Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Protozoans and helminths are responsible for a significant burden of human disease, disproportionately affecting resource-limited countries worldwide. These agents are collectively referred to as parasites, implying dependence on an adversely affected host, but in this context they are no different from other infectious agents, such as bacteria and viruses.
Protozoan parasites are single-celled, microscopic organisms that generally undergo multiplication in the mammalian host. In contrast, helminthic parasites are multicellular, vary tremendously in size, and, in general, are less capable of multiplying within the mammalian or definitive host.
Protozoa and helminths have complex life cycles and have adapted to exist within the hostile environment of one and sometimes several hosts. The distribution of these infectious agents parallels the poor socioeconomic conditions in the developing world. However, with widespread travel, infections due to these agents are being seen more frequently in nonendemic settings. In addition, with the increasing number of immunosuppressed patients resulting from infection with human immunodeficiency virus (HIV) or after transplant or chemotherapy, disease manifestations can be more frequent, severe, and aggressive. Central to the clinician’s approach to the patient with a central nervous system (CNS) infection with a protozoan or helminthic organism is a thorough travel history and an increased index of suspicion. Once such an infection is suspected, an appropriate work-up can be initiated.
The protozoa and helminths that cause the bulk of CNS disease are summarized in Table 46-1 .
| Organism | Geographic Distribution | Major CNS Syndromes | Mode of Infection |
|---|---|---|---|
| Protozoans | |||
| Plasmodium falciparum | Africa, Haiti, South America, Southeast Asia, Oceania | Encephalopathy, coma, seizures | Mosquito |
| Toxoplasma gondii | Cosmopolitan |
|
Undercooked meat; contaminated water; vertical transmission |
| Trypanosoma brucei gambiense , T. brucei rhodesiense | Democratic Republic of Congo>Chad, Central African Republic ( T.b.g .); Malawi, Uganda ( T.b.r ) | Personality changes, indifference, stupor, and coma in late stages | Tsetse flies |
| Naegleria fowleri | Southern United States, Australia, Great Britain, former Czechoslovakia | Acute rapidly progressive meningoencephalitis | Fresh water |
| Acanthamoeba spp. | Cosmopolitan | Subacute, chronic meningoencephalitis | Fresh water, soil |
| Balamuthia mandrillaris | Southern United States, South America | Subacute, chronic meningoencephalitis | Soil>water |
| Entamoeba histolytica | Africa, Mexico, South America, India, Southeast Asia | Brain abscess | Fecal–oral |
| Helminths | |||
| Taenia solium | Cosmopolitan | Neurocysticercosis: seizures, hydrocephalus, chronic meningitis | Fecal–oral |
| Trichinella spp. | Cosmopolitan | Seizures, meningoencephalitis | Uncooked meat |
| Angiostrongylus cantonensis | Southeast Asia, Pacific Basin (including Hawaii) | Eosinophilic meningitis | Uncooked snails, crustacea |
| Gnathostoma spinigerum | Southeast Asia>Mexico, Central and South America | Eosinophilic meningitis | Uncooked fish, frog, bird, snake |
| Toxocara spp., Baylisascaris procyonis | Cosmopolitan | Seizures, palsies, retinal mass | Fecal–oral |
| Schistosoma species | Africa, Asia, Brazil | Seizures, cerebritis, tumor, spinal cord compression | Exposure to infected fresh water |
| Paragonimus species | Asia, Central and South America | Meningitis, mass lesion, infarction | Uncooked crustacea |
Malaria is the most important parasitic infection worldwide. In 2017 an estimated 219 million cases were reported, about 90 percent of which were from Africa. About 435,000 deaths were due to malaria, with approximately 60 percent occurring in children younger than 5 years of age. While Southeast Asia continues to see steady declines in malaria cases, declines in Africa have slowed or reversed recently.
Malaria infection is widely distributed ( Fig. 46-1 ). Five species of malaria infect humans: Plasmodium falciparum , Plasmodium vivax , Plasmodium ovale , Plasmodium malariae , and Plasmodium knowlesi , although P. knowlesi is predominantly zoonotic. P. falciparum predominates in sub-Saharan Africa (>99% of cases), Haiti, New Guinea, Southeast Asia, South America, and Oceania. P. vivax predominates in the Americas and is common in endemic areas outside of Africa. Approximately 1,700 cases of malaria are diagnosed in the United States per year, primarily in recent travelers to and immigrants from areas in which malaria is endemic.

The life cycle of Plasmodium is similar across species. The infective sporozoites are injected by female Anopheles mosquitoes into subcutaneous tissue or directly into the blood stream, and thereafter circulate to the liver to invade hepatocytes. Parasites multiply, and after 1 to 2 weeks, schizonts rupture and release thousands of merozoites, which then enter the blood stream to infect erythrocytes. Of the five Plasmodium spp. that infect humans, P. vivax and P. ovale can establish a latent liver stage, which must be treated to avoid recrudescent infection.
