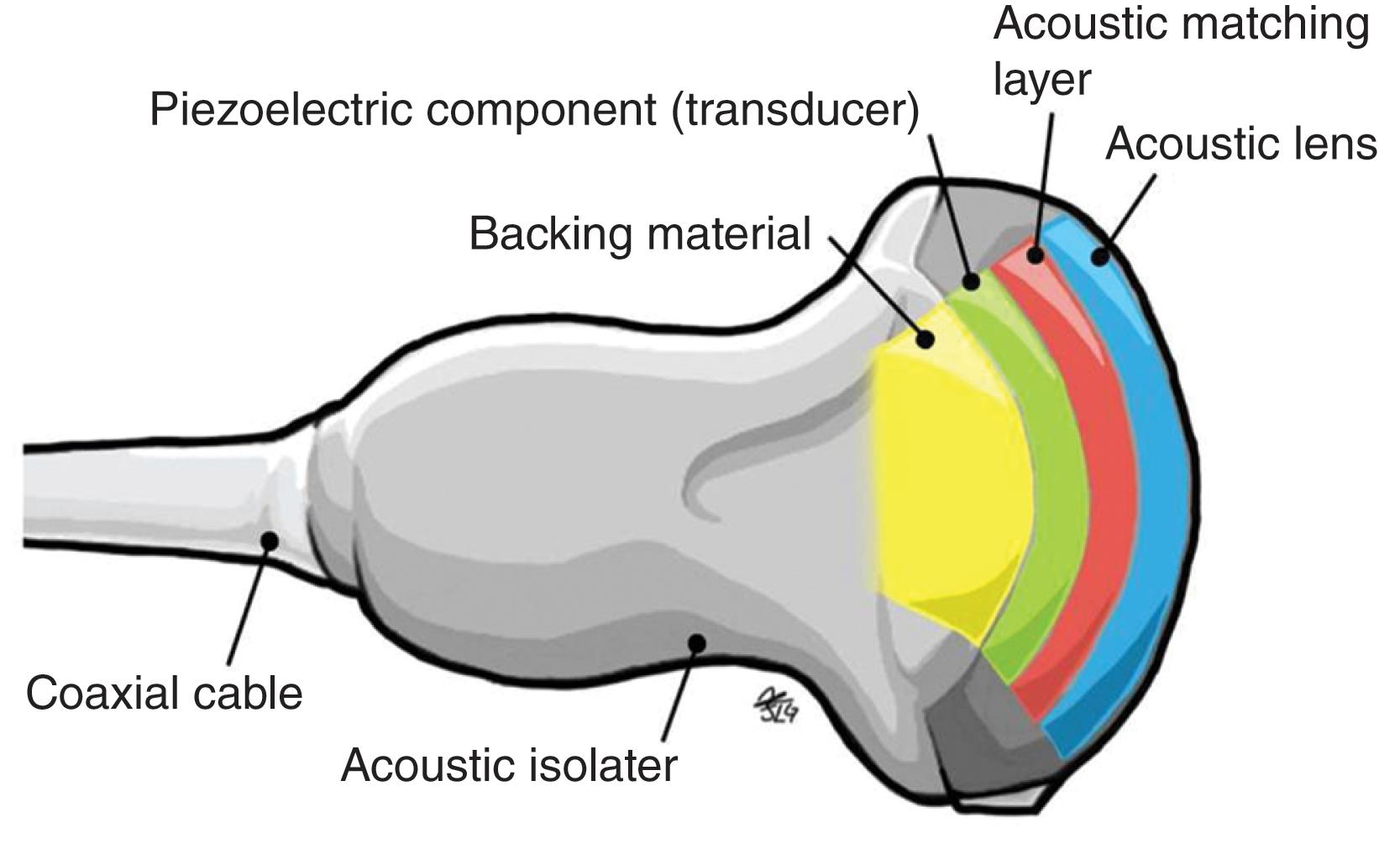
The transducer (also called “probes”) acts as both the transmitter and receiver. The ultrasound waves are generated from the mechanical movement or oscillations of crystals that are excited by electrical pulse. Of those sound waves transmitted into the patient’s body, a small percentage is reflected back to the crystals within the transducer and the reception of these reflected sound waves generates electrical impulses, which are processed to create an image. This phenomenon is known as the “piezoelectric effect” ( Fig. 4 ).
