Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The skin is the largest organ of the body and acts as a structural barrier to invasion by microbes. Skin and soft tissue infections (SSTIs) vary from minor to life-threatening. These infections may involve the epidermis, dermis, subcutaneous tissue, deep fascia, and muscle. They are the result of diverse causes, and the host may be either healthy or compromised. Prompt diagnosis helps to ensure effective treatment. Assess the severity of the patient’s illness, determine the probable pathogen, chose the appropriate antibiotic, and undertake immediate surgery when appropriate. A myriad of microbes can produce soft tissue infection, and to confound issues, there is considerable overlap of clinical presentation.
Soft tissue infections have been described throughout the medical literature since ancient times. In the first century, Celsius described the classic signs of inflammation: rubor, dolor, calor, and tumor. Necrotizing fasciitis was described in the fifth century bc by Hippocrates, though Wilson coined the term in 1952. Much of our knowledge regarding the treatment of soft tissue infection has been based on the experience gained during military conflicts and translated into civilian practice. For instance, hospital gangrene was described first by Joseph Jones, a Confederate surgeon during the Civil War.
Soft tissue infections frequently occur in all health care settings accounting for approximately 48.5 in 1000 outpatient visits in the United States. Infections of the skin and soft tissue are among the most common infections treated in hospitals, being higher in frequency than urinary tract infections and pneumonia. The total health care cost of soft tissue infections is approximately $13.8 billion annually. Studies estimate that hospitalizations due to soft tissue infections increased over time from 641,863 in 2005 to 752,770 in 2011, an increase of over 15%. Staphylococcus aureus –associated infections caused much of this increase. The mortality from soft tissue infections is relatively low at less than 1%. Necrotizing soft tissue infections (NSTIs), however, have a higher mortality rate historically at 25–30% with improvement with advances in care to 5-12% based on data acquired from large data repositories such as National Inpatient Sample (NIS) and the Nation Surgical Quality Improvement Program (NSQIP). Many single-institution studies continue to report mortalities of 8-17%. The overall incidence of NSTI remains unclear due to widely different reporting practices. Based on the NIS (1998–2010) data, the annual incidence of NSTI admission in the United States is estimated to range from 3800 to 5800.
The skin is designed to protect by maintaining a mechanical barrier to microbes. It additionally maintains temperature homeostasis, produces vitamin D, and excretes fluids, electrolytes, lipids, and acids. Figure 1 depicts the anatomy of the skin.
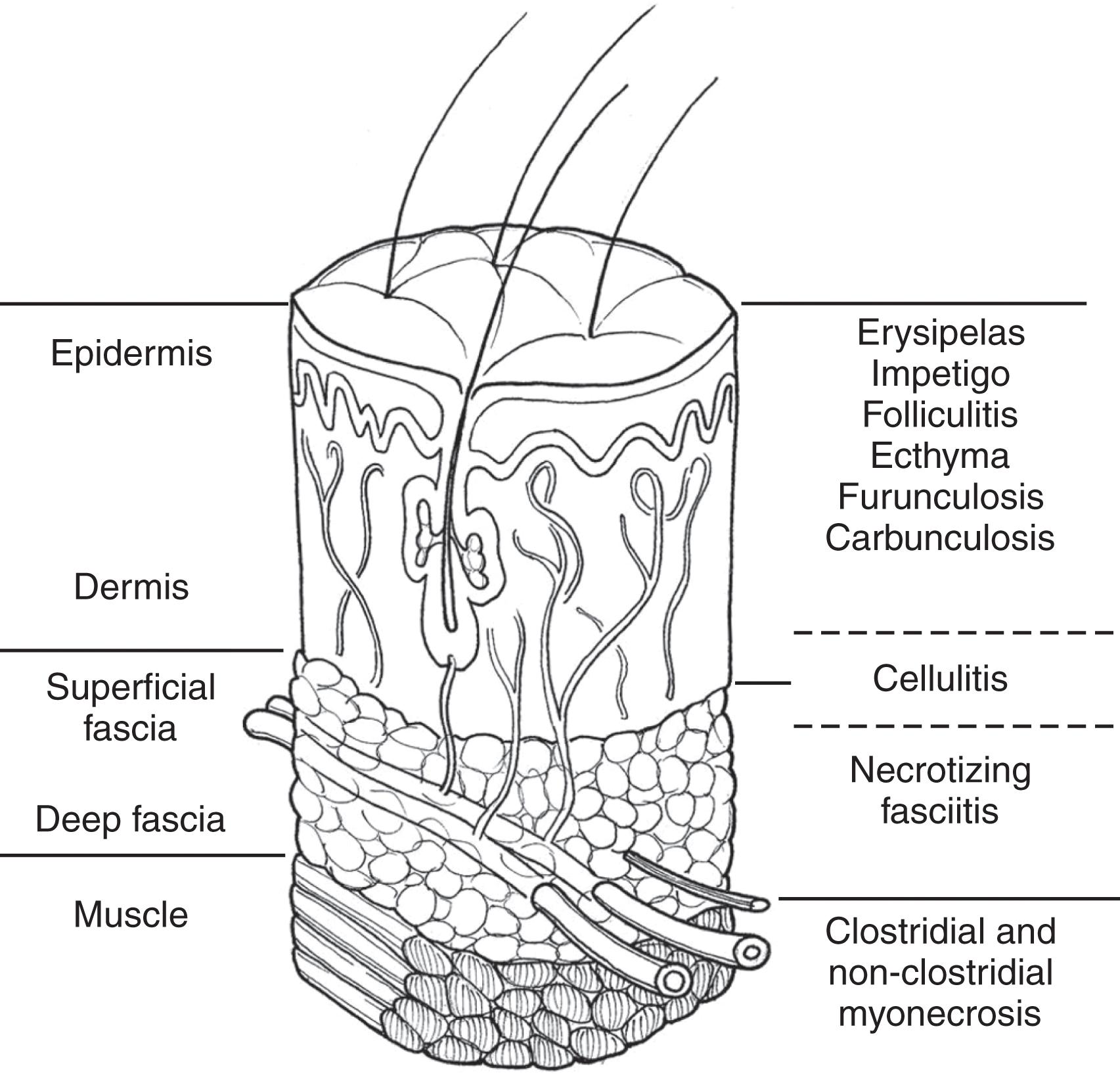
