Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
If you listen carefully to the patient, they will tell you the diagnosis. William Osler
Arguably, perilymph fistula (PLF) is the single most polarizing diagnosis in our specialty. As a resident at UCLA, I was taught that PLFs do not exist. This was ironic, because only a generation before, UCLA’s Victor Goodhill was one of the thought leaders in the pathophysiology of PLF ( Fig. 25.1 ). Goodhill coined the terms implosive (Valsalva-induced) and explosive (increased intracranial pressure) to describe the pressure changes that can result in PLF. I believe that there are three principal factors that led to the current negative perception of PLF and PLF surgery in the United States: (1) there is no clear description or understanding of what constitutes the clinical phenotype of PLF; (2) there are challenges in pre- and intraoperative diagnosis; and (3) the determination of “poor PLF surgical outcomes” occurred in the era before superior semicircular canal dehiscence (SSCD) and vestibular migraine (VM) were recognized clinical entities.
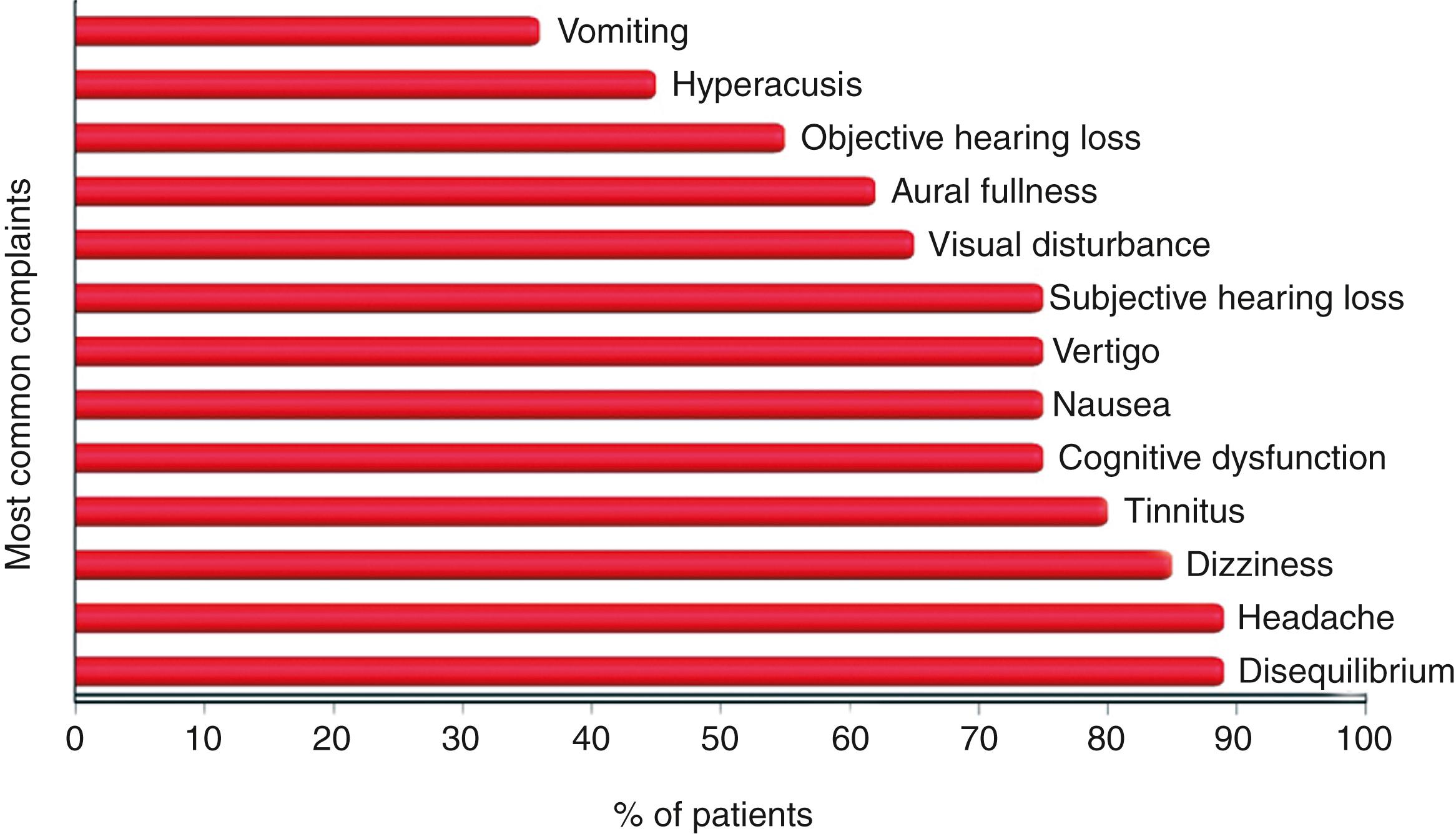
The literature has been conflicted about the frequency of symptoms and diagnostic test findings in patients with PLF. One illustrative summary that highlights the spectrum of the most common complaints from patients with PLF was published nearly a quarter century ago. Fig. 25.2 shows the percentage of these patients reporting each of the 13 most common complaints. The three most frequent complaints were disequilibrium, headache, and dizziness. Other important clinical symptoms included cognitive dysfunction, nausea, visual disturbance, and objective and subjective hearing loss. The review of Fig. 25.2 also demonstrates that these are extraordinarily similar to the spectrum of symptoms of patients with SSCD and VM experience. Table 25.1 outlines the contemporary spectrum of symptoms, signs, or exacerbating factors seen in third window syndrome (TWS) and the diagnostic tools and metrics available to measure these clinically observed phenomena.
| Category | Symptoms, Signs, or Exacerbating Factors | Diagnostic Tools and Metrics |
|---|---|---|
| Sound-induced | Dizziness or otolithic dysfunction (see vestibular dysfunction below); nausea; cognitive dysfunction; spatial disorientation; migraine/migrainous headache; pain (especially children); loss of postural control; falls | History; 128-Hz and 256-Hz tuning forks applied to ankles, knees, and/or elbows heard or felt in the ear or head; pneumatic otoscopy; cVEMP/oVEMP with reduced threshold with or without increased amplitude, auditory stimuli inducing symptoms; Romberg test while pure tones are delivered to individual ear or low-frequency tuning fork applied to elbow |