The intensity of the electrical pulses that act on the crystals within the transducer allows a period of silence that is sufficient to allow the reception of both superficial and deep reflected sound waves or echoes, prior to the generation of the next transmitted sound wave. A pulse consists of three phases: (1) the emitting phase, (2) the phase of balance, and (3) the receiving phase. The emitting phase occurs when the ultrasound wave is generated; the receiving phase is the period in which reception of the reflected sound waves occur; and the phase of balance is the period in which there is neither reception nor emission of ultrasound waves. The majority of ultrasound scanners have a maximum depth of examination of 20 to 30 cm, and on occasion it is important to analyze the area from different angles so as to obtain the best possible image. Sound waves have a constant speed of 1540 m per second in the body tissue. Therefore, if a wave has a reduced duration of “emission and reception” to the transducer, this indicates that the particular soft tissue, which is reflecting the sound wave, is of greater density (like fat and bones).
Transducers consist of several basic components; the crystal of the transducer is made of a piezoelectric material, typically single-crystal quartz or lead zirconate titanate. The purpose of the crystal is to convert the electrical impulses into transmitted sound waves and vice versa (piezoelectric effect) (see Fig. 4 ). Insulation material, most commonly rubber, is used for the “footprint” of the transducer and increases the focused transmission of the sound waves, thus increasing the axial resolution and depth of the sound wave penetration. An external protector or acoustic insulator is used to envelope the transducer and isolate it from any unwanted sound wave interference.
The transducer is connected to the external display via an electronic cable, which allows the transmission of electrical impulses toward and away from the piezoelectric crystals ( Fig. 5 ).
The suitability of the use of specific transducers is dependent upon the purpose of the scan being performed as well as the depth of view that is required ( Fig. 6 ). Frequencies more often used for medical imaging range from 2 to 20 MHz, with higher frequencies being used for the optimal viewing of superficial structures. This is because higher frequencies have smaller wavelengths, which are subject to greater levels of attenuation of the sound waves over