A microbacterial flora typically colonizes the skin. Usual organisms include staphylococcal species, Corynebacteria , Propionibacteria , and yeast. The number of organisms per surface area varies extending from a few hundred in drier areas to several thousand in moist areas like the axilla and groin. The indigenous microbes may prevent the growth of pathogenic species. Primary soft tissue infections occur most commonly following the disruption of the protective skin barrier. Disruptions of the protective skin barriers result from injury, surgery, bites (insect, spider, animal, or human), burns, medical injections, or parenteral drug use.
Additionally, skin cracks resulting from tinea pedis, xerosis, skin abrasion following vigorous scratching, or irritation at a hair follicle or sweat gland can lead to infection. Secondary infections occur when a skin lesion such as dermatitis exists. Chronic ulcers (diabetic, venous, or vascular) or dermatologic skin lesions like psoriasis can also become secondarily infected. Direct spread from nearby structures can result in soft tissue infections as with gastrointestinal or genitourinary perforations. Less commonly, secondary infection of the soft tissue results from hematogenous spread, as seen in the immunosuppressed and injection drug users with endocarditis.
Most SSTIs are caused by Staphylococcus aureus and beta-hemolytic streptococcus groups A, C, and G. Group B beta-hemolytic streptococcus is seen most in patients with diabetes mellitus. Gram-negative and anaerobic organisms are more likely to be found in infections following surgical procedures involving the abdominal wall or that occur around the anus. Infections caused by both gram-negative and gram-positive organisms are more likely to occur in tissues where perfusion is compromised as happens in the patient with diabetes mellitus and arterial or venous insufficiency.
The bacterial flora of patients previously treated with antimicrobials will change the likely causative organisms. Therefore, chronic infections are frequently polymicrobial. Additionally, the use of antibiotics results in the emergence of resistant strains of microorganisms. Methicillin-resistant S. aureus (MRSA) has reached endemic proportions. MRSA was identified in 1960 and was initially a problem confined to the hospitalized patient population. In the late 1990s, community-associated MRSA (CA-MRSA) appeared in patients without discernible risk factors. In a US population-based study from 2005 to 2009, the annual incidence of CA-MRSA increased by 34%, healthcare-associated MRSA by 7%, and overall skin and soft tissue infections by 4%. MRSA accounts for about 50% of all SSTIs. Paralleling the increase in MRSA prevalence, multi-drug-resistant (MDR) gram-negative bacteria are emerging as pathogens in SSTIs. Patients with chronic wounds, residents of long-term care facilities, the immunosuppressed and those with hospital-acquired infection are at higher risk of developing SSTIs caused by MDR gram-negative organisms. Pseudomonas aeruginosa, Acinetobacter species, Klebsiella , and vancomycin-resistant enterococcus (VRE) are examples. Table 1 lists the bacterial pathogens frequently isolated from cultures of SSTIs.