| Autophony | Resonant voice; chewing; heel strike; pulsatile tinnitus; joints or tendons moving; eyes moving or blinking; comb or brush through hair; face being touched | History |
| Vestibular dysfunction | Gravitational receptor (otolithic) dysfunction type of vertigo (rocky or wavy motion, tilting, pushed, pulled, tilted, flipped, floor falling out from under); mal de débarquement illusions of movement | History; Dizziness Handicap Inventory (DHI); cVEMP/oVEMP; computerized dynamic posturography; Romberg/sharpened Romberg; head tilt; nuchal muscle tightness |
| Headache | Migraine/migrainous headache; migraine variants [ocular, hemiplegic or vestibular (true rotational vertigo)]; coital cephalgia; photophobia; phonophobia; aura; scotomata | History; Headache Impact Test (HIT-6); Migraine Disability Assessment Test (MIDAS) |
| Cognitive dysfunction | General cognitive impairment, such as mental fog, dysmetria of thought, mental fatigue; impaired attention and concentration, poor multitasking (women > men); executive dysfunction; language problems including dysnomia, agrammatical speech, aprosodia, verbal fluency; memory difficulties; academic difficulty including reading problems and missing days at school or work; depression and anxiety | History Cognitive Screen: MoCA and Schmahmann syndrome scale IQ: WRIT or WAIS2 Attention: NAB, Attention Module and/or CPT3 Memory: CVLT2, WMS4, or WRAML2 Executive Functioning: WCST, TMT, D-KEFS Language: NAB, Naming Visuospatial: Benton JLO Mood/personality: Clinical interview, PHQ-9, GAD-7, ACES, BDI2, BAI, Personality Assessment Inventory (PAI), or Millon Behavioral Diagnostic |
| Spatial disorientation | Trouble judging distances; detachment/passive observer when interacting with groups of people; out-of-body experiences; perceiving the walls or floor moving | History; subjective visual vertical |
| Anxiety | Sense of impending doom | History; GAD-7; BAI |
| Autonomic dysfunction | Nausea; vomiting; diarrhea; lightheadedness; blood pressure lability; change in temperature regulation; heart rate lability | History; autonomic testing |
| Endolymphatic hydrops | Ear pressure/fullness not relieved by the Valsalva maneuver; barometric pressure sensitivity | History; electrocochleography, tympanometry |
| Hearing | Pseudoconductive hearing loss (bone-conduction hyperacusis) | Comprehensive audiometric evaluation including tympanometry, stapedial reflex testing, speech perception testing, air-conduction and bone-conduction thresholds; magnitude varies by site of dehiscence |
