Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
Targeted use of antiepileptic drugs (AEDs) is an important facet of neurosurgical practice to maximize AED efficacy as well as minimize inappropriate use of AEDs.
Ideally, patients should be managed with only one AED and at the lowest possible dose to maintain seizure freedom, most often a modest dose.
After a continuation of seizures after three appropriately selected AEDs, a patient is considered to have a less than 1% chance of being seizure free with medications alone.
AEDs are not useful in the context of “seizure prophylaxis” when a patient has never had a seizure.
A benzodiazepine such as lorazepam 4 mg is the first-line treatment for status epilepticus.
Seizures and epilepsy are a frequent presentation of various neurosurgically encountered diseases. Thus, the decision to use antiepileptic drugs (AEDs) is common in neurosurgical practice, whether as initiation of treatment, as continuation of treatment, or to change a preestablished therapy. Advances in the pharmaceutical arena in the past few decades have led to a formidable armamentarium of drug options. This is a welcome scenario on the one hand and a potentially bewildering apothecarial situation on the other. Indications for initiation of treatment vary, often with the blurring of lines between seizure prophylaxis and actual epilepsy therapy in various brain pathologies. It is important to understand that current commercially available AEDs, without exception, have only antiseizure properties and thus do not prevent the development of epilepsy. Thus, rather than rotely reviewing each seizure medication, this chapter instead provides a framework for an understanding of the most opportune times for AED usage as well as times when it is not advisable. We then continue with a practical method for starting and maintaining AEDs in routine neurosurgical care. We continue with specific situations in which additional AED considerations may be made and conclude with a discussion of when and how treatment may be stopped.
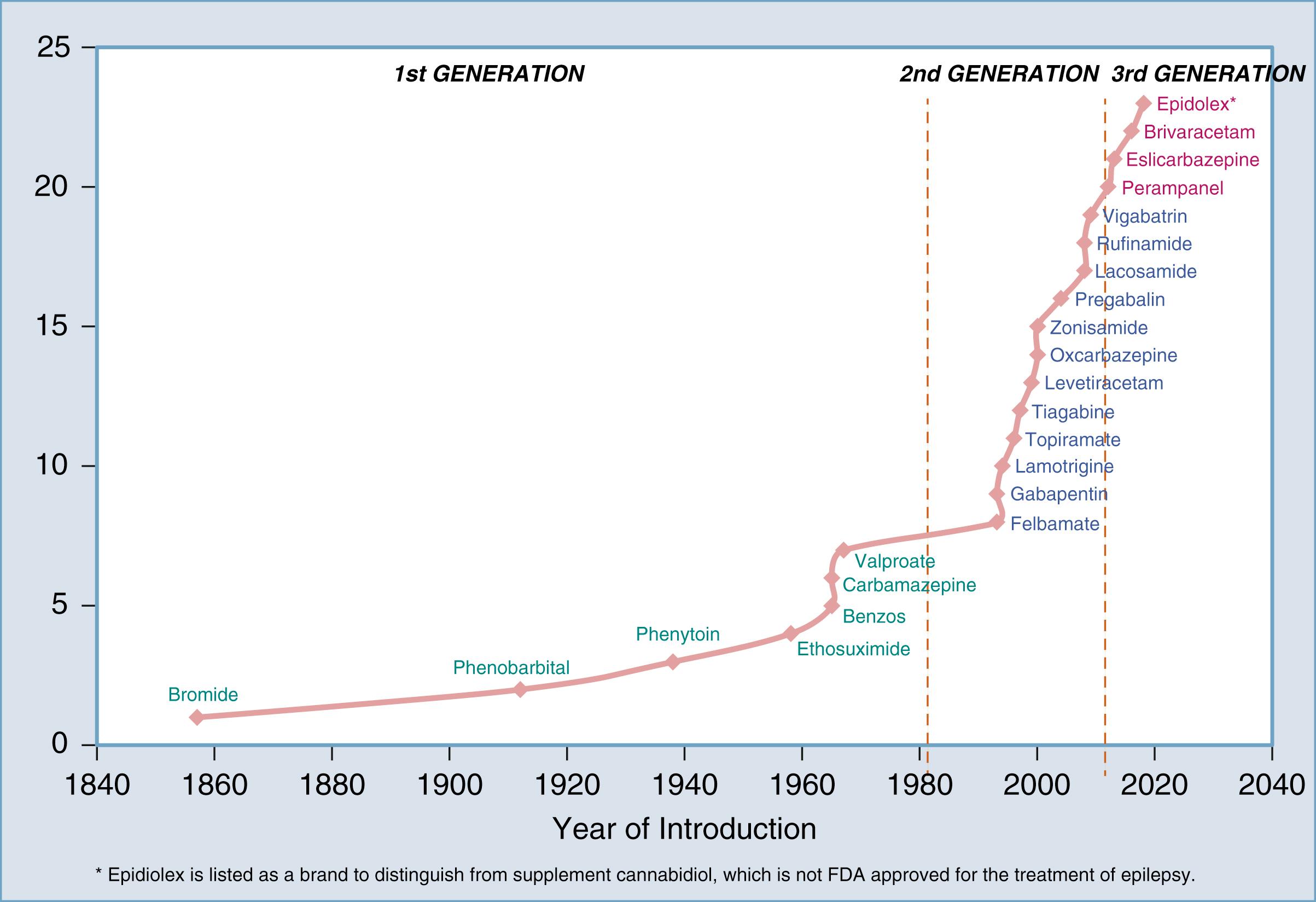
The advent of what can be considered modern AED pharmacotherapy first began in 1857 with the discovery of potassium bromide by Dr. Charles Locock in the United Kingdom. Despite the logistical difficulties posed by this compound for oral treatment, it remained the only AED until the discovery of phenobarbital’s antiseizure effects in 1912 by Dr. Alfred Hauptmann in Germany. Phenytoin was the next discovery in 1938 by Dr. H. Houston Merritt in the United States. The 1950s and 1960s brought forth other well-recognized “conventional” AEDs such as carbamazepine, valproate, and primidone. The benzodiazepines clonazepam and clobazam came into utility in 1970. There was a relative quiescent period in AED development at this point that ended in the 1990s with the development of the second-generation AEDs such as vigabatrin, lamotrigine, topiramate, zonisamide, levetiracetam, oxcarbazepine, gabapentin, and pregabalin. We currently reside in the third-generation phase of AEDs with lacosamide, perampanel, brivaracetam, and eslicarbazepine being representative examples. AEDs are broken down by generation in Figure 81.1 .
