Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Progress in understanding the causes of pediatric cancer, as well as advances in cancer-directed therapies, hold great promise for curing and extending the lives of many children diagnosed with cancer. However, as advances in medicine and technology improve the survival of children with life-threatening illnesses, attention to health-related quality of life and progress in symptom management have not kept pace with advances in disease-directed therapies. As a result a population of children exists who are living with cancer and have suboptimally controlled symptoms.
Amelioration of symptoms experienced by a child with cancer does far more than reduce the suffering caused by the troublesome symptoms. Control of physical symptoms may allow attention to be focused on other issues faced by family members who are living with a child's cancer, such as psychosocial concerns or existential distress, and it may allow them to participate in activities and interactions that are important for maximizing quality of life. In fact, symptom burden is highly associated with quality of life in children with cancer and is one of the most significant determinants of quality of life in adolescents and young adults with cancer. In some instances, improved control of symptoms may enhance the delivery of optimal cancer-directed therapy as well. Finally, optimal symptom control throughout the illness trajectory may also shape the child's and family's long-lasting impressions of their experience with cancer.
In many instances, relief from distressing symptoms is possible with a myriad of modalities available today. Pediatric oncologists play a key role in managing symptoms in their patients. Symptom management requires actively partnering with families to assess not only the presence of symptoms but also the impact of uncontrolled symptoms on their daily lives, as well as to implement appropriate symptom-directed interventions. Ideally such attention to symptoms occurs in the context of attention to the child's overall condition so that the impact of symptoms on the child's overall quality of life is appreciated.
The vast majority of children who have cancer experience multiple symptoms that could be ameliorated but are not addressed. For example, Collins and colleagues asked 10- to 18-year-old children about the symptoms they experienced during the past week. Symptoms included lack of energy (49%), pain (49%), nausea (45%), lack of appetite (40%), and itching (33%). The prevalence of uncontrolled symptoms in children with cancer is likely to stem from a variety of systemwide causes, including inadequate formal training dedicated to symptom management, a focus on cancer-directed treatment, and time constraints.
In addition, a lack of systematic research in the pediatric population, particularly with regard to non–pain-related symptoms, leads to a lack of evidence or standards on which to base interventions. Many symptom-directed interventions for children at this time are based on extrapolation from adult studies or even individual or anecdotal experiences. Some guidelines exist that are strongly evidence based, such as the National Comprehensive Care Network (NCCN) Guidelines for Pediatric Cancer Pain, which is encouraging. As advances in pediatric oncology extend into the realm of symptom management, additional guidelines for non–pain-related symptoms will become available to help pediatric oncologists attend to their patients' symptoms.
The promising survival rates that have resulted from discoveries in pediatric oncology have led to increasing attention to health-related quality of life and the human costs of cancer care. With such a shift, efforts to understand and ameliorate the myriad of symptoms experienced by children with cancer are likely to be increasingly supported in the coming years.
Pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.” This symptom is the most studied and best understood of all cancer symptoms in adults and children alike. However, despite such attention, pain is often suboptimally controlled.
Families and children confronting cancer often worry about potential pain due to the disease and its treatment. In fact, pain is the most feared problem for children with cancer. To some extent, their concern is justified. For example, Collins and colleagues demonstrated that children with cancer experience multiple symptoms, including pain. Among 160 children ages 10 to 18 years with cancer, pain was the second most common symptom, with a prevalence of 49.1%. In addition, 61.6% reported pain that was moderate to very severe in the 48 hours prior to completing the questionnaire, and 40.9% reported pain that occurred “a lot” to “almost always.” Moreover, the pain experienced by children was distressing—“quite a bit” to “very much” in 39.1% of respondents.
Pain control provides a variety of benefits beyond the amelioration of suffering. Prompt pain relief is needed to prevent central sensitization, a centrally mediated hyperexcitability response that may result in escalating pain. Uncontrolled pain also leads to a physiologic stress response with a variety of effects such as altered metabolism and immune function. Control of pain with appropriate analgesia in the perioperative period can reduce some of these effects and prevent complications.
In general, pain experienced by children with cancer may be caused by a variety of entities, including the disease itself (e.g., tumor invasion of bone, viscera, or the peripheral or central nervous system [CNS] or compression of the spinal cord), treatment (e.g., mucositis, radiation-induced dermatitis, and drug-induced neuropathy) or procedures (e.g., venipuncture, lumbar puncture, and bone marrow aspiration or biopsy, as well as postoperative pain). Authors who performed a cross-sectional analysis of pain in inpatient children with cancer found that the most frequent cause of pain was adverse effects of antitumor therapy. Evidence also indicates that children who have solid tumors outside of the CNS have more pain and higher opioid requirements.
Because the majority of pediatric cancers respond at least initially to treatment, most of the pain that children experience early in the disease trajectory is related to procedures and treatment. Later, if the cancer progresses, pain is more likely to be due to tumor extension. In a series of structured interview surveys conducted by Ljungman and colleagues, 49% of children with cancer experienced pain at diagnosis. Procedure- and treatment-related pain were the most significant types of pain at the start, and although procedure-related pain improved, treatment-related pain did not improve. In addition, measurement of pain intensity was rarely performed.
A significant barrier to pain management in children stems from the fact that research and development of evidence-based practice guidelines in pediatrics lag behind that in adults. The NCCN and World Health Organization (WHO) have developed comprehensive guidelines for managing pain in children with cancer, but in many instances, pain management in children relies on extrapolation from adult data, anecdotal reports, and personal experience.
Children may also experience incidental, non–cancer-related pain. Other pain-inducing disorders such as migraines, recurrent abdominal pain, or injuries seen in the general pediatric population can also occur in children with cancer. These causes of pain unrelated to the cancer diagnosis should always be included in the differential diagnosis of pain in children who have cancer.
Nociception is a complex process whereby actual (or potential) tissue damage is perceived as pain by an individual. In many instances pain is a protective mechanism that alerts a person to tissue injury. Nociception may be thought to occur on three levels: peripheral, spinal, and supraspinal ( Fig. 71-1 ). Through transduction, the primary afferent nociceptors, which are thinly myelinated A-delta and unmyelinated C fibers, transmit biochemical changes at the sensory nerve endings that are generated by painful (chemical, thermal, or mechanical) stimuli into electrical signals. Stimulation of such nerve endings in the periphery may be increased or decreased by molecules such as prostaglandins, leukotrienes, bradykinin, and histamine. For example, the bradykinin and histamine that accompany tissue inflammation can directly activate nociceptors, in addition to reducing their threshold and increasing their response to suprathreshold stimulation. The analgesic effect of nonsteroidal antiinflammatory drugs (NSAIDs) lies in their ability to inhibit prostaglandin synthesis and thus desensitize nociceptors.
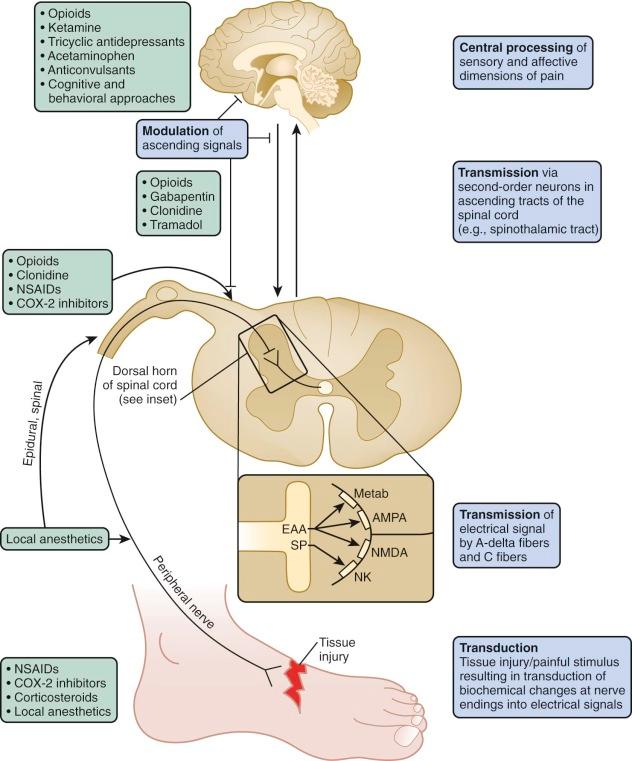
The electrical signals generated are propagated, or transmitted, to second-order neurons that synapse with sensory nociceptors in the dorsal horn of the spinal cord. Second-order neurons convey signals via ascending tracts in the spinal cord, including the anterolateral spinothalamic tracts, to supraspinal sites that include the brainstem, thalamus, and cortical areas involved in the sensory and affective dimensions of pain.