The more general term of TWS is more appropriate than is SSCD syndrome because the same spectrum of symptoms, signs on physical examination, and audiologic diagnostic findings are encountered with SSCD. These include cochlea-facial nerve dehiscence (CFD), cochlea-internal carotid artery dehiscence, cochlea-internal auditory canal dehiscence, lateral semicircular canal-superior semicircular canal ampulla dehiscence, modiolus, “PLF,” posterior semicircular canal dehiscence, posterior semicircular canal-jugular bulb dehiscence, SSCD-subarcuate artery dehiscence, SSCD-superior petrosal vein dehiscence, vestibule-middle ear dehiscence, lateral semicircular canal-facial nerve dehiscence, wide vestibular aqueduct in children, posttraumatic hypermobile stapes footplate, otosclerosis with internal auditory canal involvement, and CT– TWS (see reference 3). A common structural finding in all of these conditions is an otic capsule defect that creates a ‘third mobile window.’ In the light of our recognition that there are multiple sites where third windows occur in the otic capsule, it is interesting to note that Kohut’s definition of a PLF, from over a quarter century ago, still applies to all currently known sites producing a TWS; “A perilymph fistula may be defined as an abnormal opening between the inner ear and the external surface of the labyrinth capsule….” Hence, a fistula of the otic capsule (Kohut’s definition) can occur in any location that is in communication with the perilymph, whether an SSCD, CFD, or any of the well-established sites that can result in a TWS.
A central problem with understanding peripheral vestibular disorders or communicating associated symptoms is the use of poor, or at least imprecise, terminology. The terms vertigo, dizziness, and disequilibrium are frequently used; however, what do they mean? To answer this question, a brief review of peripheral vestibular function is necessary.
The role of the 10 vestibular receptors is to transduce the forces associated with head acceleration and gravity into a biologic signal. The central nervous system integration of these data results in the subjective awareness of the head position relative to the environment. Motor reflexes that maintain gaze and posture are generated in response to afferent vestibular inputs. Propulsion and orientation of the body in space depend on the vestibular system, vision, and proprioceptive system. Most persons can manage with only two of these systems, but not with one. Accordingly, patients with vestibular dysfunction may have additional difficulty in maintaining equilibrium when their vision or proprioception is impaired.
The vestibular system, through its signal transduction by the peripheral end-organs and their afferent neurotransmission, constantly signals the position of the head in space and effects a continuous adjustment of the musculature of the body. More specifically, it signals the acceleration and deceleration of motion. The otolith organs are capable of signaling only linear acceleration or deceleration, whereas the cristae within the semicircular ducts are able to signal angular acceleration or deceleration. Constant motion and acceleration cannot be detected by the vestibular system.
