Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Many neuromuscular disorders in children are single gene disorders, and therefore genetics plays a primary role in the diagnosis of many neuromuscular conditions. Establishing a definitive molecular genetic diagnosis provides clarity about prognosis; suggests possible therapeutic strategies; allows patients, families, and providers to access appropriate clinical trials, research studies, centers of excellence, and family support organizations; and provides parents and other family members with critical information to accurately determine risk of recurrence and reproductive options to increase the probability of having a healthy child in the future. Increasingly, the exact type of mutation may also suggest possible therapeutic strategies, and it will become increasingly important to identify the exact mutation(s) in clinically affected individuals even when the clinical diagnosis is not in question. Molecular genetic diagnostics have been revolutionized by the decreased cost of sequencing and elimination of patents on genes, and improvements in genetic diagnostics should allow for increasingly efficient, expedient, and cost-effective methods to establish a diagnosis so patients can move beyond diagnosis and on to treatment.
This chapter will briefly review the basic principles of genetics and the principles of molecular genetics, and then focus largely on the clinical application of genetics to provide new approaches to diagnosis, counseling, and treatment. Given the uneven access to clinical genetics services outside major academic medical centers, the burden of the increased demand of genetic evaluation will fall largely on the neurologist who diagnoses and manages the neuromuscular patient. Thus, neurologists need to be conversant with the technologies and principles of genetic evaluation, testing, and counseling.
The human genome consists of approximately 3 billion base pairs of DNA packaged into 23 pairs of chromosomes. The 23 pairs of nuclear chromosomes consist of 22 non-sex chromosomes, called autosomes, and two sex chromosomes, the X and the Y. In addition, there are approximately 16,500 base pairs of DNA located on circular double-stranded molecules within mitochondria, referred to as the mitochondrial genome. Recessively inherited diseases require mutations in both copies of the gene whereas dominantly inherited conditions require mutations in just one of the two copies of the gene. X-linked conditions such as Duchenne muscular dystrophy more commonly manifest in males since males have only a single X chromosome and lack the second copy of most of the genes on the X chromosome as a backup if there is a mutation in an X-linked gene. Mitochondria are exclusively maternally inherited, so mutations of the mitochondrial genome are maternally inherited.
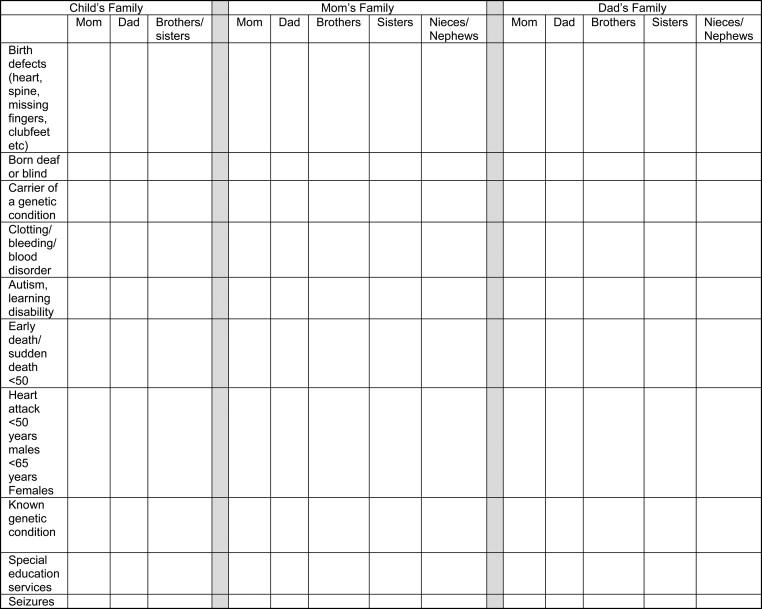
Taking a three-generation family history should be a standard part of any initial clinical evaluation. This is most efficiently performed by having patients or their parents complete a brief tabular questionnaire about the family history at home or in the waiting room so the provider can review the pertinent positives while taking the medical history. Some practices have a patient portal to facilitate collection of family history data. A sample family history screening tool is shown in Figure 2.1 , and an online tool is also available. It is important to remember to ask about deceased relatives, since some people forget to mention them when a family history is taken. Individuals who have had multiple partners should be noted, and full siblings should be distinguished from half siblings as this may provide important clues to a dominantly inherited disorder such as myotonic dystrophy. Consanguinity can be screened by asking if the child’s parents are related by blood, which is common in some cultures. The ethnic background of both sides of the family should be noted since hereditary diseases vary in prevalence by ethnicity.
Assessment of recurrence risk is extremely important for many families who are considering having more children and is often difficult in small families. Genetic testing can be helpful to confirm the diagnosis, establish a precise risk of recurrence, and provide the family with options for future family planning. For an autosomal recessive disorder, parents are usually both asymptomatic carriers for the condition, and neither parental side may report a prior family history of the condition. If the affected child has no siblings, or is a member of a small family with no affected siblings, parents will often dismiss the possibility of a genetic etiology because they have never observed this condition within their family. However, parents who are asymptomatic carriers of an autosomal recessive condition have a 25% risk of recurrence with each pregnancy.