For example, descending inhibitory pathways from the thalamus and brainstem modulate excitatory transmission through such inhibitory neurotransmitters as serotonin, norepinephrine, and endogenous opioids. Several pharmacologic interventions such as opioids and tricyclic antidepressants (TCAs) exert their analgesic effects through such inhibitory processes at the spinal and supraspinal levels.
Pain is a complex sensory and emotional experience that involves nociception but is modified by a range of contextual and psychological factors. Because the experience of pain is subjective, the degree of tissue injury and therefore nociceptive input does not necessarily correlate with the intensity of pain experienced. Interventions that may reduce the perception of pain include hypnosis and relaxation techniques.
Pain that results from stimulation of intact neurons by impulses reflecting tissue injury or inflammation is called nociceptive pain . On the other hand, pain resulting from abnormal excitability of neurons (for example, due to neuronal damage) is called neuropathic pain . Even if tissue damage initially accompanied neuropathic pain, neuropathic pain may persist long after the damage has resolved. Nociceptive pain often may be distinguished from neuropathic pain by its characteristics. Neuropathic pain frequently is described as burning or shooting and is often associated with paresthesias or allodynia (i.e., elicitation of pain by normally nonpainful stimuli such as light touch).
As a subjective experience, many factors have an impact on a child's experience of pain. Recognizing such factors can facilitate an understanding of the pain as it is experienced by the child and facilitate development of an effective pain treatment plan. The experience of pain shapes learning in infancy and throughout life. The neurobiologic mechanisms underlying nociception develop during the third trimester. Specific cortical responses to noxious stimuli can be demonstrated in 32-week preterm infants using evoked potentials or near-infrared spectroscopy. The nature of pain as a conscious experience in young infants remains a subject of speculation and controversy.
Factors influencing the perception and meaning of pain that occurs after infancy are both individual and contextual and include developmental and cognitive factors (e.g., understanding, control, expectation, and relevance) and behavioral and emotional factors (e.g., anxiety, fear, frustration, anger, guilt, and isolation), as well as familiar and cultural factors. A variety of other factors including age, gender, pain acceptance, and pain tolerance have been hypothesized to influence pain perception in the pediatric population and have been summarized elsewhere.
Importantly, previous encounters with pain may heighten the experience of subsequent encounters with pain. For example, children newly diagnosed with cancer who had inadequate procedural analgesia when undergoing a first bone marrow aspirate or lumbar puncture had more severe distress during subsequent procedures, even when efficacious pain relief was subsequently provided. This finding highlights the need to provide effective analgesia to prevent both present and future painful experiences.
Pain assessment and measurement provide the foundation for addressing pain effectively. Regular assessment of pain may improve pain management and should be conducted in a developmentally appropriate manner. When permitted by the child's developmental status, self-report is considered the gold standard in pain assessment. Because self-report can include bias and error, behavioral observations, physiologic changes, and clinician/parental report may be incorporated into the pain assessment. However, these methods also have inherent limitations. For example, tachycardia may reflect fever or intravascular volume depletion rather than pain.
Physiologic and behavioral signs and symptoms can indicate pain, but lack of these signs does not indicate absence of pain, particularly in chronically or very ill children, in whom these indicators are unreliable. In one study comparing a behavioral pain measure with two self-report measures, many children who reported severe pain showed few behavioral pain indicators. In addition, these signs are not necessarily specific for pain itself and may reflect distress that may or may not be related to pain. Parental and clinician estimates of the child's pain also have limitations, because clinicians and parents frequently underestimate pain.
Examples of symptom assessment tools for children of various ages and developmental capacities are demonstrated in Figure 71-2 . These tools may be helpful in assessing symptoms and measuring severity before and after interventions. However, they are not all validated specifically for the population of children with cancer. A given tool should be used consistently with a given child. When the assessment reveals the presence of pain, further inquiry regarding the nature of the pain (e.g., the character of the pain and aggravating and alleviating factors) and the meaning that the pain holds for the child and the family is in order.
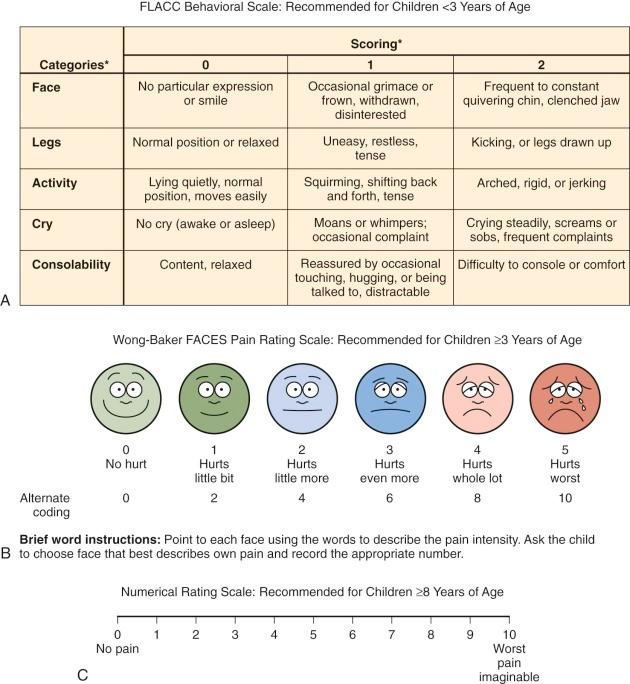
Many children 3 years of age or older are able self-report their symptoms. Because some young children, particularly those who are chronically ill, may be more mature than their chronologic age, the responses even of young children should be heeded. Self-report scales for children include faces scales, color analogue scales, visual analogue scales, the Poker Chip Tool, and numeric rating scales. With the faces scales, such as the Wong-Baker Faces Scale and the Bieri Faces Pain Scale, the child is asked to match how he or she feels with one of the faces. Color analogue scales and faces scales can be used by most children ages 4 years and older. Faces scales differ psychometrically; an example is the use of a smiling face for the “no pain” anchor in the Wong-Baker Faces Scale versus a neutral face for the “no pain anchor” in the Bieri Faces Pain Scale. Although children often report that they like using the Wong-Baker Scale, some researchers regard the use of a neutral face for the “no pain anchor,” as in the Bieri Faces Pain Scale, to be psychometrically more specific. With the visual analogue scale, a child selects a point on a line that represents the intensity of his or her pain. These scales have been extensively studied and are appropriate for children 8 years and older.
Numeric rating scales do not require any equipment, are simple to use, and are already used frequently with adults. These scales require numeracy and the ability to think and express oneself in quantitative terms and thus are most appropriate for use in children who are at least 8 years of age. Children younger than 8 years may provide an unreliable numeric response because, although they can count, they have do not have an understanding of the quantitative meaning of numbers. All quantitative scales are based on the concept of counting, which is a universal concept for children who have this developmental capacity. It is therefore possible to develop quantitative tools for pain measurement that are appropriate for children of virtually all cultures.