The peripheral vestibular system is a unique neurosensory system. At rest, the type I and type II vestibular hair cells and their primary afferent neurons have a relatively constant and symmetrical resting discharge rate of approximately 80 spikes per second. This discharge rate increases if the stereocilia are deflected toward the kinocilium of each type I or type II vestibular hair cell and decreases if they are deflected away from the kinocilium. Transduction of accelerated motion is brought about by the movement of the endolymph, which is coupled to the stereocilia and kinocilia of the neuroepithelium. All the kinocilia are oriented in the same direction relative to the long axis of each crista, and the flow of endolymph in one direction results in the same discharge characteristics for all the hair cells in each individual end-organ. A further level of redundancy exists in the push-pull organization between both sets of vestibular apparatus. For example, with rotation to the right in the horizontal plane, there is a relative flow of the endolymph to the left. The resting discharge rate from the right horizontal crista ampullaris is greatly increased as the cupula is deflected toward the vestibule (i.e., ampullipetal displacement), whereas the discharge rate from the left side decreases by an equal amount as the cupula of the left horizontal crista ampullaris is deflected away from the vestibule (i.e., ampullifugal displacement). Normally, this bilateral system is constantly at work receiving signals and passing them on to regulate the posture and movement of the body, limbs, and eyes. Under normal circumstances, the vestibular signals produced by each side are equal and opposite in magnitude bilaterally. The paired otolithic organs function by similar mechanisms, except that the type I and type II vestibular hair cells are coupled to gravitational force through the otolithic membrane and their overlying otoconia, which are polarized relative to a region called the striola . Consequently, a conscious perception of this normal vestibular activity does not occur. However, if there is an imbalance in the relative increase and decrease in afferent firing between the sides, patients experience vertigo.
Vertigo is an illusion of movement in any plane or direction. Patients are deceived so that they feel themselves move or see abnormal movement of their surroundings. In rotational receptor asymmetries, patients experience a true rotational or spinning movement. In gravitational receptor asymmetries, patients have a gravitational receptor dysfunction type of vertigo. They often describe a “rocky, wavy, tilting” perception. Other descriptors include a sensation as “being on a moving boat, the floor falling out from under them or flipping.” The terms dizziness, giddiness, or disequilibrium do not accurately capture these experiences, yet they are often used, which leads to a poor understanding of TWS otic capsule defect (i.e., PLF or SSCD) symptoms by most physicians. Patients with PLF or other TWS sites do not typically experience rotational vertigo. However, this clinical observation can be blurred by VM with true rotational vertigo being superimposed on PLF, SSCD, or other TWS sites. This will be discussed later.
Most of the symptoms that disrupt the lives of patients with PLF and/or SSCD are related to severe symptoms that are secondary to these gravitational receptor asymmetries. ,
Autonomic dysfunction occurs to varying degrees with PLF, VM, and/or SSCD; however, it is extremely common. Autonomic dysfunction also occurs with rotational receptor asymmetry. These symptoms include nausea, “cold-clammy skin,” decreased heart rate, and vomiting. Many investigators have studied the underlying mechanisms and pathways involved in this dysfunction.
Cognitive dysfunction is nearly universal in patients with PLF and/or SCD. This is uncommon in the rotational receptor dysfunction type of vertigo, as seen with benign positional vertigo, vestibular neuronitis, or other disorders producing true rotational vertigo. Patients with PLF and/or SSCD often use the following descriptors when describing their cognitive function: “fuzzy, foggy, spacey, out-of-it; memory and concentration are poor; difficulty reading—as if the words are floating on the page; trouble finding the right words; and forgetting what I wanted to say.”
Gurvich and colleagues published an excellent review of the role of the vestibular system in cognition and psychiatry. The two key anatomical regions that provide links between the vestibular system and neural networks involved in cognitive and emotional processing are the parabrachial nucleus and the hippocampus; however, many of the neuroanatomic regions that are linked to the vestibular system are also implicated in several psychiatric illnesses. The past decade has seen an increased interest in the relationship between the vestibular system and mood, cognition, and psychiatric symptoms, and studies have demonstrated that vestibular stimulation can produce changes in mood, cognition, and psychiatric symptoms. I have also seen many individuals with PLF and/or SSCD assigned a psychiatric diagnosis before their vestibular disorder was diagnosed and have observed resolution of their “psychiatric disorder” following surgical intervention. This, unfortunately is common in children. , , , The hippocampus is consistently implicated in cognition and in models of psychiatric disorders, and there is a large body of evidence supporting vestibular–hippocampal interactions.