| Rank | Organism |
|---|---|
| 1 | Staphylococcus aureus |
| 2 | Pseudomonas aeruginosa |
| 3 | Enterococcus species |
| 4 | Escherichia coli |
| 5 | Enterobacter species |
| 6 | Klebsiella species |
| 7 | Beta-hemolytic streptococcus |
| 8 | Proteus mirabilis |
| 9 | Coagulase-negative staphylococcus |
| 10 | Serratia species |
Many bacteria produce endotoxins and exotoxins that facilitate their movement along and ultimately through fascial planes. These toxins produce tissue injury, ischemia, and liquefactive necrosis. In severe cases, they can cause rapid progression of the infection by as much as 1 inch of tissue per hour. Clostridium , Staphylococcus , Streptococcus , and Vibrio are best known for this rapid spread ( Table 2 ). More importantly, these toxins stimulate neutrophils and macrophages to produce inflammatory cytokines tumor necrosis factor, interleukin-1, and interleukin-6. These cytokines produce the systemic inflammatory response that may lead to sepsis, organ failure, and death. Locally, neutrophil degranulation and superoxide release produce injury to the endothelium. Superantigens produced by the bacteria cause massive T-cell stimulation, amplifying the inflammatory response. They also activate the complement system and the coagulation cascade. These effects cause thrombosis of small vessels and further tissue ischemia and injury.
| Organism | Toxic Substance | Effects |
|---|---|---|
| Clostridia | Alpha toxin |
|
| Streptococci and Staphylococci |
|
|
| Vibrio vulnificus |
|
|
The inciting event or type of exposure predicts the bacteriology. Table 3 lists the more common associations of bacteria with risk factors.
| Risk Factor | Pathogen |
| Human bite | Eikenella corrodens |
| Cat bite | Pasteurella multocida |
| Dog bite | Capnocytophaga canimorsus; Pasteurella multocida |
| Rat bite |
|
| Animal hides |
|
| Medical leeches |
|
| Water exposure | |
| Salt water | Vibrio species |
| Freshwater | A. hydrophila |
| Hot tub | Pseudomonas aeruginosa |
| Fish tank | Mycobacterium marinum |
| Salon foot spa | Mycobacterium fortuitum |
| Reptile contact | Salmonella species |
| Injection drug use | Clostridium botulinum , anaerobes, E. corrodens, MRSA, Pseudomonas aeruginosa |
| Travel |
|
| Surgical site infections | Staphylococcus species, anaerobes, Enterococcus species, gram-negative bacilli, Streptococcus species |
| Cirrhosis |
|
| Neutropenia | Pseudomonas aeruginosa |
| Diabetes mellitus | Staphylococcus aureus, group B streptococci, anaerobes, gram-negative bacilli |
Several classification schemes have been suggested to simplify the discussion of soft tissue infection and guide the treatment; however, the terminology has become confusing over the years. In 1998 the Food and Drug Administration (FDA) classified soft tissue infections as uncomplicated and complicated by depth of infection and severity for drug development and treatment. In 2004, Eron and colleagues characterized soft tissue infections by presence of systemic manifestations and patient comorbidities to aid management, treatment, and admission decisions, as follows: class 1: patients with SSTI, but no signs or symptoms of systemic toxicity or comorbidities; class 2: patients are either systemically unwell with stable comorbidities or are systemically well but have a comorbidity that may complicate or delay resolution; class 3: patients appear toxic and unwell; class 4: patients have sepsis syndrome and life-threatening infection typically necrotizing. In 2013, the FDA coined the term “acute bacterial skin and skin structure infections” as a bacterial infection only of the skin with a lesion size area of at least 75 cm 2 based on the area of erythema, edema, or induration. In 2014, the Infectious Disease Society of America released their guidelines categorizing soft tissue infections by severity and the presence of purulence or tissue necrosis. Most recently, the American Association for the Surgery of Trauma has proposed a grading system for soft tissue infections and has validated that rising grade correlates with increased mortality. For simplicity, we will use the World Society of Emergency Surgeons classification of soft tissue infections to organize the remainder of this chapter. They simply stratified infections as nonnecrotizing, necrotizing, and surgical site infections (SSIs) ( Table 4 ).