Behavioral and physiologic cues are particularly useful in preverbal children and in children who are not able to verbalize their symptoms because of cognitive impairment, developmental capacity, or sedation. Such scales may rely on facial expressions, motor or verbal responses, or combinations of behavioral and autonomic responses. Behavioral scales may actually rate distress, including fear and anxiety, rather than specifically assessing pain per se.
When using behavior to assess pain, it is important to partner with parents or other caretakers who are particularly familiar with the child, because knowing the child, having familiarity with children who have the same or similar conditions, and having a thorough grasp of the science of symptom management are all important components of effective pain relief. For example, the Paediatric Pain Profile was created to be a usable document for parents to assess and record their child's pain behavior. It is a well-validated instrument that uses behavioral cues, including changes in facial expression, vocal sounds, posture and movements, sleeping, eating, and mood, to assess pain. The Individualized Numeric Rating Scale is an individualized scale for nonverbal children. This scale is an adaptation of the numeric rating scale; it allows parents or other clinicians who know the child well to identify the child's typical pain behavior and rank that behavior on a standard scale from 0 to 10.
The Face, Legs, Activity, Cry, Consolability (FLACC) tool was originally designed to score postoperative pain in children ages 2 to 7 years but has also been validated for postoperative use in children with cognitive impairment. Use of this instrument may be advantageous because it employs a variety of types of measures such as activity and facial expression. Further work in the area of pain assessment in children with developmental or cognitive impairment is needed because children in these vulnerable populations are less likely to be assessed for pain, in addition to receiving less analgesic medication.
The previously discussed assessment scales largely reduce pain assessment to the measurement of pain intensity. Although such pain assessment is an oversimplification, the scales permit rapid evaluation of pain and rapid institution of interventions aimed at ameliorating pain. Such scales are also key outcome measures in evaluations of pain-relieving interventions. Findings from a recent study, however, highlight the fact that such a simple screening for pain intensity fails to identify many persons who have significant functional interference from pain or pain significant enough to trigger a physician visit. Regardless of the scale used, if clinical pain indicators are unclear, a trial of measures to ameliorate pain may clarify the cause of distress or pain.
Beyond use of formal assessment tools, key elements in the history include alleviating/exacerbating factors; the quality, location, onset, and severity of pain; and the degree of impact on the child's function and well-being. Understanding the child's previous experiences with pain and strategies that have been used successfully in the past to address pain are also key components of the history and may inform the treatment plan. To this end a multidimensional indicator of pain can help the clinician understand the existence, intensity, and location of pain that a child is experiencing. Use of techniques that employ more than one measure (e.g., self-report, behavioral, and physiologic) permit a more accurate pain assessment.
In addition, it is necessary to understand the holistic nature of pain, rather than pain as merely a physiologic phenomenon. For example, the meaning of the pain, the degree of distress it causes, and the impact of pain and pain treatment on the child's functional capacity and quality of life are crucial elements of the history. Exploration of these issues may elicit exacerbating and potentially modifiable factors related to the pain experience. Finally, it is important to gain a clear understanding of the beliefs that the child and family have about pain, as well as their treatment goals in terms of pain control.
Anticipation and prevention of pain is the most effective treatment approach. Just as children and parents need information regarding their cancer treatment so they know what to expect, informing them in a sensitive manner about the symptoms they may expect and strategies available for reducing their symptoms can reduce anxiety about the unknown. In many cases, without open conversations in which expectations can be addressed, the imagined reality that is substituted is far worse than the true reality. By helping children understand what will happen and how the treatment or procedure will work, their fear and anxiety may be allayed, thereby reducing the symptoms they may actually experience.
When a child reports pain, careful assessment with use of history and physical examination is imperative to generate a complete differential diagnosis. Determination of the underlying cause of pain may inform consideration of which modalities are likely to be most effective in alleviating it. Both pharmacologic and nonpharmacologic (e.g., cognitive, behavioral, physical, and supportive) approaches should be considered, including addressing factors that are likely to affect the child's experience of pain. Once a pain treatment plan is implemented, pain should be regularly reassessed. The goal of pain management should be to achieve the degree of comfort that the child finds satisfactory.
To provide optimal pain control and prevent breakthrough pain, analgesics should in most cases be administered “By the ladder, by the clock, by the appropriate route, by the child” (WHO), and behavioral, physical, and cognitive supports should be provided throughout treatment. Pain of a moderate to severe rating should be treated with analgesics that are administered around the clock, with rescue doses of the same or an alternative analgesic available. Families often find it helpful to understand that preemptive analgesia is far more effective than catch-up analgesia administered for established pain.
Finally, the dosing interval as determined by the pharmacokinetics of the agents considered and the feasibility of their administration by caregivers should also be considered.
The WHO guidelines previously provided a three-step approach to cancer-related pain in children and adults. For mild pain in a child who is not taking any analgesics, an NSAID or acetaminophen was suggested. For moderate pain, a short-acting weak opioid (e.g., codeine) was recommended, and for severe pain, a strong opioid was recommended (e.g., morphine).
The three-step WHO guidelines, which were widely publicized for cancer-related pain, provided an effective approach to pain control for many adults, with published success rates of 69% to 100%. However, the three-step ladder consisting of nonopioids, weak opioids, and strong opioids may not be appropriate for some patients with cancer, particularly those with advanced disease. In fact, studies have shown that a two-step ladder in which treatment passes directly from step I (nonopioids) to step III (strong opioids) for mild to moderate pain provides superior pain relief. Some medications historically used in step II (e.g., codeine and tramadol) are unlikely to provide better control of moderate pain compared with continuation of acetaminophen and NSAIDs (unless contraindicated) along with titrated doses of a strong opioid. Furthermore, codeine presents safety and efficacy concerns (discussed later), and few data exist to guide the administration of tramadol or other intermediate-potency analgesic agents in children. For these reasons, the most recent WHO guidelines for the management of pain in children with cancer (or other medical illness) are now based on a two-step approach.
The WHO guidelines, which provide an effective, systematic approach to pain that includes strong opioids, have facilitated other WHO initiatives, such as increasing worldwide access to essential medicines for children with painful conditions. The WHO approach, however, may not adequately emphasize a multidisciplinary approach from the start, and thus opportunities to use adjuvant medications (e.g., steroids and local anesthetics) and cognitive or behavioral interventions to reduce pain and pathologic responses to pain may be missed.
Nonopioid analgesics are appropriate for mild pain in a child who is not already receiving analgesics. Nonopioid analgesics include acetaminophen, NSAIDs, salicylates, and selective cyclooxygenase (COX-2) inhibitors.
Nonopioid analgesics are frequently used as monotherapy for mild pain and are used together with opioids for more severe pain. Unlike opioids, they do not cause sedation, tolerance, and respiratory depression. When given as an adjuvant to opioids, they enhance analgesia. To this end they may be opioid-sparing (i.e., decreasing the amount of opioid required for adequate analgesia), thereby also limiting opioid-associated adverse effects. A recent randomized double-blind study of infants who underwent noncardiac surgery demonstrated that children randomly assigned to receive intermittent intravenous (IV) paracetamol with morphine as needed for rescue required only about one third as much rescue morphine as children randomly assigned to receive an IV placebo. Combinations of nonopioids and opioids are available, although dosing of such a combination is often limited by the maximum dose of the nonopioid.
Salicylates and NSAIDs nonspecifically inhibit cyclooxygenase enzymes, thereby blocking production of a variety of prostaglandins that mediate pain, inflammation, fever, and platelet function, protect gastric mucosa, and maintain a physiologic distribution of blood flow in the liver and kidneys. The permanent effects of aspirin on platelet function via permanent acetylation of cyclooxygenase are of particular concern in patients who have thrombocytopenia because the antiplatelet effect lasts even after the drug has been metabolized. Choline magnesium salicylate (Trilisate) is a salicylate that provides many of the same benefits as those provided by other salicylates or NSAIDs without known antiplatelet activity. Salicylates, however, have been associated with Reye syndrome in children younger than 2 years.