Another possible explanation for why TWS patients experience cognitive dysfunction, spatial disorientation, and recovery of function after surgical intervention is that intermittent aberrant otolithic input to the cerebellum creates an episodic but reversible cerebellar cognitive affective syndrome. Schmahmann conceptualizes cerebellar cognitive affective syndrome as dysmetria of thought and emotion. He describes impairment of executive function (planning, set-shifting, verbal fluency, abstract reasoning, and working memory), spatial cognition (visual-spatial organization and memory), personality change (blunting of affect or disinhibited and inappropriate behavior), and language deficits (agrammatism and aprosodia). These clinical features closely fit what TWS patients describe and their neuropsychologic testing measures. ,
Smith et al. and Zheng et al. have reported that the modulation of memory, but not spatial memory, occurs with vestibular lesions and can be influenced by galvanic vestibular stimulation. , These findings may lead to additional treatment strategies that may accelerate or maximize recovery after repair of an otic capsule defect such as PLF or SSCD.
We published a study incorporating pre- and postoperative quantitative measurement of cognitive function in a cohort of patients with PLF and/or SSCD. Seventeen patients (13 adults, four children) with clinical SSCD spectrum underwent surgical management. We completed neuropsychology test batteries preoperatively and every 3 months postoperatively for up to 1 year. These included the Beck’s Depression Inventory-II (BDI-II), Delis-Kaplan Executive Function System (D-KEFS), Wide Range Intelligence Test (WRIT FSIQ), and Wide Range Assessment of Memory and Learning (WRAML-2), including the four domains of verbal memory, visual memory, attention/concentration, and working memory. We statistically compared pre- versus 3 months postoperative (post-3) and post-3 versus the most recent cognitive and neurobehavioral functions. There was a highly significant improvement in the BDI-II scores at pre- versus post-3 ( P = .0006), but no further improvement at most recent ( P = .68). There was a statistically significant improvement in D-KEFS at post-3 ( P = .023) and most recent ( P = .023). For the WRAML-2 (pre- vs. post-3; post-3 vs. most recent): verbal ( P = .02; P = .008), visual ( P = .24; P = .10), attention/concentration ( P = .05; P = .048), and working memory ( P = .27; P = .007). Overall, there was a marked improvement in cognitive and neurobehavioral functions postoperatively. The delay in performance improvement measured in some domains may represent brain reorganization. Delayed improvement in specific domains may represent an opportunity for additional interventions to accelerate recovery. These interventions may include galvanic stimulation, the use of a ketogenic diet, or neurocognitive therapy approaches.
Gizzi and coworkers have reported that there is no causal relationship between vestibular disease and cognitive dyfunction. They studied 200 patients with “dizziness”— half with a history of brain trauma and half without. They concluded that in patients with postconcussive dizziness, cognitive complaints are likely due to neurologic injury or affective disturbance, and in patients with dizziness without brain trauma, cognitive complaints are likely due to concurrent affective disturbance. These findings conflict with our observations; however, in our series, we have been studying cognitive dysfunction before and after intervention so that each subject has comparative data. I also record video clips of consenting patients before and after intervention to further capture this obvious dysfunction in ways that complement standardized neuropsychology testing.
We also published a comorbidity study and noted a high rate of psychological comorbidity ( n = 6). The Millon Behavioral Medicine Diagnostic (MBMD) and the clinical psychology examinations were the most useful in identifying these comorbidities. Factitious disorder, functional neurologic symptom disorder (formerly conversion disorder) dissociative motor disorder variant, somatic symptom disorder, attention deficit hyperactivity disorder (ADHD), dissociative identity disorder (DID), major depressive disorder (MDD), and posttraumatic stress disorder (PTSD) were represented in 6 of the 12 participants in the comorbidity cohort. Suicidal ideation was also common ( n = 6). These findings underscore the challenges in sorting out the TWS symptoms caused by the dehiscence, those resulting from other comorbid conditions, or those resulting from interactions between the two factors.
Patients with PLF, VM, and/or SSCD often use the following descriptors when describing their altered spatial orientation: “trouble judging distances; feeling detached and separated or not connected, almost like watching a play when around other people, and even an out-of-body experience (in more severe gravitational receptor asymmetries).” Several groups have begun studying this phenomenon. Clinically, this spatial disorientation reverses postoperatively; however, Baek et al. reported that spatial memory deficits following bilateral vestibular loss may be permanent. There is also evidence that simulation of the vestibular system is necessary to maintain normal spatial memory. Deroualle and Lopez have explored the visual-vestibular interaction and in their 2014 review of the topic conclude that vestibular signals may be involved in the sensory bases of self-other distinction and mirroring, emotion perception, and perspective taking. Clinically, patients with PLF and/or SSCD recognize changes in their personality. Smith and Darlington argue that these changes in cognitive and emotional occur because of the role of the ascending vestibular pathways to the limbic system and neocortex play in the sense of spatial orientation. They further suggest that this change in the sense of self is responsible for the depersonalization and derealization symptoms such as feeling “spaced out,” “body feeling strange,” and “not feeling in control of self.”