The actual mechanism of AEDs is at the level of cellular ion channels and the neurotransmitters that affect such channels. Thus, it is vital to understand that seizure generation begins at the single neuron level. Influx and efflux of sodium, potassium, chloride, and calcium ions occur through their respective channels. In a seizure, there is sustained and abnormal synchronization among neurons that gives rise to the seizure. Thus, modulating those ion channels is the mechanism by which AEDs work. Medications that exert influence on the sodium channel include classic AEDs such as phenytoin and carbamazepine as well as newer AEDs such as lamotrigine and zonisamide. Chloride channels are modulated via GABA receptors, which are affected by benzodiazepines and phenobarbital among others. Many AEDs, including valproate, exert influence over multiple ion channels. Lastly of note, levetiracetam (and its new counterpart brivaracetam) has a unique mechanism that affects the synaptic vesicle 2A (SV2A) receptor without direct ion effects. Table 81.1 provides a reference for stratifying AED by mechanism of action.
| Sodium channel | Carbamazepine, eslicarbazepine, felbamate, lacosamide, lamotrigine, oxcarbazepine, phenytoin, rufinamide, topiramate, valproate, zonisamide |
| Calcium channel | Ethosuximide, gabapentin, lamotrigine, oxcarbazepine, pregabalin, phenobarbital, phenytoin, topiramate, valproate, zonisamide |
| GABA | Benzodiazepines, phenobarbital, carbamazepine, gabapentin, pregabalin, tiagabine, topiramate, vigabatrin, valproate |
| Glutamate | Carbamazepine, felbamate, oxcarbazepine, phenobarbital, topiramate |
| Carbonic anhydrase | Topiramate, zonisamide |
| Other | Brivaracetam, carbamazepine, lacosamide, levetiracetam, perampanel, phenytoin |
Benzodiazepines act via the GABA receptor, which increases the frequency of Cl – channel opening, thus hyperpolarizing the neuron. This reduces neuronal firing and seizure-related spontaneous and coordinated neuronal synchronization. Benzodiazepines are of particular importance in the treatment of status epilepticus, for which they remain the first-line treatment. Crucially, the benzodiazepine’s target GABA receptor becomes internalized during prolonged seizures and subsequent status epilepticus, which can explain tachyphylaxis and diminished effectiveness of benzodiazepines in prolonged status epilepticus after the first 24 to 48 hours.