No particular NSAID or route of administration has been found to be superior over another. Even ketorolac, the only parenteral NSAID widely used in the United States, is no more effective than orally administered NSAIDs, especially if doses are compared in an equitoxic range. A single NSAID dose is roughly equivalent to 5 to 10 mg of intramuscular morphine in adults and has fewer adverse effects. NSAIDs are commonly regarded as having a ceiling effect with no added benefit at supramaximal doses, although the doses commonly recommended (based on safety concerns) remain well below the ceiling in most cases. NSAIDs can cause nephropathy, gastritis, and bleeding from reversible platelet dysfunction, a characteristic that may limit their use in patients with cancer who are thrombocytopenic. COX-2 inhibitors (e.g., celecoxib) selectively block production of prostaglandins that mediate inflammation and pain without impairing platelet function and with fewer effects on gastric mucosal integrity compared with traditional NSAIDs, particularly with short-term use. Concerns have been raised regarding effects of COX-2 inhibitors on the risk of cardiovascular events in adults. Evidence does not currently indicate that these concerns extend to children with neoplasms, including the small subgroup of children with tumors or vascular anomalies who have an increased risk of thrombotic events.
Acetaminophen is a nonopioid analgesic that reduces pain and fever. It may act in part by inhibiting prostaglandin synthesis in the CNS. Unlike salicylates and NSAIDs, which inhibit peripheral cyclooxygenases, it does not have peripheral antiinflammatory properties. Acetaminophen is available in a variety of oral formulations, as an IV formulation (approved in the United States for children ≥2 years), and as a rectal suppository, although rectal administration should be avoided in neutropenic patients. When not contraindicated, rectal administration is helpful for children unwilling or unable to take oral medications, although its absorption is slow and variable, peaking at 70 minutes. Because rectal absorption is less efficient, single doses of 30 to 40 mg/kg can be administered. Subsequent doses should be smaller (20 mg/kg), and the interval between doses should be increased to 6 to 8 hours. Dosing of oral or IV acetaminophen is limited to 75 mg/kg/day or a maximum of 4 g/day (whichever is smaller) because of the risk of hepatic toxicity.
Guidelines for initial opioid dosages are presented in Table 71-1 and are discussed in greater detail in this section. Ultimately, the correct dose of opioid is the dose that provides the desired analgesia with acceptable adverse effects. In general, intolerable adverse effects are usually the dose-limiting factor, as opposed to opioids having a ceiling effect. This situation should be explained to parents, as well the fact that, with rare exceptions, children with cancer who receive opioids for pain control do not become addicted to opioids. Addiction is an aberrant psychiatric condition in which the person exhibits maladapted, drug-seeking behavior. True addiction is comparatively rare in patients with cancer-related pain. Children who exhibit exaggerated pain behaviors (e.g., demanding pain medication and engaging in manipulative behaviors) are far more likely to be demonstrating pseudoaddiction, in which case their behavior is a reflection of poorly controlled pain.
| Drug | EQUIANALGESIC DOSES | USUAL STARTING IV OR SUBCUTANEOUS DOSES AND INTERVALS | Ratio of Parenteral to Oral Dose | USUAL STARTING ORAL DOSES AND INTERVALS | |||
|---|---|---|---|---|---|---|---|
| Parenteral | Oral | Child <50 kg | Child ≥50 kg | Child <50 kg | Child ≥50 kg | ||
| Morphine | 10 mg | 30 mg (long term) 60 mg (single dose) |
Bolus: 0.1 mg/kg every 2-4 h Infusion: 0.03 mg/kg/h |
Bolus: 5-8 mg every 2-4 h Infusion: 1.5 mg/h |
1 : 3 (long term) 1 : 6 (single dose) |
Immediate release: 0.3 mg/kg every 3-4 h Sustained release: 20-35 kg: 10-15 mg every 8-12 h 35-50 kg: 15-30 mg every 8-12 h |
Immediate release: 5-20 mg every 3-4 h Sustained release: 30-45 mg every 8-12 h |
| Oxycodone | NA | 15-20 mg | NA | NA | NA | 0.1-0.2 mg/kg every 3-4 h | 5-10 mg every 3-4 h |
| Methadone † | 10 mg | 10-20 mg | 0.1 mg/kg every 4-8 h | 5-8 mg every 4-8 h | 1 : 2 | 0.1-0.2 mg/kg every 4-8 h | 5-10 mg every 4-8 h |
| Fentanyl | 100 µg (0.1 mg) | NA | Bolus: 0.5-1.0 µg/kg every 1-2 h Infusion: 0.5-2.0 mg/kg/h |
Bolus: 25-50 µg every 1-2 h Infusion: 25-100 µg/h |
NA | NA | NA |
| Hydromorphone | 1.5-2 mg | 6-8 mg | Bolus: 0.02 mg/kg every 2-4 h Infusion: 0.006 mg/kg/h |
Bolus: 1 mg every 2-4 h Infusion: 0.03 mg/h |
1 : 4 | 0.04-0.08 mg/kg every 3-4 h | 2-4 mg every 3-4 h |
| Meperidine (pethidine) ‡ | 75-100 mg | 300 mg | Bolus: 0.8-1.0 mg/kg every 2-3 h |
Bolus: 50-75 mg every 2-3 h |
1 : 4 | 2-3 mg/kg every 3-4 h | 100-150 mg every 3-4 h |
* Doses are for patients older than 6 months of age. In infants younger than 6 months, initial per-kilogram doses should begin at roughly 25% of the per-kilogram doses recommended here. Higher doses are often required for patients receiving mechanical ventilation.
† Use of methadone requires additional vigilance because it can accumulate and produce delayed sedation. If sedation occurs, doses should be withheld until sedation resolves. Thereafter, doses should be substantially reduced; the interval between doses should be extended to 8 to 12 hours, or both.
‡ Use of meperidine, especially long-term use, should generally be avoided if other opioids are available, because its metabolites can cause seizures.
Addiction should be distinguished from dependence, that is, a physiologic response to opioids in which abrupt removal leads the patient to experience symptoms of withdrawal. It may be helpful to draw a parallel to blood pressure medication, the abrupt cessation of which causes an undesired rebound hypertensive effect because the body has adjusted to the presence of the antihypertensive drug. Addiction should also be distinguished from tolerance, which is another physiologic response of the body to the presence of opioids that requires increasing doses to achieve the same analgesic effect. The need to adjust dosing to account for tolerance is not an indication of addiction. Other processes that can resemble tolerance, including opioid-induced hyperalgesia, are discussed later in this chapter.
Opioids do not need to be saved for cases of extreme pain, because increasing pain can often be managed by increasing the opioid dose. In addition, when escalating doses provide marginal benefit, rotating to an alternative opioid may provide better analgesia. It may be helpful to explain to parents that good pain control from the start may improve pain control overall and actually minimize the amount of opioid that is ultimately needed because when good pain control is achieved, the need to use large doses required to catch up to uncontrolled pain can be avoided.
Some children who have cancer, particularly advanced cancer, require extremely high doses of opioids. For example, in a sample of children with advanced cancer, Collins and colleagues found that in some patients the opioid infusions ranged more than 100-fold from 3.8 to 518 mg per hour of morphine equivalent.
Some studies conducted in vitro and in animal models have demonstrated that opioids may promote cancer cell growth by affecting processes such as tumor cell proliferation and migration. For example, opioids at physiologically relevant concentrations promote tumor angiogenesis. In addition, the opioid receptor antagonist naloxone and the COX-2 inhibitor celecoxib inhibit angiogenesis, tumor growth, and metastasis in rodents. However, other studies demonstrate growth-inhibiting effects of opioids and have been reviewed elsewhere. No evidence to date demonstrates opioid-associated promotion of tumor growth in humans. These preliminary findings should not, by themselves, lead to the conclusion that use of opioids to relieve cancer-related pain should be avoided. The data accumulated to date regarding the potential risks of opioids and the benefits of adequate analgesia are insufficient to recommend limiting opioid use for analgesia.