Invasion of erythrocytes by merozoites requires specific surface receptors on the parasite and erythrocyte. In contrast with other Plasmodium species, P. falciparum uses multiple redundant pathways to invade, including sialic acid-dependent glycophorin pathways and nonsialic acid-dependent ones. In addition, P. falciparum can invade erythrocytes of all ages, and parasitemia can reach high levels. The magnitude of parasitemia was previously thought to relate to the degree of morbidity and mortality, but other factors such as the degree of metabolic stress in nutritionally deficient children, presence of parasitic coinfections, and other less-understood mechanisms may play significant roles in adverse outcomes.
Within the erythrocyte, merozoites eventually develop into schizonts, which rupture to release merozoites capable of infecting new erythrocytes. Only the asexual erythrocytic stages are directly deleterious to the host, and the mechanisms involved in the development of clinical manifestations are related to fever, anemia, and tissue hypoxia, as well as to host factors including immunopathologic events.
While high fever and rigors are the hallmarks of acute malaria, some patients with acute malaria, often those under age 5, will develop severe malaria, which is defined as having clinical or laboratory evidence of organ dysfunction (e.g., renal failure, pulmonary edema, coma). Most severe malaria, including cerebral malaria, occurs in patients with P. falciparum , though P. vivax can also cause severe malaria.
Cerebral malaria occurs in 0.5 to 1 percent of P. falciparum cases and is associated with a 15 to 20 percent mortality. Significant neurologic sequelae develop in around 10 percent of cases. Sequestration of parasitized erythrocytes within the capillaries of the cerebral cortex is typically observed, but is likely not the only pathogenic mechanism. While the pathogenesis of cerebral malaria is incompletely understood and remains highly speculative, it is postulated that parasitized red blood cells express parasite-derived variant surface antigens, causing the erythrocytes to adhere to endothelial cells of venules, resulting in microvascular obstruction. Specific var genes, the gene family that encodes the malarial cytoadherence protein P. falciparum erythrocyte membrane protein 1, and host receptors are postulated to play key roles in cerebral malaria pathogenesis. In addition, recent data suggest that endothelial cells are a major site of pathology as these cells show signs of activation, including characteristic morphologic changes, upregulation of surface adhesion molecules and antigens, and production of numerous cytokines and other mediators of inflammation.
The World Health Organization (WHO) defines cerebral malaria as unarousable coma in a person with P. falciparum asexual parasitemia in whom other causes of encephalopathy have been excluded. Children under 5 have the highest rates of cerebral malaria, though adults and older children can also develop the disease. As noted earlier, it remains unclear as to what host or parasite factors determine the development of cerebral malaria. Presenting features may include seizures (15 to 20% in adults, 80% in children), disturbances of consciousness, acute delirium, meningismus, and, infrequently, focal neurologic abnormalities, including pyramidal signs, cranial nerve abnormalities, or movement disorders. Decorticate posturing or decerebrate posturing is more common in children and may indicate increased intracranial pressure or hypoglycemia. Adults are more likely than children to present with diffuse encephalopathy and no focal neurologic signs. As severe malaria affects other organs, concomitant findings can include hepatosplenomegaly, pulmonary edema, renal dysfunction, severe anemia, and hypotension. Hypoglycemia is often also seen in cerebral malaria and severe malaria, especially in pregnant women. Other causes of CNS infection and encephalopathy must be excluded, and biochemical screening of blood and examination of cerebrospinal fluid (CSF) are mandatory.
Although cerebral malaria should be suspected in any person with impaired consciousness, fever, and recent travel to or residence in a P. falciparum -endemic country, confirming the diagnosis can be difficult. While demonstration of P. falciparum parasites in thick and thin blood smears should establish the diagnosis, one must consider the possibility of another cause for the impaired consciousness with coincident parasitemia. This concern is especially high for children from countries with high levels of asymptomatic parasitemia. In addition, the sensitivity and specificity of microscopy are operator dependent and can be problematic in nonendemic countries where laboratory technicians are not experienced in microscopic evaluation, or in resource-limited settings where diagnostic equipment is substandard or unavailable.
Several point-of-care diagnostics (now called rapid diagnostic tests or RDTs) based on immunoassays that detect specific parasite proteins have been developed and field-tested. These tests use a small amount of patient blood to determine if the patient is infected with a malaria species and if that species is P. falciparum ; they are rapid and inexpensive. However, these tests are considered complementary to microscopy as they have poor sensitivity in patients with low parasite burdens; cannot determine the level of parasitemia; and do not differentiate between the non- falciparum species of malaria. Like microscopy, RDTs do not distinguish between cerebral malaria and coincidental parasitemia.
When cerebral malaria is a consideration, lumbar puncture should be performed to exclude other causes of encephalopathy. In cerebral malaria, CSF examination occasionally reveals elevated protein concentration or mild pleocytosis, especially if seizures have occurred. Hypoglycorrhachia is not a feature and indicates other causes.