shorter distances when compared with lower frequencies. Transducers used for viewing superficial tissues tend to use frequencies ranging from 6 to 13 MHz and have a maximum depth of imaging around 6 cm. Transducers utilizing lower frequencies (between 3 and 5 MHz) allow transmission of sound waves to greater depths and therefore are suitable for use in imaging abdominal and pelvic regions and thus are used in the performance of FAST.
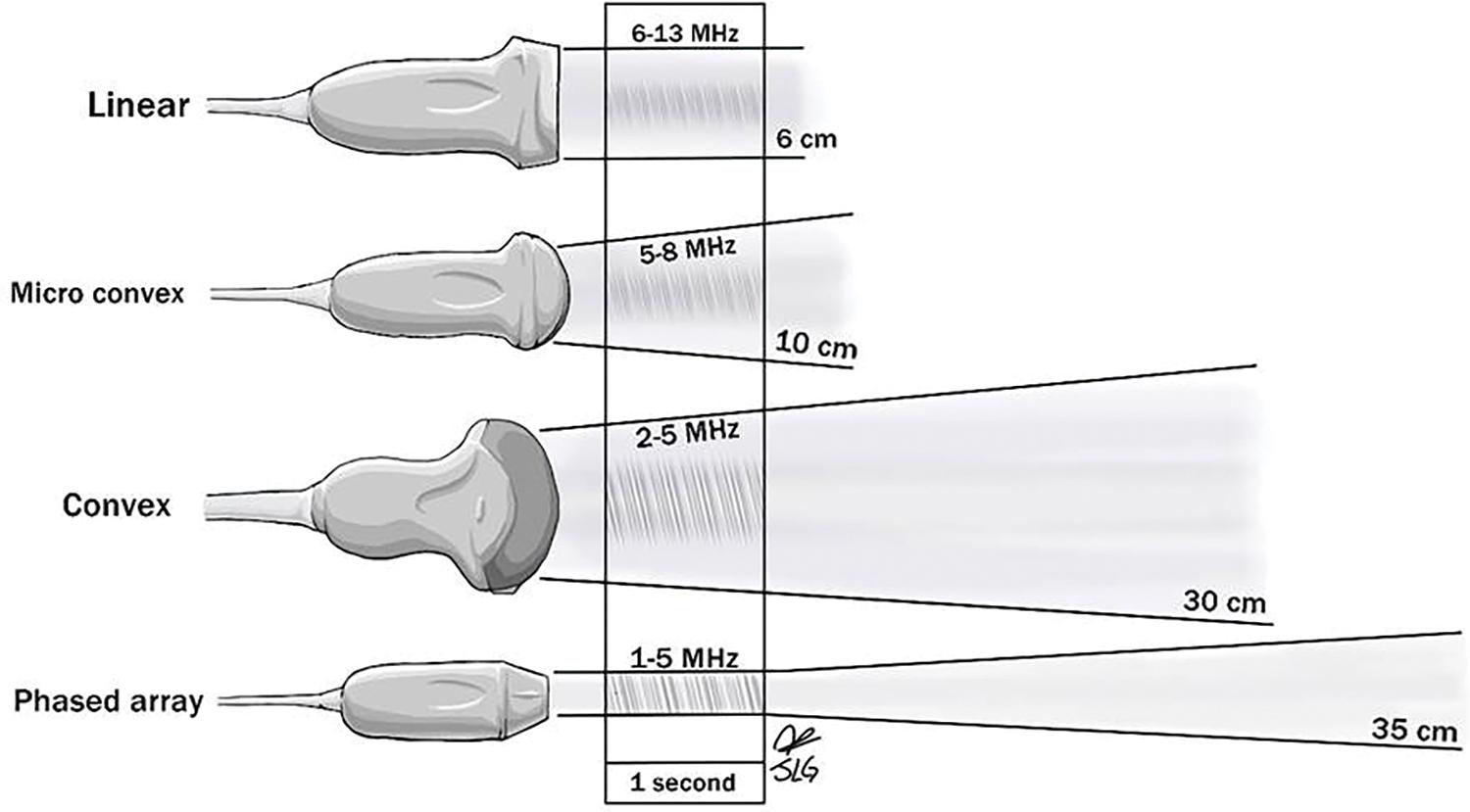
The shape of the transducer “footprint” is an important factor in determining when its use is appropriate. Linear scanners allow the transmission of sound waves that run parallel to each other and remain the same distance regardless of the depth of transmission, and thus, linear scanners are best at visualizing superficial structures and soft tissues. Curved surface scanners transmit ultrasound waves in a fan-shaped manner, thus increasing the width of view perceived. When used in combination with better-penetrating lower-frequency sound waves, curved scanners provide the most appropriate view with which FAST may be accurately performed.
Modern ultrasound machines come with preset optimized combinations of different-shaped transducers and wavelength frequencies to be used when scanning different parts of the body. Therefore, the operator often has to merely select the appropriate viewing settings on the machine and choose the appropriate transducer prior to performing the scan. While performing FAST, it may be necessary to make real-time adjustments of the setting of the ultrasound equipment so as to fine-tune the quality of the images obtained.
Detailed knowledge of the various means of improving the quality of the ultrasound picture increases the accuracy of the treatment decisions made based upon them and therefore potentially avoids errors in diagnosis:
Acoustic power/output: This controls the voltage applied to the piezoelectric crystal; increasing the power output increases the intensity of the sound wave created. Typically, the lowest level of power output is used to obtain suitable images for interpretation, and it is recommended to use low-power output and short periods of time in potentially sensitive tissues such as the eyes, given the potential harmful effect on the retina.
Cineloop: A sequence of images stored digitally that may be reviewed upon completion of the examination where it is possible to go forward or backward and select the image of greatest interest.