Elements of renal clearance such as glomerular filtration and tubular secretion increase in the first few weeks of life, such that renal clearance commensurate with adult clearance is achieved by 8 months. When compared with children and adults, neonates and infants have reduced hepatic clearance as a result of hepatic enzyme immaturity. In addition, children 2 to 6 years of age have higher clearance than do adults because of a larger liver mass to body weight ratio. As a result, drugs may need to be administered more frequently in children than they are in adults. Other age-related differences, such as changes in body composition and plasma concentrations of drug-binding proteins, can also influence pharmacokinetics.
The usual starting opioid is morphine because of its low cost, wide availability, multiple routes of administration, and familiarity to clinicians. Because of its long history of use in children, it should be considered the first-line opioid in this population unless specific reasons exist to consider alternatives. Alternative opioids including oxycodone, hydromorphone, and semisynthetic and synthetic compounds such as fentanyl and methadone can be used in children. Use of alternative opioids may be predicated on availability, route of administration, presence of organ impairment, and the patient's prior experience with particular opioids.
Metabolites of morphine, oxycodone, and hydromorphone (discussed later) may accumulate during renal failure. Some metabolites have analgesic activity, which may lead to delayed opioid toxicity (opioid neurotoxicity will be discussed later). For this reason, dosing intervals may need to be increased in patients with renal dysfunction. The pharmacokinetics of alternative opioids such as fentanyl and methadone are not changed with renal impairment, making these opioids better choices in this setting.
Because glucuronidation is relatively well preserved in persons with liver failure, opioids metabolized by glucuronidation (i.e., morphine, hydromorphone, and buprenorphine) are generally better choices than those metabolized by oxidation via liver cytochromes (e.g., oxycodone, fentanyl, and methadone) in this setting. However, because of shunting in persons with liver cirrhosis, the bioavailability of glucuronidated opioids may be increased. For this reason, initial opioid doses should be lower in persons with hepatic impairment.
Tramadol is an atypical analgesic with some direct noradrenergic and serotonergic agonist action and with an active metabolite that is a weak opioid. Tramadol has numerous drug-to-drug interactions that should be considered. For example, it can produce seizures by itself, but this risk is greatly increased when tramadol is administered in combination with several classes of antidepressants. It is available in oral immediate and extended-release preparations and in combination formulations with acetaminophen.
Although codeine has historically been recommended as a weak opioid for mild to moderate pain, it has several limitations. The variable expression of the CYP2D6 enzyme that biotransforms codeine leads to unpredictable levels of the active metabolite, morphine. In the fetus, CYP2D6 activity is absent, and in children younger than 5 years, CYP2D6 activity is less than 25% that of adult activity. Furthermore, certain genotypes are associated with reduced enzyme activity, regardless of age. In a study of 96 children randomly assigned to receive either morphine and diclofenac or codeine and diclofenac after an adenotonsillectomy, it was found that 47% of children had genotypes associated with reduced enzyme activity and that neither morphine nor its metabolites were detected in 36% of children with reduced CYP2D6 activity genotypes who received codeine, although the study did not account for other factors influencing catalytic rate, such as gene copy number. Such “poor metabolizers” of codeine were more likely to have uncontrolled pain and to require rescue medication.
On the other hand, persons who are “ultrarapid metabolizers” may possess multiple copies of the CYP2D6 gene responsible for codeine metabolism or have genotypes associated with rapid metabolism and may therefore be at risk for adverse effects such as respiratory depression from rapid generation of morphine from codeine. Several pediatric deaths from codeine administered after a tonsillectomy and/or an adenoidectomy have resulted from such ultrarapid metabolism. It is for this reason that the U.S. Food and Drug Administration (FDA) has issued a “boxed warning” and deemed codeine contraindicated in this setting.
In a variety of painful conditions in children, provision of codeine provides no benefit greater than that of NSAIDs. When codeine, ibuprofen, and acetaminophen monotherapy were compared in children with musculoskeletal trauma who presented to an emergency department, ibuprofen provided superior analgesia. In the posttonsillectomy setting, codeine in combination with acetaminophen caused more nausea with no difference in pain or postoperative bleeding. A meta-analysis also found that weak opioids (i.e., codeine) in combination with NSAIDs fail to provide superior analgesia to that provided by NSAIDs alone but do have significantly more adverse effects. Based on these findings, in our view, very few instances exist in which codeine is a preferred choice among opioids.
Some opioids exhibit partial mu receptor agonist activity (e.g., buprenorphine), kappa agonist activity (e.g., nalbuphine), or mixed agonist activity (e.g., butorphanol and pentazocine). Although these opioids have predominantly agonist activity, some have significant antagonist activity as well, and thus they may reduce the effect of pure mu agonists given concurrently. In general they do not provide superior analgesia, although they may have fewer gastrointestinal (GI) or respiratory adverse effects and may be considered for individual patients who have limiting adverse effects with other opioids. Currently the greatest use of buprenorphine in the United States is for substance abuse treatment. It has been used by multiple routes for treatment of cancer-related pain in children, particularly in countries with greater impediments to the prescribing of morphine, methadone, or other opioids. Authors of a recent small prospective case series reported reasonable effectiveness and tolerability of transdermal buprenorphine for children with cancer.
Meperidine is a strong opioid that should be avoided in most cases because of its adverse effect profile and lack of superiority to the strong opioids described in the next sections. For example, repeated doses of meperidine lead to accumulation of its metabolite, normeperidine, which in turn causes neuroexcitatory symptoms including agitation, tremors, myoclonus, and seizures. In a double-blind trial comparing morphine with meperidine administered via patient-controlled analgesia (PCA), it was found that morphine resulted in better analgesia and had no more adverse effects than did meperidine. In low doses, meperidine reduces postoperative shivering or rigors associated with amphotericin infusion.
Morphine is the most frequently prescribed opioid in children and is the best studied opioid in this population. It offers flexibility in terms of routes of administration. It is also available in a controlled-release formulation, and multiple randomized controlled trials have shown that this formulation can effectively control cancer-related pain when administered every 12 hours. Morphine is extensively metabolized by glucuronidation in the liver to morphine 3-glucuronide (M3G) and morphine 6-glucuronide (M6G). M3G does not bind mu receptors and has no analgesic activity but may contribute to some of the neuroexcitatory adverse effects of morphine. On the other hand,M6G does bind mu receptors and is a potent analgesic.
Oxycodone is a semisynthetic derivative of morphine. Although it is frequently categorized as a weak opioid appropriate for mild to moderate pain (and is frequently administered in combination with acetaminophen), this categorization is a reflection of its use at low doses. In fact, a meta-analysis showed that oxycodone is as efficacious an analgesic as morphine or hydromorphone. This meta-analysis also showed no difference between oxycodone and morphine in terms of adverse effects such as dry mouth, sedation, or nausea. Oxycodone itself has no ceiling effect or dose limit, although dosing may be limited when it is administered in combination with nonopioid agents such as acetaminophen. Although it is available in parenteral formulations in other countries, in the United States it is only available in an oral formulation. The oral formulation is available in an extended-release preparation.
Oxycodone is predominantly metabolized by CYP3A4 to inactive products. A secondary pathway involving CYP2D6 may lead to generation of the active opioid oxymorphone in patients with ultrarapid metabolizing variants. Although cases of apnea and death associated with excessive conversion from oxycodone to oxymorphone have been published, the prevailing impression is that pharmacogenomic variation overall leads to less variance in effect for oxycodone compared with codeine. Oxycodone pharmacokinetics have been studied in children, but there is a need for additional pediatric pharmacokinetic/pharmacodynamic studies that include oxymorphone assay, pharmacodynamic end points, and contemporary genotyping.