A variety of abnormalities in laboratory tests may be observed in cases of P. falciparum malaria, including a normocytic, normochromic hemolytic anemia, leukopenia, monocytosis, thrombocytopenia, proteinuria, azotemia, elevated liver enzymes, and disseminated intravascular coagulation. With severe P. falciparum malaria, lactic acidosis, elevated blood creatinine, and hypoglycemia may be observed. Hypoglycemia, which is often seen in children and pregnant women or as a result of intravenous quinine therapy, may be responsible for deteriorating neurologic status and so must be recognized and treated.
Currently there is no diagnostic role for brain imaging in cerebral malaria, but there are studies using imaging to try to discern abnormalities specific to cerebral malaria, including prognostic factors and even pathophysiology. Fundoscopy should be performed since the presence of malaria retinopathy is the only reliable clinical feature that distinguishes cerebral malaria from other CNS pathologies.
Severe malaria, including cerebral malaria, should be treated parenterally. The standard of care is now intravenous artesunate. To obtain artesunate in the United States, the CDC Malaria Hotline should be called (770-488-7788 anytime or 855-856-4713 Monday through Friday 9 a.m. to 5 p.m. EST). The dose of intravenous artesunate is weight based. For adults/children greater than 20 kg the dose is 2.4 mg/kg, while for children less than 20 kg the dose is 3.0 mg/kg. Appropriate doses are given at 0, 12, 24, and 48 hours. While awaiting the delivery of intravenous artesunate the CDC lists a number of enteral alternatives that can be given. After the course of artesunate is administered, an oral course of antimalaria drugs should be given. If oral medications cannot be given, then 7 days of intravenous artesunate, doxycycline, or clindamycin can be given.
After initiation of antimalarial therapy, patients with P. falciparum infection must be closely monitored for complications and response to treatment. Seizures should be treated with anticonvulsants, and frequent glucose monitoring is essential to manage hypoglycemia. Maintaining adequate fluid balance is critical in preventing further morbidity, especially in those with renal failure or pulmonary edema. Blood transfusions can be used to correct anemia, but can lead to significant fluid overload. The CDC no longer recommends exchange transfusions.
In an attempt to decrease the mortality and morbidity associated with severe malaria and cerebral malaria, many adjuvant therapies have been tried. Measures that have failed to show clinical improvement or made outcomes worse include broad immunosuppression (e.g., corticosteroids, intravenous immunoglobulins, cyclosporine), anti-TNF treatment (e.g., antitumor necrosis factor alpha antibodies or pentoxifylline), and prophylactic phenobarbital, mannitol, and antithrombotics (e.g., aspirin, ibuprofen, N -acetylcysteine, heparin). Other adjunctive therapies for severe malaria, not specifically cerebral malaria, are currently in trials or their outcomes have not yet been reported.
Toxoplasma gondii is an obligate intracellular protozoan that naturally infects most warm-blooded animals from birds to humans. The prevalence of T. gondii infection in humans worldwide is estimated to be 25 to 30 percent but varies greatly by geographic location and socioeconomic status. Felids (i.e., cats) are the definitive hosts in which the sexual life cycle occurs, while the asexual life cycle occurs in intermediate hosts such as humans. Humans commonly become infected with T. gondii via ingestion of food or water contaminated with the persistent forms of T. gondii (i.e., bradyzoite-containing tissue cysts from intermediate hosts or sporozoite-containing oocysts excreted in the feces from acutely infected felids). As implied by being the “T” in the TORCH acronym for congenital infections, T. gondii can also be vertically transmitted in newly infected pregnant women, leading to the recommendation for pregnant women to avoid contact with cat feces. Infections have also been acquired through blood transfusions, organ transplantation, and accidental inoculation in laboratory workers, though these routes are quite rare.
Upon entry into humans, the sporozoite or bradyzoite, depending on the infective stage ingested, transforms into a tachyzoite that invades host cells and disseminates via the lymph and blood. The tachyzoite invades host cells and forms a protective parasitophorous vacuole within the cytoplasm. Like most intracellular microbes, the parasite modifies the host cell via the secretion of a number of effector proteins. How T. gondii crosses the blood–brain barrier to enter into the brain and retina has not been definitively determined, but evidence exists that T. gondii : (1) passes from the vasculature between endothelial cells; (2) infects endothelial cells and then ruptures across the basolateral side of the cell; or (3) is carried into the brain by infecting an immune cell that then travels across the blood–brain barrier. Tachyzoites infection is controlled by an intact T-cell response (CD8 cells more than CD4 cells) which produce interferon-γ. In skeletal and cardiac muscle and in the brain and retina, parasites switch to the bradyzoite form and establish cysts, which appear to provoke less of an immune response ( Fig. 46-2 ). These cysts persist as reservoirs for reactivation or transmission.

Approximately 80 percent of primary toxoplasmosis infection is asymptomatic in immunocompetent adults. Nontender cervical adenopathy is most frequently seen among symptomatic individuals, but generalized adenopathy and a mononucleosis-like illness may occur. In the immunocompetent patient, the course of toxoplasmosis is usually benign and self-limited. A minority of patients can develop persistent lymphadenopathy or ocular toxoplasmosis. Ocular toxoplasmosis was previously thought to arise primarily from congenital infection, but more recent work suggests that it arises equally often from postnatally acquired T. gondii infection. Ocular lesions usually present as a focal, necrotizing retinitis with intense vitreal inflammation, showing a characteristic “headlight in fog” appearance. Relapses of chorioretinitis are frequent and are evident in the areas around the chorioretinal scars ( Fig. 46-3 ).