| Grade 1 | Grade II | Grade III | Grade IV | Grade V | |
|---|---|---|---|---|---|
| Clinical criteria | Folliculitis, erysipelas, impetigo, simple cellulitis | Necrotizing, blistering, or bullous cellulitis or skin necrosis | Subcutaneous abscess | Fasciitis | Myonecrosis |
| Imaging criteria | Superficial inflammation with no subcutaneous stranding | Subcutaneous stranding but no abscess | Well-defined (walled off) subcutaneous fluid collection with surrounding inflammation | Inflammation extending to fascia; likely air along fascia margins | Air deep to fascia likely poor perfusion of muscle |
| Operative criteria | N/A | N/A | Well-defined subcutaneous fluid collection | Clear involvement of fascia with healthy, viable muscle underneath | Extension of necrosis into muscle and deeper tissue |
| Pathologic criteria | Acute inflammation involving epidermis only | Acute inflammation involving epidermis and dermis | Acute inflammation involving epidermis, dermis, and subcutaneous fat with cultures positive for organisms if available | Acute inflammation involving epidermis, dermis, subcutaneous fat, and muscular fascia with cultures positive for organisms if available | Acute inflammation into deeper muscle and deeper tissue |
Nonnecrotizing soft tissue infections include impetigo, folliculitis, simple abscess, erysipelas, cellulitis, and simple and complex abscess. Superficial infections are limited anatomically to the epidermis and the dermis of the skin ( Fig. 1 ). These infections can occur spontaneously or secondary to minor trauma. Impetigo usually presents with vesicles that leak, producing a thick yellow crust. Systemic symptoms are unusual. Lesions are typically located on the face, neck, and extremities. S. aureus and Streptococcus pyogenes are the most common causative agents. Bullous impetigo, like impetigo, is typically caused