Increasingly, couples considering having children are offered carrier screening for a variety of autosomal recessive conditions, and some obstetricians include carrier screening for spinal muscular atrophy (SMA) in that panel. However, it is important to remember that screening tests are usually not comprehensive and are not 100% sensitive; therefore, even if parents have had normal carrier screening for a condition within the child’s differential, the child could still have the disease due to a mutation not included within the carrier screening panel or due to technical limitations of the carrier screening test.
Autosomal recessive conditions are more frequent when there is a family history of consanguinity, but consanguinity does not guarantee that the condition will be recessive. When the child has two copies of the same mutation, the child is homozygous for the mutation. When the child has two different mutations, the child is a compound heterozygote. Occasionally, a child will be found to carry more than two genetic variants in a gene, and the inheritance of the variants can be most readily determined by testing the parents to determine which variants are in cis (contained on the same copy of the gene and inherited together from one parent) and which are in trans (contained on separate copies of the gene and inherited from each parent separately).
Individuals with an autosomal dominant disorder have a 50% chance of passing the mutation on to any offspring, as is the case for conditions such as myotonic dystrophy or Charcot-Marie-Tooth type IA. In children, a significant percentage of severe and lethal diseases in childhood that are dominantly inherited are due to de novo or new mutations in the child. Although genetic testing from blood in the parents is normal, there is a small chance that one of the parents is a gonadal mosaic and has multiple germ cells carrying the mutation even though the blood cells do not carry the mutation. A 1% risk of recurrence is given to such parents who have a child with a de novo mutation. It is important to note that if a child carries a de novo mutation for a dominant condition, the mutation becomes a germline mutation in the child and the risk of recurrence for that child’s children will be 50%.
For X-linked disorders, the recurrence risk depends solely upon the mother’s genetic status and does not rely upon the father’s genetic status. The lack of male-to-male transmission of the genetic disease is the hallmark of X linkage. If the mother is a carrier for the genetic mutation, there is a 50% risk that her sons (regardless of her partner) will inherit the condition and will be symptomatic while 50% of her daughters will be carriers and unlikely to have classical symptoms for most disorders due to the random process of lyonization or X inactivation in the relevant tissue. However, milder manifestations in female carriers are more common. As an example, some women who are carriers for Duchenne muscular dystrophy will have a mild elevation of CPK and a small percentage will develop signs and symptoms of cardiomyopathy, although most will not develop clinically significant muscular dystrophy. For some X-linked disorders, again using Duchenne muscular dystrophy as an example, there is a high frequency of spontaneous mutations. Women who have sons with spontaneous mutations have a 1% risk of recurrence in future pregnancies due to the small chance of gonadal mosaicism as described previously.
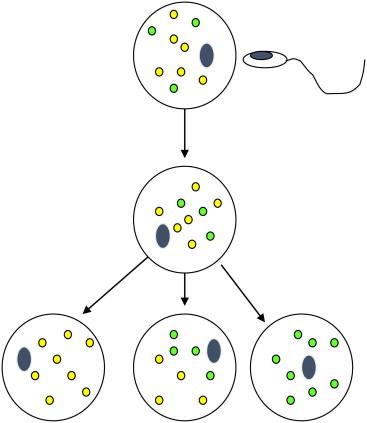
Risk assessment in mitochondrial disorders is more difficult because the genes responsible for mitochondrial function can be encoded in the nuclear or mitochondrial genes, so that inheritance can be either autosomal, rarely X linked, or maternal. The majority of proteins involved in oxidative phosphorylation in the mitochondria are encoded in the nucleus, but there are 13 proteins involved in oxidative phosphorylation, as well as a full set of transfer and ribosomal RNAs encoded in the mitochondrial genome (see Chapter 41 ). Because the brain and skeletal muscle have high energetic demands, clinical manifestations of mitochondrial disorders are often neuromuscular. Nuclear encoded genes lead to straightforward risk of recurrence, but mitochondrial encoded genes are difficult to predict in the next generation due to heteroplasmy; that is, the mitochondria are, in fact, a population of organelles with only a subset of the organelles carrying the mutation ( Figure 2.2 ). Only the maternal contribution is relevant for disorders encoded within the mitochondria since all mitochondria in the embryo and fetus are derived from the egg. From one egg to the next, the percentage of mitochondria with the mutation varies and is not predictable, and within the developing fetus as cells divide by mitosis the distribution of mutant mitochondria between tissues can vary and give rise to significant heterogeneity in severity and affected organs. Thus, prenatal diagnosis for mutations encoded within the mitochondria is imprecise and can be inaccurate.