Congenital infection arises most commonly as a result of primary infection of the mother during gestation or 3 months prior to conception. Congenital infection can also occur in a chronically infected mother who is severely immunocompromised (e.g., AIDS) during gestation, or in a chronically infected pregnant woman who becomes infected with a new, highly virulent strain to which her prior infection does not confer immunity. Infection acquired in the first trimester is severe and can be associated with spontaneous abortion. Infection later in pregnancy shows more variable severity depending on individual factors. In the newborn, the presence of hydrocephalus, retinochoroiditis, or intracranial calcifications is suggestive of congenital toxoplasmosis and should be evaluated accordingly. Most infants are asymptomatic at birth but may present with overt symptoms, including severe developmental delay later in life.
Toxoplasmic encephalitis most commonly occurs as a result of reactivation of a latent infection which occurs in the setting of significant compromise of T-cell function (e.g., AIDS, organ transplant, and hematologic malignancies). In addition, immunosuppressants including steroids, mycophenolate mofetil, and adalimumab have been linked to rare cases of toxoplasmic encephalitis. In the immunocompromised, primary infection with T. gondii usually results in systemic or lung symptoms, rather than CNS involvement. Immunodeficient individuals with toxoplasmic encephalitis usually manifest with encephalitis, meningoencephalitis, or mass lesions ( Fig. 46-4 ); therefore, toxoplasmic encephalitis often presents as a nonspecific decrease in mentation, seizures, or with focal neurologic findings. Frank meningismus is rare.

The diagnosis of primary toxoplasmosis in immunocompetent hosts, transplant patients, and pregnant women is typically established through serology, either with a positive IgM to IgG seroconversion in paired sera or a twofold increase in IgG titers. Prenatal diagnosis can be confirmed by amniotic fluid polymerase chain reaction (PCR) or fetal ultrasound consistent with findings of congenital toxoplasmosis. Most disease in immunocompromised hosts is reactivation disease, and the presence of IgG antibodies is suggestive of the diagnosis. Among HIV patients, toxoplasmic encephalitis usually occurs after the CD4 count has fallen to less than 100/mm 3 , although those with CD4 counts less than 200/mm 3 are at risk for toxoplasmic encephalitis. If the patient is taking appropriate trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis, then even in the setting of a low CD4 count, toxoplasmic encephalitis is unlikely. Histology of brain biopsy specimens confirms the diagnosis but is impractical in most cases. PCR of serum, CSF, and biopsy tissue has been performed with varying degrees of success. CSF PCR is very specific, but the sensitivity is low (50%). For retinochoroiditis, the diagnosis is usually made by fundoscopic exam. Serology of aqueous humor in parallel with serum or PCR of the aqueous and vitreous can be used in cases where the clinical diagnosis is ambiguous.
Cranial imaging should be performed in patients with suspected CNS toxoplasmosis. Neuroimaging usually reveals multiple ring-enhancing or solid lesions typically located in the gray matter; in HIV patients the cerebral cortex is more common than the basal ganglia, and thalamic locations are more likely than cerebellar ( Fig. 46-4 ). In those with severe immunosuppression, atypical presentations with a lack of enhancement may occur. In infants, computed tomography (CT) is the method of choice, although ultrasound can be used. CSF findings are nonspecific and include mononuclear pleocytosis, elevated protein, and normal glucose. It should be noted that in the setting of mass lesions in the brain parenchyma, lumbar puncture is generally not recommended or indicated.
Definitive diagnosis of toxoplasmosis is made in the presence of a compatible clinical syndrome, consistent findings on magnetic resonance imaging (MRI), CT, or other imaging modalities, and detection of the protozoan in a clinical sample. AIDS patients with multiple enhancing cerebral lesions and with a positive IgG antibody should be presumed to have toxoplasmic encephalitis and started on empiric therapy and monitored for clinical and imaging improvement by 2 to 3 weeks following initiation of therapy. Biopsy is reserved for refractory cases, non-AIDS immunocompromised patients, or in AIDS patients with single lesions or those who are IgG negative.
Suspected or confirmed cases of ocular toxoplasmosis that is active and threatens vision should be treated. Several regimens have been used to treat it successfully, with a combination of pyrimethamine, sulfadiazine, and leucovorin being the most common regimen. Other regimens, including intravitreal clindamycin, are reviewed elsewhere. The use of systemic steroids in combination with antiparasitic drugs is indicated for those with severe vitreous inflammation, affected vision, lesions close to the optic nerve or fovea, and large active lesions.
Management of infected pregnant women varies considerably. Confirming a diagnosis of acute infection is essential due to the potential toxicity of the drugs used for treatment and for guiding decisions about termination of the pregnancy. Treatment indications and regimens for pregnant women and children with congenital toxoplasmosis are reviewed elsewhere.