by S. aureus. Small vesicles that coalesce to form large bullae are characteristic. The peak incidence is in childhood at ages 2 to 5, though it does occur in older children and adults. Generally, skin colonization may precede the development of this infection by an average of 10 days. It occurs mostly on exposed areas such as the face or extremities. The lesions may be multiple but are well localized. Ecthyma is a deeper ulcerated form of impetigo.
Folliculitis develops as a superficial inflammation of the hair follicle. The lesions appear as pustules or papules, commonly on the extremities, scalp, or beard, and involve the epidermis. Moist heat application will usually promote drainage at this stage. Hot tub folliculitis is associated with immersion in inadequately chlorinated pools or hot tubs, and diffuse pustules are seen. P. aeruginosa is the classic causative agent of hot tub folliculitis. Swimmer’s itch is a folliculitis that develops after freshwater exposure.
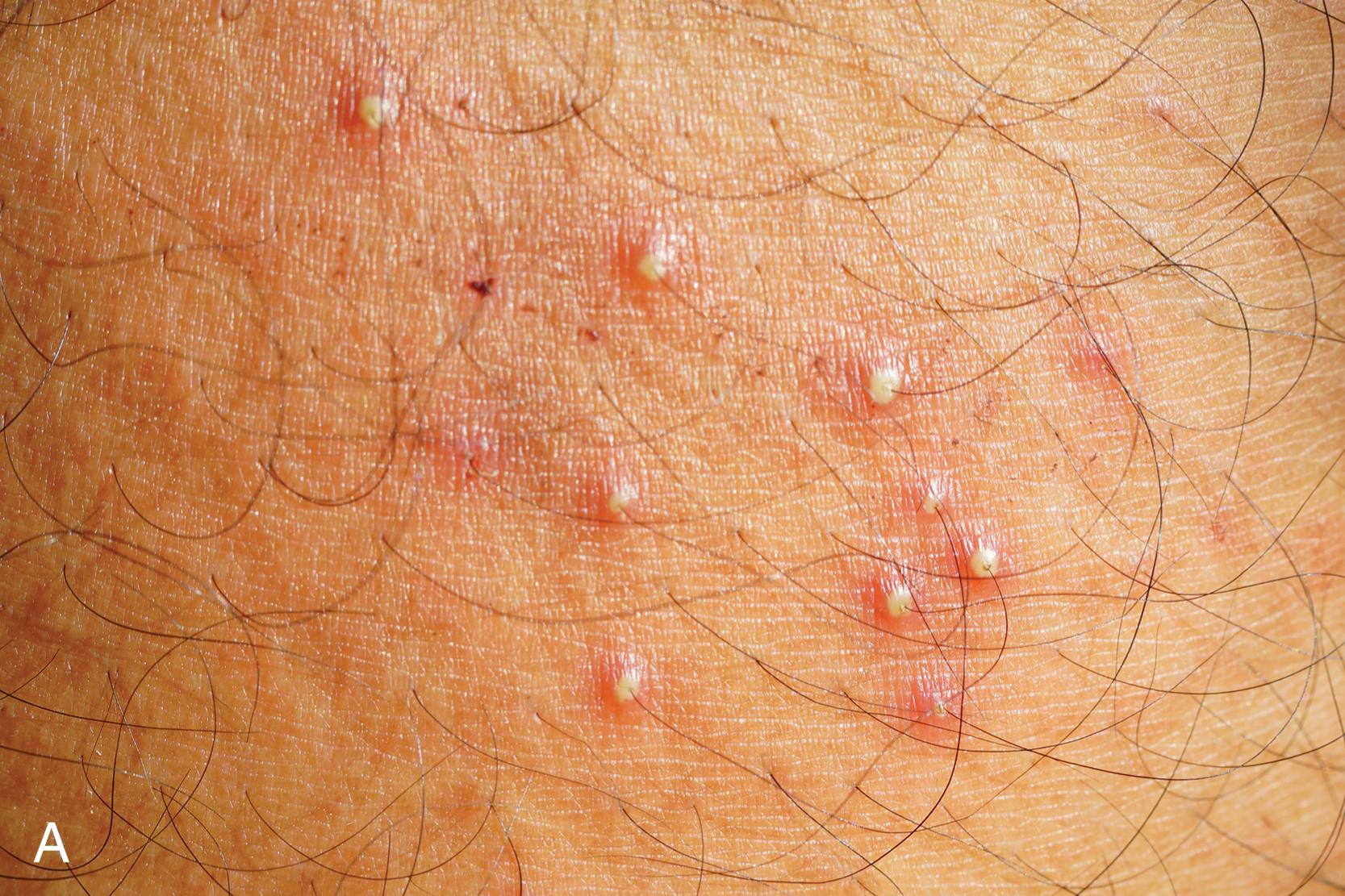
Furuncles, deeper inflammatory nodules, can develop from folliculitis when the suppuration extends through the dermis and into the subcutaneous tissue. S. aureus is often the causative agent. Carbuncles are coalescing furuncles formed by connecting sinuses. The nape of the neck is the most common anatomic location. Furuncles and carbuncles require incision and drainage ( Fig. 2 ). Systemic antibiotics are necessary when there is surrounding cellulitis or associated fever. When recurrent infection with resistant S. aureus occurs, patients must be meticulous in their hygiene. Thorough cleansing of areas that may harbor the organism using antimicrobial soaps and thoroughly laundering towels and bed sheets may be necessary. Nasal decontamination with mupirocin ointment twice daily for 5 days may be useful in cases of recurrent infection.