Hydromorphone may be administered orally, intravenously, or subcutaneously. Although it was previously thought that hydromorphone has less neurotoxicity, hydromorphone metabolites have recently been shown to convey neuroexcitatory adverse effects. One advantage of hydromorphone compared with morphine is that its higher potency allows for smaller subcutaneous volumes to be delivered when this route of administration is utilized.
Fentanyl may be given intravenously with rapid onset of action and short duration of action (20 to 30 minutes). For this reason fentanyl is frequently used as an analgesic for brief, painful procedures. Rapid administration of fentanyl may cause chest wall rigidity that requires reversal with naloxone or even neuromuscular blockade and positive pressure ventilation. In occasional patients, fentanyl may be better tolerated than other opioids, in part because it is associated with less histamine release and creates no metabolites that may produce neurotoxicity.
Fentanyl transdermal patches last 72 hours and are a convenient parenteral mode of drug delivery that is preferred by many adults. These patches have also been used successfully in children who have chronic pain. Although wide within-individual variability in fentanyl absorption exists, intraindividual absorption is reported to be stable. In addition, hyperhidrosis, hypertrichosis, and the localization of patches on the skin do not appear to affect fentanyl absorption. Because the onset of action is at least 12 hours, and because some fentanyl remains in the system for 72 hours after patch removal, transdermal fentanyl lacks flexibility for close titration for rapidly changing pain severity. The smallest patch size (12 µg/h) may be too large a dose for some children. For children as young as 2 years who had previously taken opioids and had developed some degree of tolerance, transdermal fentanyl was found to be a safe and well-tolerated alternative to oral opioid treatment. The reservoir design of the patch prevents the patch from being cut to adjust the dose delivered. Transdermal fentanyl should be avoided in patients who have not previously taken opioids because it may result in respiratory depression.
In addition to rapid onset of action and transdermal application, fentanyl provides several other benefits. When renal function is impaired, fentanyl does not accumulate to the same extent as morphine. Some studies have demonstrated that transdermal fentanyl appears to cause less constipation than does oral morphine, but it is unknown whether this observation is related to the route or the drug.
In adults or children already receiving 60 mg/day of morphine, oral transmucosal fentanyl citrate (OTFC, or Actiq) provides extremely rapid control of incident pain, with an onset of action of 5 to 10 minutes. This rapid onset of action is due to its lipophilic nature, as well as because this route bypasses first-pass hepatic metabolism. Because of the rapidity of its onset of action, OTFC has been used to provide analgesia for brief, painful procedures without the requirement for IV access. OTFC is also quite useful for patients who have breakthrough pain. In a double-blind, double-dummy, randomized, crossover study of adult patients with cancer who had incident (breakthrough) pain, it was found that OTFC reduced pain intensity more effectively than did immediate-release oral morphine, and OTFC was favored over immediate-release morphine sulfate by more patients after the study. No conversion ratio is available for OTFC, and careful titration is needed to determine the correct dose. No correlation exists between the effective OTFC dose and the around-the-clock dose of an opioid. For this reason the lowest strength (200 µg) should be tried first. If inadequate pain relief is achieved in 20 minutes, this dose may be repeated.
The fentanyl buccal tablet is another preparation of fentanyl that is rapidly absorbed through effervescent action through the oral mucosa. Patients should be taking at least 60 mg/day of oral morphine so they have opioid tolerance to safely receive a fixed dose of fentanyl with such rapid onset of action through the oral mucosa. The need for this degree of tolerance is highlighted by recent experiences with buccal/sublingual fentanyl tablets, which, when (inappropriately) administered to opioid-naive patients, may result in life-threatening respiratory depression.
Methadone may be given orally (it is available as a tablet or liquid) or intravenously. One advantage to methadone in the pediatric population is that it is the only long-acting opioid widely available in liquid formulation. In addition, it is relatively inexpensive to manufacture, costing 90% less than extended-release morphine. Methadone also exhibits unique receptor-binding properties in that the l-isomer binds mu opioid receptors and the d-isomer binds the N-methyl-d-aspartate (NMDA) receptor. Because the NMDA receptor is involved in opioid tolerance, opioid hyperalgesia, and neuropathic pain, it may be a useful opioid in these clinical situations, which are discussed later. For these reasons, administration of methadone for analgesic purposes has become more popular in recent years.
The variable pharmacologic half-life of methadone, which ranges from 12 to 150 hours, may result in delayed toxicity (e.g., sedation and hypoventilation) that can occur many days after initiation of the drug. The analgesic half-life of methadone is commonly cited as 4 to 6 hours, although some patients can require minimal rescue analgesia with dosing at 8- or 12-hour intervals. In addition, equianalgesic dose conversion from other opioids is variable and depends in part on the dose of the previous opioid. Its potency relative to morphine is highly dependent on the previous morphine dose. A very convenient website, www.globalrph.com/narcoticonv.htm , can be used to assist in dose conversions. It should be emphasized that even with these calculations, enormous individual variability exists, and close follow-up is required to avoid delayed oversedation.
Methadone is metabolized through several cytochromes and therefore interacts with a variety of other medications. Individual variation in cytochrome expression may account in part for significant blood concentration variability in patients. Methadone, in conjunction with other medications, may prolong the QTc interval. It is unclear whether this phenomenon explains the otherwise unexplained increased incidence of sudden cardiac arrest in adults receiving methadone therapy. Until the potential for methadone to pose cardiotoxicity is better understood, it should be used cautiously in children who have underlying cardiac conditions or those at risk for prolonged QTc. Although methadone has several advantages, it also has some unique features that require familiarity with this agent for safe and effective use. The majority of pediatric oncologists rarely, if ever, prescribe methadone. Lack of familiarity with methadone pharmacodynamics, effectiveness, and dosing equivalence are the most common reasons cited for prescribing other opioids rather than methadone.
Oxymorphone is the active metabolite of oxycodone and is available as a rectal suppository. An extended-release oral formulation has also recently been approved.
For the opioid-naive patient, recommended starting doses are listed in Table 71-1 . For infants younger than 6 months, the starting dose should be roughly one fourth the weight-scaled dose suggested in the table and titrated to effect. Opioids should be administered with caution in patients who have disordered control of respiration, altered mental status, or altered drug metabolism. This is not to say, however, that opioids should be withheld from these patients. In fact, opioids can be safely delivered and adequate analgesia achieved with careful titration to effect.
Medication should be given by the simplest, most effective, and least distressing route. Other considerations that should guide the choice of route include the severity and type of pain, the ability of the child to tolerate a given route due to developmental or personal factors, and the ability of the caregivers to administer medication via certain routes.
When possible, the oral route of administration should be attempted first. In general, the time for opioids to reach peak effect is about 60 minutes with the oral route. Most extended-release preparations are available in tablets or capsules. For children who cannot swallow tablets or capsules but who would benefit from an extended-action preparation or agent, liquid methadone can be used. If the child has a gastrostomy tube, ultra–extended-release morphine (given every 24 hours) may be suspended (but not crushed) and administered via a gastrostomy tube. Ultra–extended-release morphine allows once-daily oral dosing, but unintended chewing or crushing may lead to overdose from immediate release of the morphine. Opening the capsules and sprinkling the drug onto applesauce may be an appropriate administration technique for adults but should be avoided in young children. Ultra–extended-release morphine may be considered for adolescents, but in younger children who may be at risk for accidentally chewing the capsules, these preparations should generally be avoided.
For intermittent dosing, oral opioids prepared as concentrated drops may provide analgesia without the requirement of swallowing larger volumes of liquid. Concentrated morphine may be helpful for children who are unable to reliably take oral opioids because of their neurodevelopmental capacity or nausea and vomiting.