AIDS patients with CD4 counts less than 100 cells/mm 3 who develop reactivation toxoplasmic encephalitis should always be treated with at least two agents. The first-line treatment for toxoplasmic encephalitis is usually a combination of pyrimethamine and a sulfonamide. These antifolates are active against tachyzoites and are synergistic in combination. Pyrimethamine is lipid-soluble and readily penetrates the brain parenchyma even in the absence of inflammation. The usual regimen for adults is an oral loading dose of 200 mg of pyrimethamine followed by 50 to 75 mg daily for 3 to 6 weeks as well as sulfadiazine at a dose to 1 to 1.5 g orally every 6 hours for 3 to 6 weeks. The lower doses are for patients weighing less than 60 kg and the higher dose for those who weigh 60 kg or above. While patients are taking pyrimethamine and for 1 week after, patients should also receive folinic acid 10 to 20 mg orally daily; folinic acid alleviates some of the hematologic side effects associated with pyrimethamine. For those who are sulfa allergic, clindamycin orally or parenterally 600 mg every 6 hours or atovaquone 1,500 mg orally twice a day can be used in combination with pyrimethamine and folinic acid. In areas where pyrimethamine is not readily available, TMP-SMX (15 to 20 mg/kg daily of the trimethoprim component in three divided doses) has been found to be noninferior to pyrimethamine-based regimens.
For other alternative acute treatment options as well as for chronic treatment options for HIV/AIDS patients, please see the NIH guidelines. Most non-HIV/AIDS immunocompromised patients will be treated with the same acute treatment regimens as listed above, but the chronic phase may differ and should be managed in conjunction with advice from other relevant medical specialists.
Trypanosoma brucei is the causative agent of human African trypanosomiasis, which is also known as “sleeping sickness.” Two distinct forms exist: western (chronic) sleeping sickness, due to infection by T. brucei gambiense , and eastern and southern (acute) sleeping sickness, caused by T. brucei rhodesiense. The tsetse fly, or Glossina , is the vector for T. brucei . Fewer than 1,000 new cases of human African trypanosomiasis were reported to the WHO in 2018, which represents a marked drop in cases over the last 20 years driven by sustained and focused efforts to treat patients with human African trypanosomiasis.
The majority of T. b. gambiense cases occur in the Democratic Republic of Congo, with the Central African Republic and Chad reporting far fewer cases. The disorder has a subacute course and is perpetuated mainly through chronically infected humans. East African trypanosomiasis ( T. b. rhodesiense ) occurs in the eastern and southern parts of equatorial Africa, especially Malawi and Uganda, and is typically acquired from zoonotic sources. It has a more fulminant course, with death occurring within a few weeks after infection, thereby decreasing the possibility of human reservoirs for infection. Most cases in travelers have been due to T. b. rhodesiense.
T. brucei undergoes several developmental stages in the tsetse fly over a life cycle of about 3 weeks. Upon ingestion with a blood meal, blood stream trypomastigotes become procyclic trypomastigotes in the insect midgut and divide rapidly. These then leave the midgut and transform into epimastigotes, eventually making their way into the fly’s salivary glands. They change into metacyclic trypomastigotes, which are injected into the human or mammalian host with the blood meal. The metacyclic trypanosomes transform into the bloodstream form and invade extracellular spaces, including the blood, lymphatics, tissue fluids, and, eventually, the CNS via the CSF ( Fig. 46-5 ).