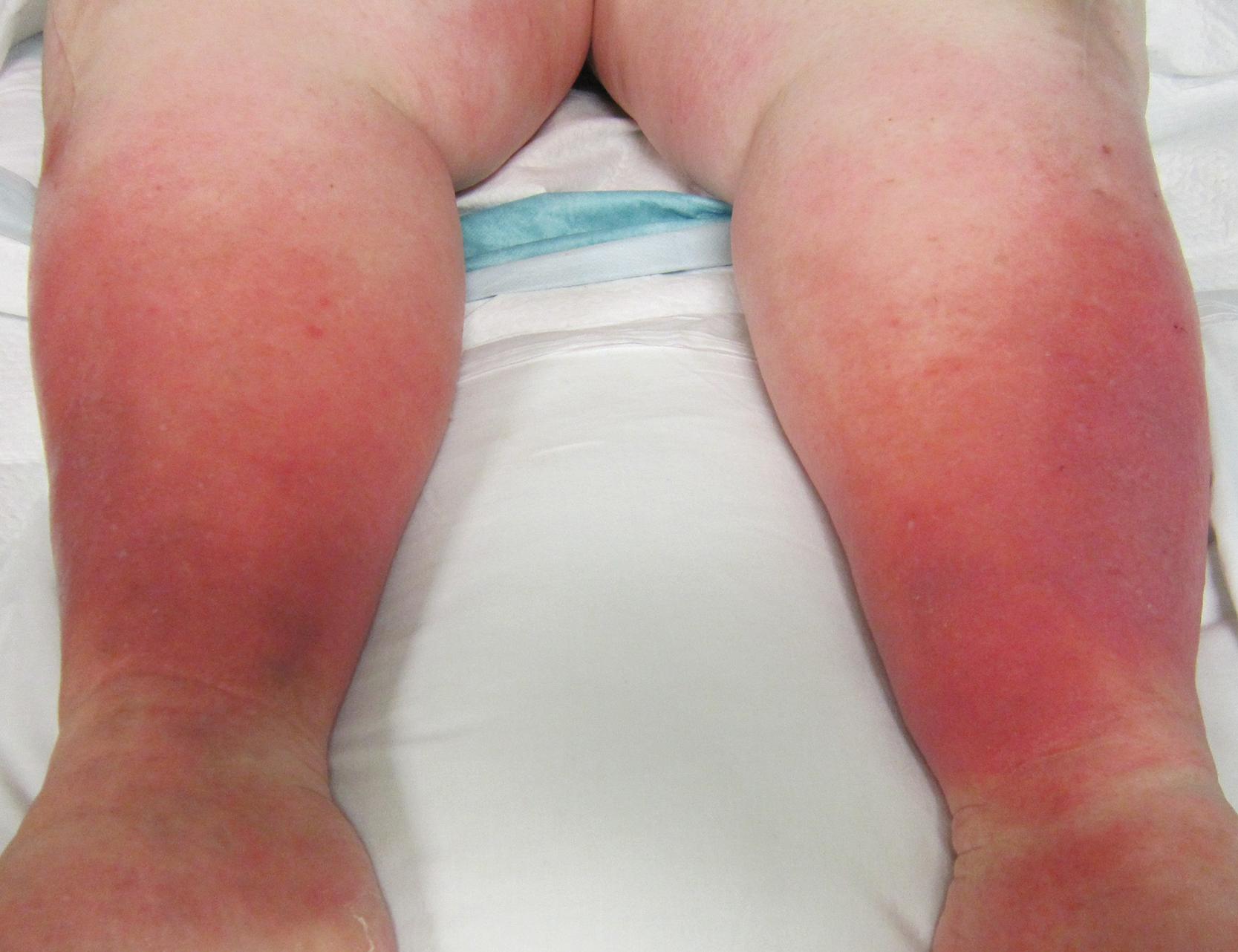
“Erysipelas” is a term frequently used interchangeably with cellulitis. A raised clear line of demarcation between healthy and involved skin characterizes erysipelas. Group A hemolytic streptococcus usually causes it, but groups C or G also may. S. aureus is also a cause. It is most common in children or the elderly. Historically, the face was the most common site, but currently, the extremities are more often affected. Cellulitis is an inflammation of the subcutaneous tissue and is, therefore, deeper than erysipelas ( Fig. 1 ). There is erythema, pain, and edema of varying severity. Lymphangitis and regional lymph node swelling may be present. The skin may have a peau d’orange appearance due to the edema surrounding the hair follicles, which remain attached to the dermis producing a dimpled appearance. Vesicles and bullae of the skin may occur. Petechiae and ecchymoses may be seen on the inflamed skin. A portal of entry for the bacteria is usually present, which may be as mundane as a crack in the skin from dryness or tinea pedis. Systemic symptoms may manifest and include fever, chills, malaise, and (infrequently) organ failure. Streptococcus (groups A, B, C, and G) and staphylococcus are frequent culprits. Areas of compromised venous or lymphatic drainage are prone to this infection, and recurrence is frequent. Pelvic radiation, or having had lymphadenectomy, mastectomy, or venectomy makes cellulitis more likely to occur. Figure 3 shows a patient with bilateral lower extremity cellulitis. Other infectious organisms may be responsible in special circumstances ( Table 3 ).