For all modes of inheritance, the genetic information for a gene is referred to as the genotype; the manifestations of that gene are referred to as the phenotype, and in medicine this largely refers to the clinically observable signs and symptoms of disease. For most pediatric neuromuscular conditions, the penetrance or the probability of expressing a manifestation of a genetic mutation is usually high and often 100%. However, in many cases, manifestations may not be present at birth, so penetrance can be age dependent. In genetic conditions, the phenotypic manifestations may differ from person to person, a phenomenon referred to as variable expressivity .
Aside from mutations in single genes, genetic abnormalities may result from contiguous gene defects of entire chromosomes or parts of chromosomes. Children can be born with extra or missing chromosomes (called aneuploidy), often associated with neonatal hypotonia. With the exception of the X and Y chromosomes, having a missing chromosome tends to be lethal during embryonic or fetal development. Trisomy is lethal for most autosomes during the prenatal period but is compatible with life for chromosomes 13, 18, and 21, although each of these is associated with moderate to severe intellectual disability. Chromosome rearrangements may occur within a chromosome, such as deletion, duplication, or inversion, or may involve exchange of genetic material between a pair of chromosomes, referred to as translocation. Translocations are balanced when no genetic material is gained or lost overall. Balanced translocations are less likely to result in disease compared to unbalanced translocations, but disruption of a gene at the translocation breakpoint can lead to clinical manifestations that are often quite specific and well defined. Deletions are generally more severe than duplications, and in general the larger the deletion or duplication and the greater the number of genes involved, the more severe are the clinical manifestations. New, extremely sensitive technologies called chromosome microarrays are now available to detect segmental aneuploidies or copy number variants associated with microdeletions or microduplications.
For a small number of genes, the parent of origin is paramount because only one copy of the gene is expressed. Whether the maternal or paternal copy of the gene is expressed is consistent for each imprinted gene, but some genes along a chromosome segment will be maternally expressed and paternally imprinted (silenced) while other genes in close proximity on the same chromosome will be paternally expressed and maternally imprinted (silenced). An example of imprinting is the Prader-Willi syndrome/Angelman syndrome locus on chromosome 15. Prader-Willi syndrome often presents as neonatal hypotonia and is due to defects in the paternal copy of the SNRPN locus on 15p, including deletions of the paternal locus, two maternal copies of chromosome 15 (uniparental disomy for chromosome 15), or rarely mutations in the imprinting locus. Angelman syndrome is due to defects in the maternal copy of the nearby UBE3A gene on chromosome 15p, including deletions of the maternal gene, two paternal copies of chromosome 15, mutations in UBE3A , or rarely mutations in the imprinting locus. The imprint is erased and reset at each generation. Imprinting disorders, when inherited, can thus have a peculiar pattern of inheritance because of appearing to skip generations. However, most imprinted disorders in pediatric neuromuscular conditions arise as de novo mutations, and there is rarely a family history of the disorder.
Understanding the basic anatomy of a gene is necessary to appreciate the nature and consequences of genetic mutations and to understand the limitations of genetic tests. A prototypical gene is shown in Figure 2.3 . Transcription begins with the binding of RNA polymerase to the promoter, and the full length of the gene is then copied into RNA. There may be enhancers of gene expression located at some distance from the promoter located upstream or downstream of the gene, or embedded in the middle of the gene in an intron. The gene is composed of exons and introns. After transcription is complete, the introns are removed by splicing to produce the messenger RNA (mRNA), which is exported from the nucleus and translated into a protein in the cytoplasm. The sequences at the junction of the intron and exon are critical for correct splicing, and consensus sequences mark the splice donor and splice acceptor sites. Splice acceptors always end in an AT sequence, preceded by a “consensus sequence,” and donors begin with GC, followed by a conserved consensus sequence. Mutations in these sequences at the splice junctions can result in aberrant splicing, leading to an abnormal mRNA and abnormal protein product. Mutations can also occur deep within introns or as synonymous changes (which do not change the amino acid encoded by the codon) within the exons leading to alternative splice sites that can also lead to aberrant splicing.
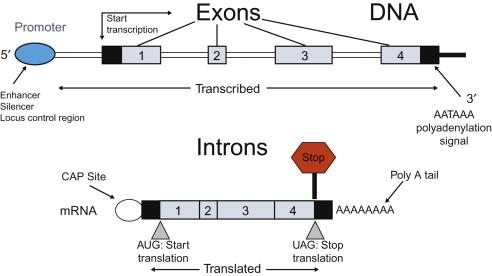
Splice mutations at the splice junction are readily detected by most molecular genetic methods used in genetic testing. However, with the exception of the small number of genetic tests that are preformed directly on the mRNA, mutations leading to aberrant splicing outside the splice junction can easily be missed because they may not be included within the target for sequencing. Mutations in enhancers are also frequently missed by most genetic tests because they have not been well defined and are not included in the sequence analyzed in most genetic tests.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here