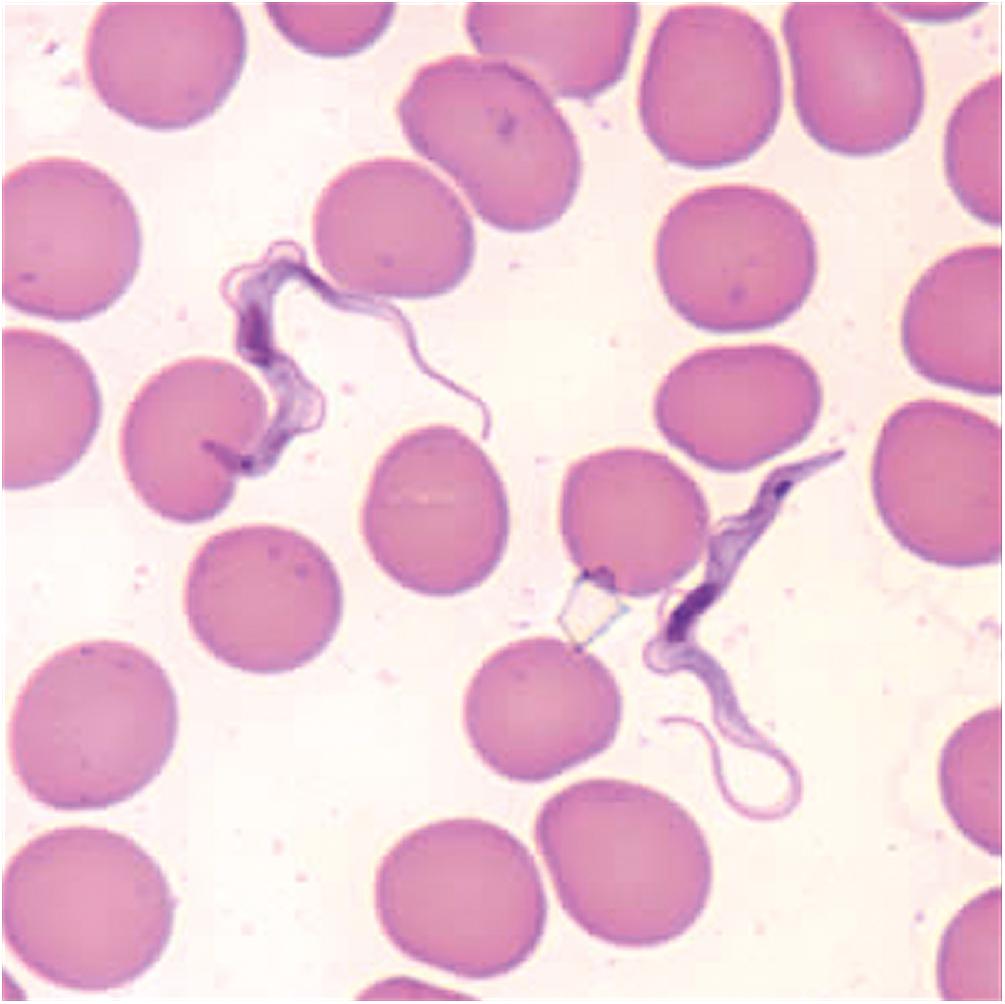
Blood stream trypomastigotes evade the immune system by antigenic variation of their variant surface glycoproteins. In addition, T. b. gambiense and T. b. rhodesiense are resistant to killing by human apolipoprotein A1, which is a major trypanolytic factor that protects humans against other T. brucei subspecies infection. T. b. gambiense and T. b. rhodesiense ’s resistance to this protein underlies their ability to cause systemic human infections.
Clinically T. brucei disease is divided into two stages: the hematolymphatic stage (stage 1) and the meningoencephalitic stage (stage 2), with T. b. gambiense having a more prolonged course (mean time to death 3 years) and T. b. rhodesiense being relatively rapid (death within 6 months). While progressive untreated disease generally results in death, asymptomatic carriers and spontaneous cures have been reported for T. b. gambiense infections. The earliest clinical manifestation is a local, hard, painful lesion (trypanosomal chancre) at the site of the insect bite, which is more common in T. b. rhodesiense infection and rare with T. b. gambiense . Histologic examination of the chancre shows a lymphocytic infiltrate with dividing trypanosomes. Shortly afterward, widespread dissemination of the organism occurs via the blood stream and lymphatics. Lymphadenopathy of the posterior cervical regions (i.e., Winterbottom sign) is typical in T. b. gambiense infection, while T. b. rhodesiense more commonly causes submandibular, axillary, and inguinal lymphadenopathy. The first stage of T. b. gambiense infection can result in days to months of intermittent fever, headache, hepatosplenomegaly, and endocrine dysfunction. For T. b. rhodesiense , the first-stage symptoms are similar but less prolonged. Of note, travelers to endemic areas are more likely to present with chancres for either T. brucei infection and the incubation period is often shorter (<3 to 4 weeks). With progression of the disease, the systemic episodes decline in severity. The second stage occurs as the trypanosomes cross the blood–brain barrier and enter the brain. In this stage, sleep and neuropsychiatric symptoms predominate. These symptoms include daytime sleepiness, night-time insomnia, and abnormalities on polysomnography. In addition, a range of behavioral changes can also occur, including personality changes (e.g., emotional lability, aggression, apathy, or mania), confusion, and frank dementia. As the disease progresses, the neurologic manifestations become more severe, culminating with coma and death.
Definitive diagnosis relies on observation of trypanosomes in aspirates from lymph nodes or concentration techniques used on blood or CSF. For T. b. rhodesiense , since the parasitemia level is higher, if concentration techniques cannot be done, motile trypanosomes may be visualized on nonconcentrated thin or thick blood smears in the early stages. The card agglutination trypanosomiasis test, which detects specific antibodies, is of value for screening of at-risk patients for T. b. gambiense but usually requires parasitologic confirmation as all areas of the world now have very low prevalence rates (<0.1%). CSF examination is essential for stage determination but cannot be used for definitive diagnosis of T. brucei disease unless trypanosomes are seen on microscopic examination.