Necrotizing soft tissue infections (NSTI) can affect any soft tissue layer. Deeper infections are more frequently life- and limb-threatening. Multiple terms are used in the literature to describe these potentially life-threatening infections. Table 5 lists a variety of terms that have been found in the medical literature to describe these infections. NSTIs involve the subcutaneous fat, and superficial and deep fascia, with variable skin involvement. In severe cases the muscle may also be involved and either inflamed or frankly necrotic. There is often vast tissue destruction that, if not fatal, is mutilating. An organism’s ability to cause infection depends on the organism’s virility and the resistance of the host. In about 80% of cases, the infection has spread from a discernible lesion. Table 6 contains a list of potential sites. The remaining 20% will not have an identifiable origin. Though most affected by NSTIs have comorbidities, both the compromised and the uncompromised host can be affected. Multiple reviews have identified risk factors associated with increased susceptibility, including increasing age, diabetes mellitus, peripheral vascular disease, obesity, chronic renal failure, HIV, alcohol abuse, and intravenous drug use. Non-steroidal anti-inflammatory drugs (NSAIDS) were strongly associated with the occurrence of NSTI in a small case series from 2008. No direct causation has been determined, however. In 2018 the FDA added a new warning to sodium-glucose cotransporter-2 inhibitors used to treat type 2 diabetes mellitus. This warning is based on 12 cases of Fournier’s gangrene that were identified through reports submitted to the FDA and in the literature. No association has been identified with other classes of hypoglycemic drugs.
| Meleney’s synergistic gangrene | Hospital gangrene |
| Streptococcal gangrene | Fournier’s gangrene |
| Gas gangrene | Acute dermal gangrene |
| Suppurativa fasciitis | Necrotizing erysipelas |
| Phagedena | Phagedena gangrenosum |
| “Flesh-eating disease” | Necrotizing fasciitis |
| Clostridial cellulitis |
| Site | Etiology |
|---|---|
| Perineum | Anal abscess |
| Bartholin’s abscess | |
| Genitourinary infection | |
| Postgenitourinary tract surgery or catheterization | |
| Postanal procedure | |
| Bulky neoplasm | |
| Extremity | Diabetic foot ulcer |
| Venous stasis ulcer | |
| Arterial insufficiency ulcer | |
| Injection drug use | |
| Traumatic wound or fracture | |
| Animal or human bite | |
| Decubitus ulcer | |
| Trunk | Surgical site infection |
| Extension from intra-abdominal source | |
| Decubitus ulcer | |
| Strangulated hernia | |
| Head and neck | Dental abscess |
| Pharyngeal/tonsillar abscess | |