Suppositories containing hydromorphone and morphine may be administered rectally. In addition, controlled-release morphine tablets may be given rectally. Although the published potency of rectal opioids approximates that of oral opioids, the pharmacokinetic properties of morphine, that is, first-pass metabolism to the active metabolite M6G by the portal circulation, should be considered when considering rectal administration. For example, Wilkinson and colleagues determined that the area under concentration-time curves for morphine metabolites were approximately twice those achieved after rectal administration. The maximal concentration of morphine and its metabolites was lower and the time to achieve peak levels was longer for rectal administration. In this study, the variation in morphine kinetics did not correspond with altered pain ratings. Although it is reasonable to start with 1 : 1 (oral to rectal) equianalgesic dosing of morphine, adjustments in the dose or dosing interval should be anticipated.
Fentanyl and buprenorphine are the only opioids manufactured in transdermal formulations. The transdermal route for delivery of fentanyl or buprenorphine provides some advantages. Other opioids such as morphine may be compounded as transdermal formulations, but absorption of these other opioids via this route is unreliable, and alternative means of opioid administration are almost invariably available.
Use of the IV route, when appropriate, may provide rapid and reliable administration. In general, the time for morphine to reach its peak effect is about 15 minutes via this route. The IV route is frequently used for patients who have severe pain or for children who are unable to take oral medications, such as children who are in the final stages of life.
All IV opioids may be administered subcutaneously, although methadone may cause local irritation if infused continuously. Delivery of opioids by this route dose adds approximately 30 minutes to the time of peak effect obtained by IV administration. This route is simple to use and requires only a portable syringe driver to administer the medication through a butterfly needle. In addition, it confers consistent delivery and easy titration without the requirement for IV access. Needles are changed every week or more often if skin irritation occurs, and this task can often be performed by a family member. In our experience, children can absorb 2 mL per hour, whereas adults can absorb 3 to 5 mL per hour. Higher doses of opioids may exceed these volume limits. In such cases, switching to a more potent opioid, such as hydromorphone, may be necessary.
PCA can provide a continuous IV or subcutaneous infusion of opioid to provide basal control of pain, as well as a bolus, which provides relief from breakthrough pain. The PCA delivery system allows patients to manage their pain themselves, and no lag time exists between the request and delivery of a bolus dose for uncontrolled pain, increasing their sense of control over their pain. In one study comparing continuous infusion morphine with PCA, it was found that patients who used PCA required less total opioid while receiving equivalent control of the pain of mucositis associated with bone marrow transplantation.
Because opioid-induced sedation generally occurs before respiratory depression, it is rare for a patient to administer boluses to himself or herself to the point of respiratory depression. PCA delivery has been used in the pediatric population with safety and efficacy. PCA does not increase the incidence of opioid-related complications, including sedation. Clinical protocols for calculating the PCA commencement opioid dose and subsequent opioid-dose escalation can facilitate the safe and efficacious implementation of PCA. Thorough assessment of PCA use includes the total daily dose, ratio between continuous and bolus opioid, amount of baseline and breakthrough pain, and response to the bolus dose. A common recommendation is to adjust the continuous (basal) rate to supply roughly two thirds of the patient's daily opioid requirement. Although this starting point is reasonable for most oncology patients with persistent disease-related pain, individual circumstances exist in which the parameters should be modified. For example, in the setting of brief, severe, episodic pain, it may be reasonable to use a lower basal rate and give more generous boluses. In postoperative care, considerable variation exists in the use of basal infusions. Our practice is to use them for operations expected to result in more severe pain and/or in patients who are likely to underdose themselves. Conversely, we tend to avoid basal infusions or use very low basal rates for patients who have less painful surgery, for patients who have received other nonopioid methods of analgesia (e.g., peripheral nerve blocks or plexus blocks), or for patients who have factors that increase respiratory risks.
PCA has been used successfully in children as young as 4 years of age. PCA may also be administered by surrogates, commonly as nurse-controlled analgesia or parent-controlled analgesia, or collectively as PCA-by-proxy. In theory the safety of PCA is maximized when the patient self-administers the medication, because when the patient falls asleep, he or she stops pushing the button. Because the proxy assesses the child's pain and provides the bolus dose, the safety feature inherent in a PCA to prevent respiratory depression is overridden, but when it is used appropriately it is associated with only rare respiratory or neurologic complications. Overall, the safety of nurse-controlled analgesia is well established and is widely used for both opioid-tolerant and opioid-naive infants and children. Greater controversy persists with parent-controlled PCA, although experience with this arrangement is mounting. Our general practice is to greatly limit its use for routine care of opioid-naive postoperative infants and children but to use it widely for opioid-tolerant infants and children in palliative care, especially at home. Guidelines to increase the safety of PCA by proxy have been proposed.
Opioids may be administered intrathecally and are considered later in the “Invasive Approaches to Pain Management” section.
Regardless of the route utilized, if the patient has continuous pain, a regimen providing continuous pain control should be implemented. To establish a patient's true analgesic needs, short-acting opioids, which can be easily titrated, may be administered for the first 24 to 48 hours. Based on the amount of opioid required during the initial interval, a longer acting opioid may then be substituted, with provision of an as-needed short-acting opioid available for incident or breakthrough pain.
Whatever the regimen, ensuring proper follow-up assessment is critical to ensure appropriate analgesia and to evaluate for potential opioid-associated adverse effects. Because constipation is a predicted and preventable adverse effect of opioids, a bowel regimen to prevent this adverse effect should always be instituted and adjusted as necessary whenever opioids are started or escalated.
Breakthrough pain is a transitory exacerbation of pain that occurs when pain is otherwise relatively controlled or stable at baseline. Patients with chronic cancer-related pain and superimposed breakthrough pain have worse overall pain, more impaired functioning, and higher psychological distress than do patients with chronic cancer-related pain alone. Breakthrough pain may occur as a result of incident pain (i.e., pain due to a stimulus, such as movement or coughing) or end-of-dose failure, or it may be spontaneous in nature (such as lancinating pain attacks in persons with postherpetic neuralgia).
In a prospective study of pediatric inpatients with cancer it was found that 57% experienced one or more episodes of breakthrough pain during the preceding 24 hours, with each episode lasting seconds to minutes and most commonly characterized by the children as “sharp” and “shooting.”
Breakthrough pain may be challenging in that its onset may be unpredictable and rapid, and it may be more severe than pain typically experienced at baseline. For these reasons, use of a pain diary may be particularly important in detecting patterns and factors associated with breakthrough pain. If end-of-dose breakthrough pain occurs, the total daily around-the-clock dose may be increased by 25%, although this increase may lead to intolerable adverse effects. Alternatively, the dosing interval may be shortened.
Breakthrough pain should be approached as other types of pain are approached, with (1) consideration of the underlying triggers and interventions aimed at the underlying problem, (2) optimization of the scheduled analgesic regimen, and (3) use of adequate analgesics for episodic pain, that is, rescue medication. The rescue dose to treat such breakthrough pain should be the equivalent to the dose used every 4 hours, or 5% to 10% of the total daily opioid dose. For predictable incident pain, a short-acting opioid given just prior to the activity may be helpful. When the breakthrough pain has neuropathic features, consideration should be given to adjunctive use of analgesics with specificity for these types of pain, that is, anticonvulsants or antidepressants, as will be discussed later.
OTFC may safely provide very rapid pain relief in patients receiving the equivalent of 60 mg/day of oral morphine, as was previously discussed. It may be especially useful when breakthrough pain is unpredictable and short-lived. Other hydrophilic agents such as morphine are often administered sublingually for breakthrough pain but have a longer onset of action. A recent small study indicates that the rapid onset of action of methadone may make it a useful agent for treatment of breakthrough pain.
When a patient who is already receiving oral or IV opioids has severe pain that persists despite a dose of breakthrough opioid medication, the dose should be increased by 50% to 100% and repeated. Once adequate analgesia is reached, the total amount given over 4 hours to determine the “effective dose” for every 4 hours should be calculated. If more than two rescue doses are used in a 24-hour period, the total standing dose should be increased.