Drug treatment is dependent on the stage of illness and the type of trypanosomiasis. The first stage is defined as patients with the diagnosis of T. brucei infection with a CSF leukocyte count of 5/mm 3 or less and no trypanosomes visualized on CSF (i.e., no obvious CNS involvement), and the second stage is defined as those with CSF leukocyte counts greater than 5/mm 3 or trypanosome visualization. For T. b. gambiense , with the approval of fexinidazole, second-stage disease is now subdivided in nonsevere (CSF WBC less than 100/mm 3 ) or severe (CSF WBCs greater than 100/mm 3 ). In T. b. gambiense infection, for people age 6 or older and body weight greater than or equal to 20 kg, those with first stage or nonsevere second stage should be treated with fexinidazole, while those with severe second stage (regardless of age or weight) should be treated with a nifurtimox-eflornithine combination therapy. Further details about T. b. gambiense treatment, including alternative therapies, are defined elsewhere. For T. b. rhodesiense , first-stage disease is treated with suramin and second-stage disease with melarsoprol. Melarsoprol is reserved for T. b. rhodesiense second-stage disease because it has a high rate of serious adverse reactions, including an encephalopathy syndrome that has a high mortality rate.
Primary amebic meningoencephalitis is an acute, rapidly progressive, necrotizing meningoencephalitis caused by the free-living ameba Naegleria fowleri . Despite the widespread distribution of N. fowleri , the disease is relatively rare and is associated with exposure to warm fresh water. In the United States, over 80 percent of the cases have occurred in the summer months and primarily in the most southern states, although Kansas, Missouri, and Minnesota have recently reported cases.
Infection is acquired when water containing the ameba is accidentally inhaled. Thus, most cases of N. fowleri are associated with recreational activities in warm freshwater lakes, though cases of N. fowleri have occurred in children bathing or playing in untreated well water or underchlorinated municipal water, as well as in adults who used N. fowleri –contaminated water for nasal irrigation. The ameba rapidly invades the olfactory neuroepithelium, gaining access to the brain through the cribriform plate. Naegleria infection initially involves the superficial gray matter with eventual extension to the deep matter and cerebellum. Prominent involvement of the frontotemporal areas, olfactory bulbs, and subarachnoid space has been observed. Neutrophil invasion, extensive necrosis, and vascular invasion are typical. The pathogenesis of infection likely involves multiple mechanisms, including secretion of lytic enzymes, elucidation of apoptotic factors, production of membrane pore-forming proteins, and direct feeding by the amoeba, resulting in a marked cytopathic effect.
Patients usually present within 5 days from exposure, although incubation periods of up to 14 days have been reported. The symptoms are commonly consistent with acute bacterial meningitis, including severe headache, fever, lethargy, nausea, and vomiting. Olfactory involvement, manifesting as nasal stuffiness or changes in smell, may be a clue to the etiology. Seizures, nuchal rigidity, and confusion, followed by coma, usually develop within days. Focal neurologic signs are rare. The patient then rapidly deteriorates and death occurs within a week. Brainstem herniation due to inflammation and increased intracranial pressure is the usual cause of death.
CSF findings are typical of acute bacterial meningitis with an elevated opening pressure, neutrophilic pleocytosis, low to normal glucose, and elevated protein levels. Elevated CSF red blood cells occur late in the course. If N. fowleri is suspected, a wet mount of CSF should be performed as it may show mobile trophozoites. Ideally the wet mount should be done rapidly after CSF collection as ameba may adhere to the container. Giemsa or Wright stain can help differentiate the trophozoite from host cells ( Fig. 46-6 ). N. fowleri can also be identified through PCR of CSF or brain tissue or via commercially available next-generation sequencing of DNA or RNA obtained from CSF or brain tissue. Ameba can also be detected by immunohistochemical means on formalin-fixed brain.

Only a few survivors of primary amebic meningoencephalitis have been reported, likely because of delays in diagnosis, the rapid progression of symptomatic disease, and the lack of clarity regarding best treatments. Most survivors have received high-dose intravenous amphotericin as well a combination of additional drugs such as fluconazole, azithromycin, rifampin, and miltefosine. In addition, treatment should address elevated intracranial pressure and brain edema with the use of adjunct therapies such as dexamethasone, CSF drainage, hyperosmolar therapy, and even induced hypothermia. The Centers for Disease Control and Prevention Emergency Operation Center (open 24/7, 770-488-7100) can help with diagnostic and treatment recommendations. Of note, a recent case of supposed primary amebic meningoencephalitis, originally diagnosed as viral meningitis, with spontaneous recovery of the patient, was determined to be caused by a Paravahlkampfia species. This case raises the possibility that the rare survivors of primary amebic meningoencephalitis, in times prior to definitive molecular diagnostics, were infected with other free-living ameba rather than N. fowleri .
Granulomatous amebic encephalitis is a subacute, indolent, usually fatal meningoencephalitis caused by either of two species of free-living ameba, Acanthamoeba spp. or Balamuthia mandrillaris .