| Surgical site infection |
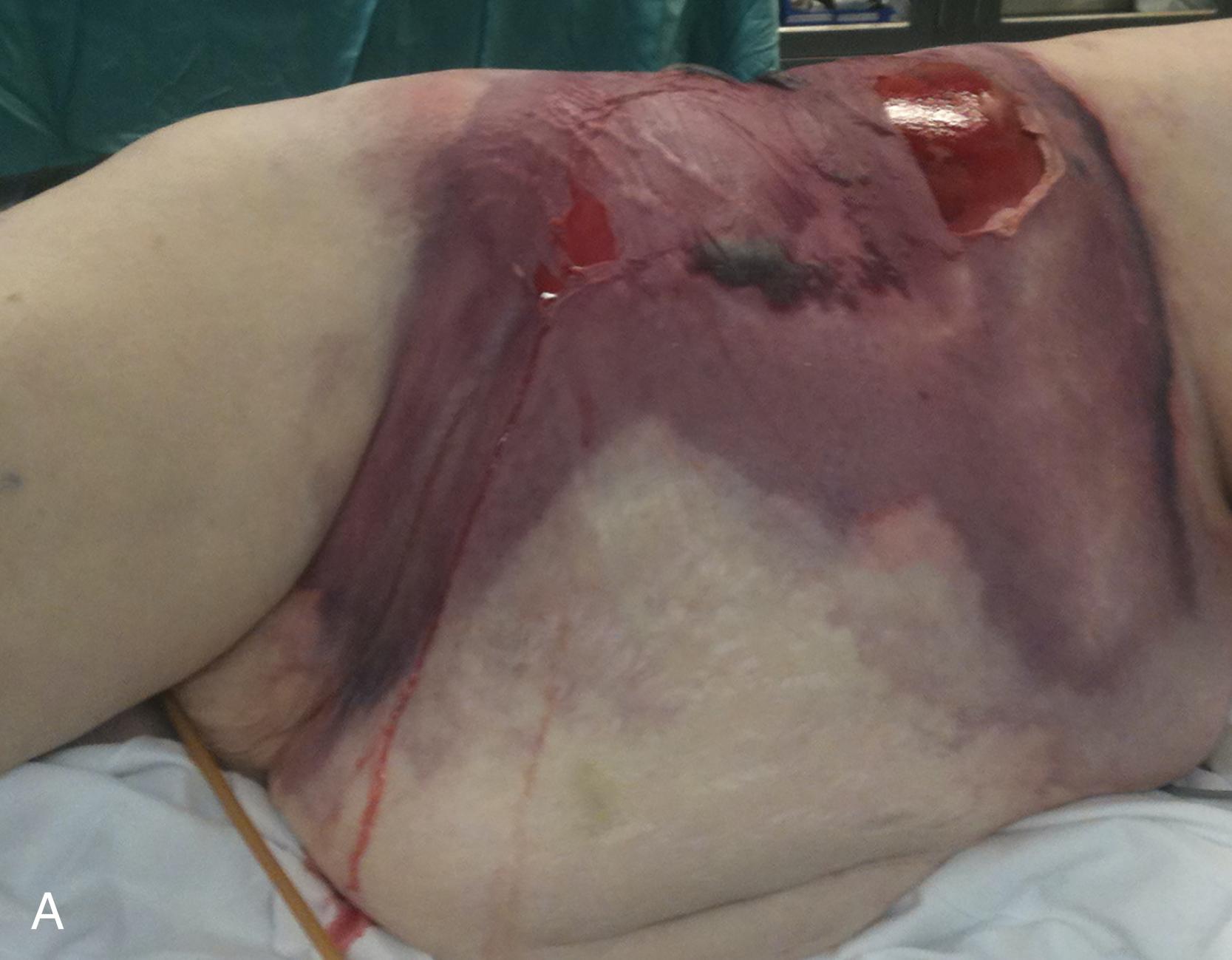
Exceptionally virulent bacteria such as group A streptococci (GAS) can affect otherwise healthy hosts. These bacteria produce exotoxins that result in the release of cytokines, leading to a robust systemic response. At the cellular level, the toxins and enzymes (such as hyaluronidases and lipases) facilitate the spread of bacteria along fascial planes and through the subcutaneous tissue. A thin, watery, foul-smelling fluid (dishwater drainage) is often found on exploration. The skin necrosis that may accompany this infection results when thrombosis of the skin’s nutrient vessels occurs ( Fig. 4A ). Microscopic findings are usually severe subcutaneous fat necrosis, severe inflammation of the dermis and subcutaneous fat, vasculitis, endarteritis, and local hemorrhage. The fascia may be edematous and suppurative, and thrombosis of vessels may be seen. Figure 4B shows classic intraoperative findings of liquefaction necrosis involving the superficial fascia/subcutaneous fat.
NSTIs are often classified by the bacteria that cause the infection. Type I is a polymicrobial infection and is the most common variety. Typically, this infection is seen in patients with comorbid conditions. Gram-negative enteric bacteria are seen in combination with gram-positive organisms and anaerobes. As many as 15 different organisms have been cultured from such patients, with an average of 5. Type II is GAS either alone or in combination with S. aureus. This type takes a more virulent course and occurs in younger, healthier patients. Clostridial infections classically produce air in the soft tissues, although the polymicrobial type I infections may also produce air as well. Clostridial infections are distinguished by the presence of air diffusely within the muscles. Vibrio vulnificus and Aeromonas hydrophilia should be suspected when the patient has been exposed to shellfish (i.e., ingesting raw oysters) or saltwater and are considered to be type III by some.
Pyomyositis occurs when there is pus within muscle groups. Clinically patients report severe pain often initially thought to be secondary to muscular strain leading to misdiagnosis and treatment with rest and nonsteroidal anti-inflammatory. Without treatment, the pain worsens and muscle spasm progress and, if not suppressed, fever develops. Early in the course, abnormalities may not be palpable as the infection is deep. As the infection spreads and the muscle necroses, woody firmness or even bulging of an abscess may be appreciated. The organism isolated is usually S. aureus.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here