Vestibular disorders can produce anxiety; however, the classic sense of impending doom only occurs with the most severe gravitational receptor asymmetries. It is nonetheless quite unnerving to patients because it is a unique type of anxiety and characteristically patients have no insight as to why they feel that way or what makes them feel that way. Much work has been completed to understand the underlying mechanisms and pathways involved in this dysfunction. ,
In Minor’s review of 65 patients with SSCD, 54 (83%) had vestibular symptoms elicited by loud sounds, and 44 (67%) had pressure-induced (sneezing, coughing, and straining) symptoms. This is also characteristic of patients with PLF, SSCD, and CFD.
In SSCD, one of the most disturbing auditory symptoms is autophony, an unpleasant subjective discomfort of one’s own voice during phonation. Often, patients describe their voice as “echo-like” or “resonant.” This is also very common in PLFs. Just as in SSCD, some patients with PLF can also hear their eyes move or blink. There appears to be decreased hearing thresholds for bone-conducted sounds. Bhutta has postulated that patients who hear their eyes move do so via transdural transmission of extraocular muscle contraction. If this is the case, further credence is given to the hypothesis that some cases of PLF represent an otic capsule defect in an area such as the modiolus creating a third window, just as in SSCD and CFD. , ,
VM (migraine-associated dizziness) is becoming recognized as a distinct clinical entity that accounts for a high proportion of patients with vestibular symptoms (for a review, see Furman et al. ). It is so common that VM should be considered in any patient presenting with dizziness, vertigo, or disequilibrium. A temporal overlap between vestibular symptoms, such as vertigo and head-movement intolerance; and migraine symptoms, such as headache, photophobia, and phonophobia; is a requisite diagnostic criterion. Physical examination and laboratory testing are usually normal in VM but can be used to rule out other vestibular disorders with overlapping symptoms such as PLF, CFD, or SSCD. The pathophysiology of VM is incompletely understood, but plausibly could include the neuroanatomic pathways to and from the central vestibular structures and neurochemical modulation via the locus coeruleus and raphe nuclei. In the absence of controlled trials, the treatment options for patients with VM largely mirror those for migraine headache. These treatment approaches include the prophylactic prevention of migraines with: (1) antiseizure medications such as topiramate (Topamax) or zonisamide (Zonegran); (2) calcium channel blockers such as verapamil (Verelan); (3) tricyclic antidepressants such as nortriptyline (Pamelor), or beta-blockers for children, such as propranolol (Inderal). Approximately one-third of VM patients have endolymphatic hydrops, which is typically bilateral.
VM patients do not have sound-induced dizziness and nausea or autophony; however, when these patients have endolymphatic hydrops, they can have sound sensitivity that borders on the Tullio phenomenon. Therefore, when a high-resolution temporal bone CT with color 3D volume rendering shows no evidence of SSCD, I treat every patient suspected of having PLF as a VM patient since medical management, if successful, avoids unnecessary surgery. Typically, PLF patients will have some improvement with medical management, and then regression as the dose is increased, resulting in switching to another class of medication. Ultimately, the patients never come under control, and reassessment leads to a decision for surgical intervention.