In contrast with the reduction of neuronal firing via benzodiazepines, it is worth examining an inverse system that increases neuronal firing: glutamate, N- methyl- d -aspartate (NMDA) receptors, and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainite receptors. Glutamate is the primary excitatory neurotransmitter that acts via both NMDA and AMPA receptors. Acute cerebral injury can cause increased NMDA/AMPA receptor activation, which then subsequently causes increased neuronal hyperexcitability with an increased likelihood of focal epilepsy. Indeed, both imaging and pathologic studies have suggested increased NMDA receptor density in epileptogenic tissue. Therefore, AEDs that diminish this pathway may be of antiseizure potential. This has resulted in the development of the AMPA-blocking AED perampanel , that is in current clinical use.
The overall effectiveness of AEDs in epilepsy patients is well understood. Perhaps the best-known cohort comes from the Kwan and Brodie publications. This cohort follows newly diagnosed epilepsy patients in Glasgow, Scotland. This cohort showed that 47% were seizure free with use of the first AED, 13% became seizure free with the second AED, and 3.7% became seizure free with a third AED. Unfortunately, trials after a third AED showed a 1% or less likelihood of becoming seizure free. , The seizure-free rates have proved durable over long-term follow-up. , Based on these data, the concept of drug-resistant epilepsy has evolved and is defined as the failure of two adequately selected and dosed AEDs. As such, it is critical to set expectations early for patients regarding AEDs and their effectiveness. From a neurosurgical perspective, it is then important to consider surgical therapy once the diagnosis of drug-resistant epilepsy is reached, as suggested by a practice parameter endorsed by multiple professional societies.
A necessary prelude to prescribing an AED is the establishment of a specific seizure or epilepsy diagnosis, particularly because not all seizures represent epilepsy. In the acute hospital setting and in neurosurgical practice in particular, acute symptomatic seizures (seizures due to acute brain injury or systemic compromise of less than 1-week duration) are commonly seen in traumatic brain injury (TBI), tumor, and vascular, anoxic, infectious, or iatrogenic injury. Systemic perturbations such as hyponatremia, hypoglycemia, sepsis, and organ failure also often play a part as “provoking” factors, either solely or in concurrence with acute brain conditions. These are in sharp contradistinction to remote symptomatic seizures in which the seizures are usually caused by epileptogenesis following chronic evolution of a remote brain injury. The latter’s definitions vary, although it is likely that the first occurrence of a seizure more than 3 months after injury likely represents epilepsy and requires long-term AED treatment; acute symptomatic seizures rarely require long-term treatment. , A recent study was able to quantify the risk difference when comparing a first seizure after stratifying by acute symptomatic versus remote symptomatic. People with an acute symptomatic seizure had an 18.7% chance of subsequent epilepsy compared with a 64.8% chance in those with a remote symptomatic etiology.
The use of AEDs when an epilepsy diagnosis is established is straightforward. An epilepsy diagnosis, classically defined as at least two unprovoked seizures occurring >24 hours apart, is also now defined as one unprovoked seizure with at least a 60% risk for recurrent seizure. This amended definition is directly applicable to neurosurgical practice. In pragmatic treatment terms, there are mainly two types of epilepsy diagnoses: (genetic) generalized epilepsy and (acquired) focal epilepsy. In neurosurgical practice, it is reasonable to assume that most new epilepsy diagnoses fall into the focal epilepsy category because most neurosurgical seizure patients have some sort of focal brain lesion. As in all neurological and neurosurgical diseases, a last step in diagnosis should be taken in assigning an anatomic localization (e.g., right temporal lobe epilepsy).
A second clear indication for AED initiation is the setting of status epilepticus. Status epilepticus is practically defined as at least 5 minutes of continuous clinical or electrographic seizure activity or alternatively as recurrent seizures without return to baseline. Sometimes status epilepticus is obvious with presentation as convulsive status epilepticus with symmetric, rhythmic shaking of the extremities. Contrarily, nonconvulsive status epilepticus can sometimes be challenging to diagnose and may require a high index of suspicion. Patients can present with pure encephalopathy, with symptoms ranging from mild confusion to coma. Other times, there can be subtle motor manifestations such as unilateral extremity twitching or abnormal ocular movements. EEG is indicated as a way to guide treatment or even establish the diagnosis in the context of nonconvulsive status epilepticus, as 11% to 21% of patients in various types of ICU can have nonconvulsive seizures.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here