For patients receiving continuous IV opioids, a rescue dose for breakthrough pain should be provided that consists of 50% to 200% of the hourly infusion every 15 minutes as needed. For the sake of simplicity, use of multiple different short-acting opioids is discouraged.
The response to acquired tolerance to an opioid is usually to increase the opioid dose. When this approach is not feasible because of opioid adverse effects such as neuroexcitability, rotating to a different opioid may be an option. Because the NMDA receptor plays a role in opioid tolerance, methadone, with its attendant NMDA antagonist properties, may control pain that is otherwise refractory to opioids. The addition of adjuvant NMDA receptor antagonists such as ketamine may also reverse opioid tolerance. In animals, as well as in some patients, chronic administration of opioids can generate a condition of generalized hyperalgesia, meaning that new painful stimuli produce a greater intensity of pain than would have occurred with the same stimulus for that subject in an opioid-naive state. Opioid-induced hyperalgesia is discussed in greater detail later.
Opioid rotation, or switching, may also be indicated as a result of intolerable adverse effects or route of administration. Equianalgesic tables are useful for conversion from one opioid to another and are widely available. Although no evidence exists to demonstrate the superiority of one opioid over another in such a switch, the strategy of changing opioids may be successful in alleviating dose-limiting adverse effects or opioid tolerance in children. For example, Drake and colleagues found that opioid rotation resolved adverse effects in 90% of children without loss of analgesia or the need to increase morphine equivalents. In fact, because of the phenomenon of incomplete tolerance, the equianalgesic dose of the second opioid should be decreased by 20% when a patient is transitioned from one opioid to another. The phenomenon of incomplete tolerance is particularly pronounced when converting to methadone, likely because of the ability of the d-isomer of methadone to act as an NMDA receptor antagonist.
Opioid withdrawal is a physiologic response. Sudden discontinuation of opioids in a patient who has been taking long-standing opioids may prompt a withdrawal syndrome characterized by irritability, restlessness, dysphoria, anxiety, muscle aches, sweating, piloerection, diarrhea, nausea, vomiting, yawning, and sneezing. Withdrawal may be prevented by tapering opioids. Opioid doses may be safely cut in half without precipitating withdrawal. Continued tapering may be achieved by halving the dose every 3 days. An alternative strategy is to reduce the total daily dose by 10% per day. Maintaining the rescue dose at its original dose during the tapering process allows for effective treatment of breakthrough pain that may occur as the dose is tapered.
Nonrespiratory adverse effects from opioids include constipation, nausea, pruritus, somnolence/sedation, and, particularly at high doses, neurotoxicity. A prospective study of pediatric oncology patients found that in children receiving morphine, postoperatively, 38% had vomiting, 32% had nausea, and 24% had constipation. The high incidence of nausea and vomiting in this population is likely confounded by postoperative nausea and vomiting.
Opioid-associated sedation typically self-resolves within a few days of the institution of opioids. In some cases the sedation observed when instituting opioids is not an adverse effect of opioids per se but rather a result of an exhausted patient finally able to sleep once pain is controlled. Nausea experienced by some patients upon starting opioids similarly self-resolves within a few days and is often relieved with use of an antiemetic. Switching to a different opioid due to nausea within the first 3 days of opioid therapy may be premature. Opioid-associated urinary retention is an uncomfortable symptom that may respond to a switch to an alternative opioid. Anecdotally, urinary retention in some patients may respond to selective alpha-1A antagonists such as tamsulosin, which are commonly prescribed for adults with lower urinary tract outflow obstruction. Urinary retention, frequency, or urgency should prompt a focused consideration of a range of oncologic and neurologic causes, as well as effects of other medications in addition to opioids, such as anticholinergics and antihistamines. Management of opioid-associated sedation/fatigue, constipation, pruritus, and nausea are further discussed in the sections of this chapter dedicated to these specific symptoms.
If adverse effects persist despite appropriate interventions, rotation to a different opioid may be helpful. Overall, at this point no clear evidence exists that one particular opioid has a different adverse effect profile than another opioid. Patients may exhibit different sensitivities to different opioids because of individual variability in opioid receptors, pharmacokinetics, and metabolism. Inroads have recently been made in understanding which genetic variants may influence a particular person's response to opioids. For example, certain variants of the multidrug resistance 1 gene (formerly MDR1, now called ABCB1 ) or the gene encoding catechol-O-methyltransferase (COMT) are associated with CNS adverse effects, such as drowsiness or confusion. A very promising recent approach to reducing opioid adverse effects involves identifying opioid agonists with “ligand biasing” of actions on opioid receptors that preferentially act via G proteins rather than via beta-arrestin signaling.
Opioid-associated adverse effects may be also improved with a reduction in opioid dose, which may be achieved without loss of analgesic effect if a coanalgesic medication is added. For example, when gabapentin and morphine are combined they provide better analgesia from neuropathic pain at lower doses of each drug than either does as a single agent.
Opioid-induced neurotoxicity is typically encountered when opioid doses are rapidly increased, when high doses of an opioid are used, or in the setting of renal failure. The patient may describe increased sensitivity to pain (hyperalgesia), pain in response to nonpainful stimuli (allodynia), worsening pain despite increasing doses of opioids, or pain that appears to spread. Other findings of neurologic hyperexcitability such as myoclonus, delirium, or seizures may also be present. Opioid-induced hyperalgesia is likely due to accumulation of neurotoxic opioid metabolites such as M3G or hydromorphone-3-glucuronide. Such metabolites activate presynaptic calcium channels that release glutamate, which in turn activates NMDA receptors and depolarized postsynaptic neurons in the CNS.
If signs or symptoms of opioid neurotoxicity develop, the opioid should be decreased or changed to one with potentially less neurotoxic effects, such as fentanyl or methadone. The addition of a nonopioid analgesic may lessen the amount of opioid required. An alternative mode of analgesia such as intrathecal, regional, or local analgesia may be used in place of systemic opioids. If needed, parenteral ketamine, an NMDA receptor antagonist, may also be initiated to reduce NMDA receptor-mediated neurotoxicity.
Myoclonus is frequently relieved by a benzodiazepine such as diazepam. Opioids may also play a role in the development of delirium; the reader is referred to the section in this chapter dedicated to mental status changes.
Respiratory depression occurs as a result of opioid receptor blockade of CO 2 chemoreceptors in the medulla. The risk of respiratory depression from opioids is indeed small when opioids are dosed and titrated judiciously. Scenarios in which opioids may cause respiratory depression are the development of renal failure or a sudden decrease in pain, such as after a neurolytic block if opioid doses are not adjusted.
Respiratory depression characterized by hypopnea alone is not in and of itself an indication for opioid reversal with naloxone. In fact, administration of oxygen and reduction of the subsequent dose may be the only actions needed. For a true respiratory emergency, naloxone should be administered by (1) diluting the 0.4 mg/mL ampule in 10 mL saline solution and (2) administering 1 mL IV or subcutaneously every 3 minutes until respiratory depression improves. If the patient is taking long-acting opioids, repeated doses of naloxone or a naloxone infusion may be required, with the hourly dose being the dose initially required to overcome respiratory depression. Overadministration of naloxone will block opioid receptors, resulting in withdrawal that may be characterized by severe pain and sympathetic instability.
Radiotherapy is commonly used to relieve pain such as localized bone metastases. In a review of 13 randomized trials in adults with painful bone metastases who received either radiotherapy or radioisotope injection for pain, it was found that 27% achieved total pain relief and 42% attained a 50% level of pain relief. The largest trial found that the median duration of complete pain relief was 12 weeks. A variety of fractionation schedules were used, and no clear difference between schedules was found.
TCAs and serotonin and norepinephrine reuptake inhibitors (e.g., duloxetine) may relieve neuropathic pain and are discussed in more detail later.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here