Acanthamoeba spp. have been isolated across the globe from air, water, and soil. Balamuthia also is ubiquitous, though it is more commonly isolated from soil than water. Like N. fowleri , based on serology positivity, many people are presumed to be exposed to Acanthamoeba spp. and Balamuthia with the development of clinical disease being relatively rare. Humans are thought to become actively infected with either agents of granulomatous amebic encephalitis via the lower respiratory tract or broken skin. While Acanthamoeba disease primarily occurs in immunocompromised people, Balamuthia disease occurs more often in the immunocompetent, though it can also occur in the immunocompromised. In the United States, Balamuthia infection is seen more often in males, those of Hispanic race, and residents of the southern latitudes of the United States. The increased risk of Balamuthia infection in males and Hispanics may be due to an increased risk of exposure (i.e., more likely to be exposed to soil) or yet-to-be-defined genetic risk factors. Several cases of Balamuthia have occurred via transplantation of organs from persons not recognized to be infected with Balamuthia .
Acanthamoeba granulomatous amebic encephalitis has an insidious onset of symptoms, and presents with both nonspecific complaints, such as mental status changes and fever, as well as focal abnormalities including seizures, hemiparesis, and ataxia. As the disease progresses, increased intracranial pressure eventually causes lethargy and coma, with death occurring due to brain herniation. Balamuthia disease can present either with subacute or chronic symptoms as described earlier and can result in death within a week or over months after the onset of symptoms.
Space-occupying or ring-enhancing lesions, either single or multiple, on neuroimaging are characteristic of granulomatous amebic encephalitis. Rarely, Balamuthia infection can also demonstrate hemorrhage within the ring-enhancing lesions. CSF, when obtained, typically shows a lymphocytic pleocytosis, increased protein concentration, and slightly low or normal glucose level. Unlike primary amebic meningoencephalitis, identifying ameba in the wet mount is uncommon but has been reported. Serologic tests for either Acanthamoeba or Balamuthia are not helpful. Tissue diagnoses are most common, and unfortunately often occur at autopsy. To definitively distinguish the causative agent of granulomatous amebic encephalitis, either ameba-specific immunohistochemistry on tissue sections or molecular diagnostics (e.g., PCR, next-generation sequencing) on RNA or DNA isolated from tissue or CSF must be done. Of note, Acanthamoeba spp. and Balamuthia can both present with preceding skin lesions, which should be biopsied if present.
Akin to primary amebic meningoencephalitis, the limited number of patients diagnosed premortem and of survivors means no definitive treatment regimens have been defined. In general, treatment of granulomatous amebic encephalitis usually involves multidrug regimens that include some combination of pentamidine, an azole (e.g., fluconazole for Balamuthia ), sulfadiazine, flucytosine, miltefosine, and amikacin (for Acanthamoeba ) or azithromycin/clarithromycin (for Balamuthia ). Even in the setting of early recognition and aggressive therapy, most patients with granulomatous amebic encephalitis will die.
Amebiasis is disease caused by Entamoeba spp., some of which are nonpathogenic and some of which cause diarrhea only. Entamoeba histolytica causes tissue-invasive disease, including cerebral abscesses. Amebiasis most commonly occurs in socioeconomically deprived communities in developing countries, travelers returning from these areas, chronically institutionalized individuals, and men who have sex with men.
Infection occurs by ingestion of cysts with sources including fecal–oral transmission and contaminated food or water. Excystation occurs in the small intestine, producing trophozoites. The parasite colonizes the colon where some trophozoites encyst and are passed in the stool to begin the cycle anew. Invasive disease begins when trophozoites invade the colonic mucosa leading to inflammatory colitis. Extraintestinal spread is rare and most frequently involves the liver, which can then lead to spread locally or distally (lungs, genitourinary system, skin, and brain). Approximately 80 percent of the patients with a liver abscess are young–middle aged men.
Cerebral abscesses occur in 1 to 5 percent of those with liver abscesses and present with the findings of a space-occupying lesion such as focal findings, seizures, mental status changes, and headache. CSF findings are nonspecific, including a pleocytosis and an elevated protein; lumbar puncture is not necessarily recommended. CT and MRI findings will be consistent with an abscess.
Cerebral amebiasis is often diagnosed clinically (e.g., cerebral abscess in a patient with an amebic liver abscess or recently treated liver abscess) as there is no specific, proven diagnostic test. In liver abscesses, PCR of the abscess fluid or antigen detection from the serum or abscess fluid can be performed. In addition, serologic testing can be a useful adjunct with the recognition that serologies can be negative early in disease and that a positive serology in a patient coming from an endemic region does not distinguish prior disease from current disease or unrelated coinfection. Stool microcopy is very low yield in these patients and is not recommended.
Like bacterial brain abscesses, the treatment is generally a combination of surgical drainage and antimicrobial therapy (metronidazole) for 6 or more weeks.
Due to their relatively large size (50 μm up to 15 m), these parasites pose a distinct problem for host immunity. Helminths capable of causing CNS disease are diverse, with complex life cycles involving both human and nonhuman animal hosts during different stages of their development. Helminths are generally identified by taxonomic class [i.e., as cestodes (tapeworms), nematodes (roundworms), or trematodes (flukes)]. Except for disease caused by Taenia solium (tapeworm), helminth infections of the CNS are rare to extremely rare. Thus, this section will focus on T. solium with very brief comments about other helminths. Table 46-1 includes a summary of the most common helminths that cause CNS infections.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here