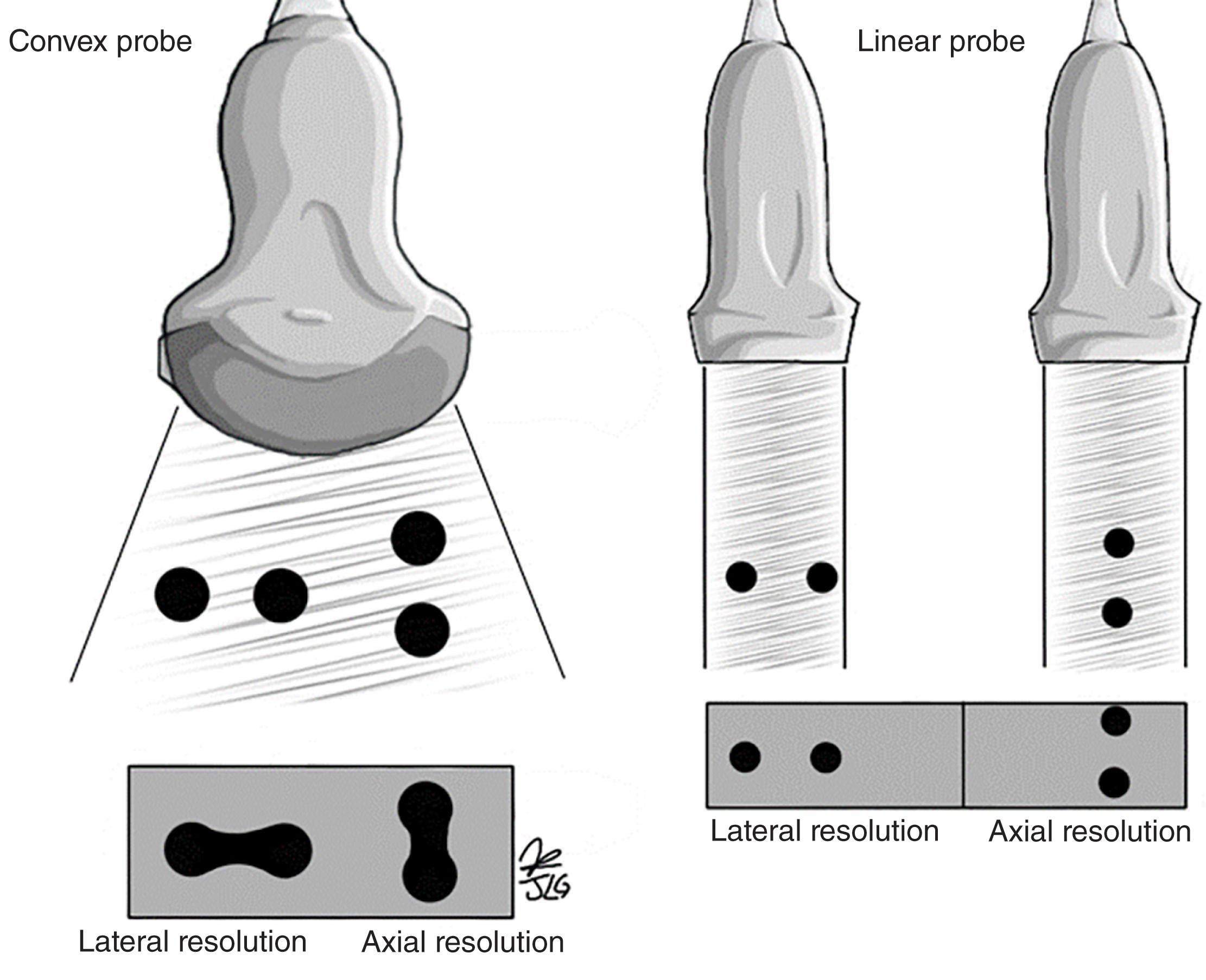
Depth: Depth determines the degree of penetration visualized upon the display screen (see Fig. 6 ). The maximum depth varies according to the type of transducer used. The greater the frequency, the lesser the depth, and the lower the frequency, the greater the depth; likewise, this affects the axial and lateral resolution of the insonated structures given the difference in wavelength between high- and low-frequency ultrasound ( Fig. 7 ).