VM is an example of the integral overlap between the vestibular pathways and migraine circuit triggers and central mechanisms for premonitory symptom generation. Information transmitted by peripheral vestibular sensory organs and the vestibular nerve to the medulla and pons is an external trigger within the migraine circuit construct proposed by Ho et al. , This model is based on the distribution of the neuropeptide CGRP, which has a complex distribution within the vestibular periphery. I have observed that migraine headache is nearly always present in patients with gravitational receptor dysfunction type of vertigo caused by PLF, CFD, or SSCD, but infrequently with the rotational receptor dysfunction type of true rotational vertigo. , This is an important concept, as PLF, CFD, and SSCD can induce migraine and its three variants, namely ocular, hemiplegic, and VM. Therefore, patients with PLF and SSCD, who normally only have the gravitational receptor dysfunction type of vertigo (disequilibrium) can experience episodes of VM and infrequent true rotational vertigo attacks. Surgical management of PLF and/or SSCD typically resolves the migraine; however, sometimes there is a marked decrease in the frequency and intensity of the migraines, as migraine has a high incidence overall. ,
If you put ‘perhaps’ before a statement and the statement turns out to be true, you will get credit for making it; if it turns out to be false, you will not be blamed. William Stewart Halsted
Clinically, patients with PLF share the same clinical phenotype as patients with SSCD. Perhaps a PLF is really an otic capsule defect in an area such as the modiolus creating a third window, as is the case with SSCD. If so, these otic capsule defects cannot be visualized using existing CT technology. Reinforcing the round window (RW) effectively closes the third window, thereby eliminating or reducing the symptoms. Thus, PLF surgery is effective in selected patients, not because a perilymph leakage from the inner ear into the middle ear has been closed, but rather because closing the third window alters the biomechanical properties of the inner ear.
This approach was recently explored in patients with SSCD who underwent RW reinforcement using a variety of materials. Silverstein et al. reported 22 patients with a confirmed diagnosis of SSCD who underwent RW reinforcement via a transcanal approach. Six surgeons from four institutions participated in this study. Various types of tissue were used, including the temporalis fascia, tragal cartilage and perichondrium, fat, loose connective tissue, Gelfoam, and/or silastic. Statistically significant improvement in all symptoms, except hearing loss, was observed in 19 patients who underwent RW reinforcement. In contrast, two of three patients who underwent the alternate treatment of RW niche occlusion experienced worsened symptoms requiring revision surgery. RW tissue reinforcement may reduce the symptoms associated with SSCD. Silverstein et al. speculated that the reinforcement technique may benefit SSCD patients by reducing the “third window” effect created by the SSCD.
Recently, 16 patients were reported with CFD; 8 of them were surgically managed with RW reinforcement alone. Overall there was a marked and clinically significant improvement in the Dizziness Handicap Inventory, Headache Impact Test, and TWS symptoms postoperatively in the CFD cohort who underwent RW reinforcement surgery. A statistically significant reduction in cVEMP thresholds was observed in patients with radiographic evidence of CFD. There was no statistically significant change in hearing in patients with CFD who underwent RW reinforcement. It was emphasized that radiographic CFD is not in itself an indication for surgery and that the most important factor in decision-making should be in the context of clinical symptoms and other diagnostic findings. Another important observation in the study was multiple sites of dehiscence in temporal bones with TWS; this finding is important to consider when faced with recurrent or incompletely resolved TWS symptoms after plugging an SSCD. Several of the patients were thought to have a CT-TWS (PLF) at the time of their surgery. Reviewing a patient’s high-resolution temporal bone CT for all the known sites of dehiscence is essential. It is also important to do so in the axial, coronal, Poschl, and Stenvers orientations.
The most important clinical task is differentiating between PLF, SSCD, other sites of TWS dehiscence, and VM.
A valuable screening study in patients with TWS symptoms, in particular autophony, is to apply a low-frequency tuning fork to a patient’s knees and elbows and ask if they can hear or feel the vibration in their head. I use both a 128- and 256-Hz tuning fork. Often, the patient can lateralize the ear in which they are able to hear the tuning fork, but sometimes they just feel that it vibrates in their head. This is true not only for patients with SSCD but also for patients with PLF and other sites of dehiscence in TWS. I also have the patient stand in the Romberg position, with the eyes closed if possible, and place the 256-Hz tuning fork on the elbow on the side of the suspected dehiscence. The patients typically experience increased sway and a sense of being pushed or starting to fall. Over time, I have used the results of these physical examination tools to help decide when to order a high-resolution temporal bone CT with color to help determine if the patient has a site of dehiscence visible on CT or a CT-TWS (PLF).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here