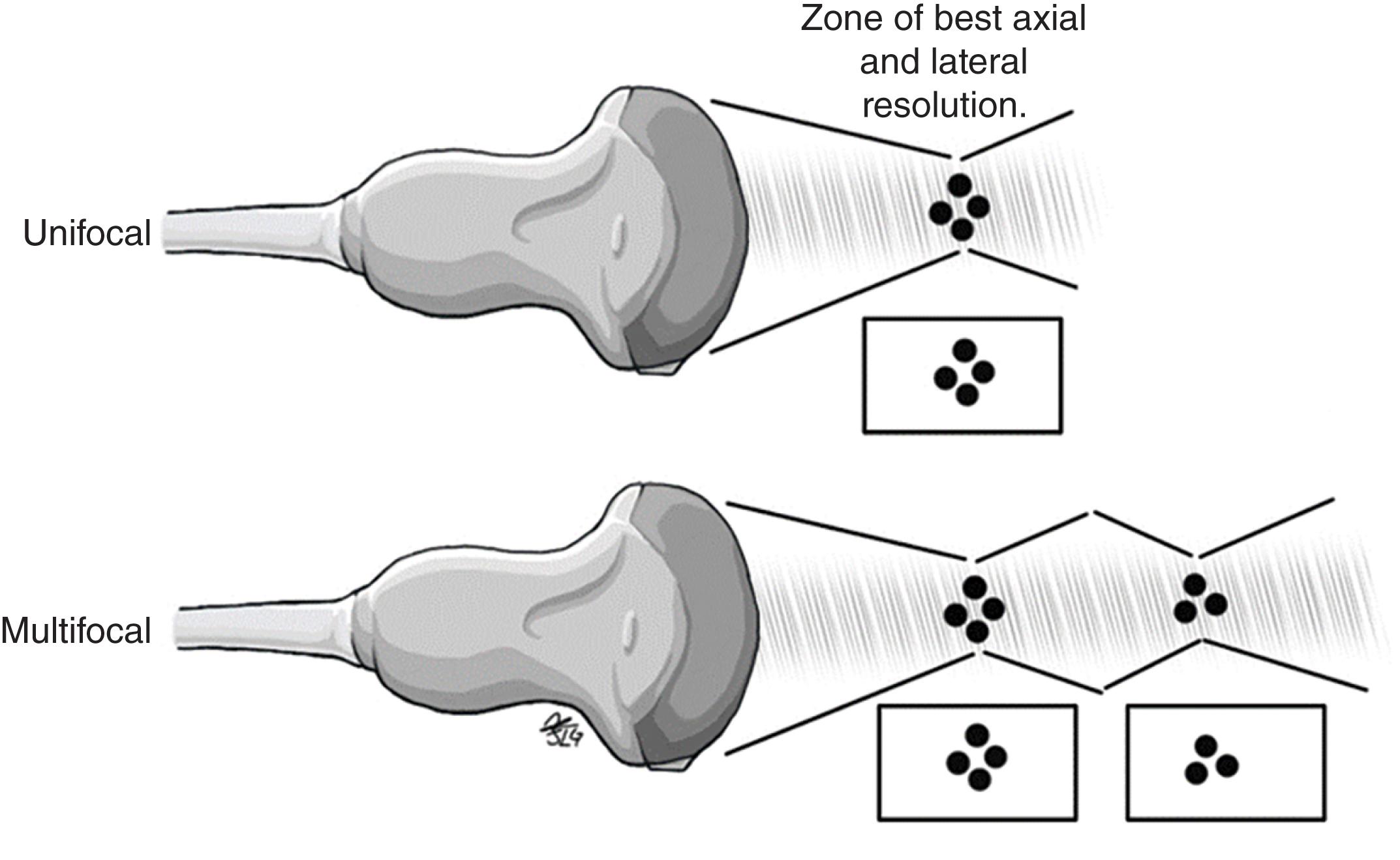
Focus: Focus allows increased image clarity and resolution at the selected depths of interest during the scan, converging as many ultrasound waves in the area of interest as possible ( Fig. 8 ). The point of focus can be manipulated on the ultrasound machine based on the interest of the examiner and the depth of the structure to be evaluated. Currently, equipment with multiple focal zones is available, providing greater definition and resolution in the multiple focal zones used. However, temporal resolution is sacrificed in these images.
Gain: Gain regulates the degree of amplification applied to all ultrasound waves returning to the transducer. Too much or too little gain can affect the quality of the images obtained. If the gain is set too high, then the image acquired will become distorted with artifact noise and there will be a loss in the contrast between different structures as all reflecting echoes become progressively brighter or “white” (hyperechoic). If gain is set too low, the perception of some real echoes will be lost and thus the quality of the image is reduced and will be dark or “black” (hypo- or anechoic).
Near gain: This controls the amplification of the echoes within the superficial field or portion closest to the probe.
Far gain: This controls the amplification of the echoes in the deep field or deepest portion to the transducer.
TGC (time gain compensation): This refers to adjustment of the gain by sectors, keeping in mind that as the ultrasound travels in depth, its energy (represented by amplitude) is depleted (i.e., the attenuation phenomenon). Therefore, as the area of interest becomes deeper, the quality of the image obtained will be lower due to the lower amplitude of the echoes obtained. In this way, having the possibility to improve the gain—especially in deep areas—optimizes the quality of the image obtained .
Zoom lens: The zoom lens allows us to magnify the image to better visualize small structures; however, this magnification can cause some distortion of the image and subsequent loss of image quality. Nonetheless, the presence of equipment with HD zoom is becoming increasingly popular to reduce distracted or pixelated images.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here