Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Soft tissue sarcomas of childhood and adolescence constitute a heterogeneous group of tumors that exhibit features of mesenchymal differentiation ( Tables 60-1 and 60-2 ). From a pediatric oncology perspective, the various types of rhabdomyosarcoma, the most common soft tissue sarcoma in younger children, are grouped separately such that the remainder of these tumors are collectively termed the nonrhabdomyosarcoma soft tissue sarcomas (NRSTS). This term seems an odd eponym, defining a category of soft tissue sarcoma by exclusion and grouping together widely disparate clinicopathologic entities distinguished by clinical features, molecular pathogenesis, and biologic behavior. However, the term NRSTS carries a unique value in pediatric oncology, enabling the study of significant cohorts of children with uncommon tumors. Sarcomas in children represent a somewhat distinct set of tumors that diverge in both incidence and histology from those in adults. The types of sarcomas that develop in adolescents and young adults are similar to those seen in older individuals; however, the relative incidence and response to therapy may differ. In addition, the term NRSTS permits the flexibility to include undifferentiated tumors and tumors that, based on treatment, are typically excluded from other groupings (e.g., desmoplastic small round cell tumor [DSRT]). Finally, by evoking those tumors prevalent in the pediatric population, the term NRSTS also permits consideration of the unique toxicities specific to children when evaluating treatment approaches and designing clinical trials.
| Intermediate (Rarely Metastasizing) | Malignant | |
|---|---|---|
| Adipocytic |
|
|
| Fibroblastic/Myofibroblastic |
|
|
| So-Called Fibrohistiocytic |
|
|
| Smooth Muscle | Leiomyosarcoma (excluding skin) | |
| Pericytic (Perivascular) |
|
|
| Skeletal Muscle | ||
| Vascular Tumors |
|
|
| Chondroosseous |
|
|
| Nerve Sheath Tumors |
|
|
| Gastrointestinal Stromal Tumors | Gastrointestinal stromal tumors, malignant (AFIP groups 3b, 5, 6a, 6b) | |
| Uncertain Differentiation |
|
|
| Undifferentiated/Unclassified |
|
| Benign | Intermediate (Locally Aggressive) | |
|---|---|---|
| Adipocytic |
|
Atypical lipomatous tumor/well-differentiated liposarcoma |
| Fibroblastic/Myofibroblastic |
|
|
| So-Called Fibrohistiocytic |
|
|
| Smooth Muscle | Deep leiomyoma | |
| Pericytic (Perivascular) |
|
|
| Skeletal Muscle | Rhabdomyoma (adult, fetal and genital types) | |
| Vascular Tumors |
|
Kaposiform hemangioendothelioma |
| Gastrointestinal Stromal Tumors | Benign gastrointestinal stromal tumor (AFIP prognostic groups 1, 2, 3a) | |
| Nerve Sheath Tumors |
|
|
| Chondroosseous | Soft tissue chondroma | |
| Uncertain differentiation |
|
Hemosiderotic fibrolipomatous tumor |
NRSTS are often characterized by distinct, consistent cytogenetic, transcriptional, and intracellular signaling changes. Exploring these molecular alterations may offer insights into the biology of mesenchymal development and the pathogenesis of these tumors, thus leading to the development of new treatment strategies.
In children younger than 5 years, rhabdomyosarcoma constitutes the most frequent type of soft tissue sarcoma (STS), however by late school age and continuing into adolescence the incidence of NRSTS rises to exceed that of rhabdomyosarcoma ( Fig. 60-1 ). The total incidence for STS in children and adolescents younger than 20 years is approximately 11 per million, which represents 7.4% of cancer in this population. NRSTS constitute about one half of these cases or 3% to 4% of childhood malignancies. Within the class of NRSTS, specific tumor types demonstrate a strong age association. Although the median age at diagnosis is approximately 13 years, NRSTS prevalent in younger children include infantile fibrosarcoma and rhabdoid tumor, whereas synovial sarcoma and malignant peripheral nerve sheath tumor (MPNST) are more common in adolescents.
Although the vast majority of NRSTS occur spontaneously, several cancer predisposition syndromes are associated with the development of STS. Families that harbor mutations of the tumor suppressor gene TP53 (i.e., those with Li-Fraumeni syndrome) are prone to the development of many types of cancer. STS as well as osteosarcoma can be associated with germline TP53 mutation. The cell cycle regulatory protein RB has also been implicated in STS development. RB was originally identified as the locus deleted in hereditary and sporadic retinoblastoma. However, retinoblastoma survivors are at risk for the development of STS, including fibrosarcoma, pleomorphic sarcoma, and leiomyosarcoma, and molecular analysis of these tumors may demonstrate homozygous RB deletion. In addition to other tumors, neurofibromatosis type 1 (NF1, von Recklinghausen disease) is associated with the development of MPNSTs and multifocal gastrointestinal stromal tumors (GISTs). Radiation therapy for optic nerve glioma in patients with NF1 increases the risk for subsequent MPNST development. Carney triad is associated with multifocal GIST (as well as paragangliomas and pulmonary chondromas). Individual Carney triad tumors are associated with specific nongermline mutations, although a possible common association with chromosome 1 changes has been reported. Carney-Stratakis dyad, characterized by paraganglioma and GISTs, is associated with germline mutations in SDHA, SDHB, or SDHC. Gardner syndrome, a phenotypic variant of familial adenomatous polyposis (FAP), results from mutation of the APC gene and is associated with an increased risk for abdominal desmoid tumor. Gorlin syndrome, caused by mutation of the Hedgehog signaling pathway regulator PTCH, is associated with undifferentiated sarcoma. Werner syndrome is caused by mutations of the deoxyribonucleic acid (DNA) helicase gene RECQL2 or nuclear lamin gene LMNA and is associated with several cancers, including STS in early and middle adulthood. Typically resulting from mutations in TSC1 or TSC2, dominantly inherited tuberous sclerosis is associated with spontaneously resolving cardiac rhabdomyomas in infants and renal angiomyolipoma, including the malignant epithelioid variant, as well as pulmonary lymphangiomyomatosis. Pleomorphic sarcoma in adults has infrequently been associated with Lynch syndrome, which is characterized by mutations in the DNA mismatch repair genes MLH1 and MLH2.
In addition to cancer predisposition syndromes, environmental exposures have been linked to sarcoma development. Although osteosarcoma predominates, radiation exposure is associated with NRSTS, including fibrosarcoma and pleomorphic sarcoma, with an incidence associated with the dose of radiation. Of the several environmental chemical exposures that have been linked to the development of sarcomas in adults, vinyl chloride exposure demonstrates the strongest association to the development of angiosarcoma. Childhood cancer survivors also appear to be at ninefold higher risk for the development of sarcomas (particularly NRSTS) in comparison with the general population, with a mean time from primary cancer diagnosis of 11 years. Although an initial diagnosis of sarcoma was associated with the greatest risk, perhaps indicating an underlying predisposition, treatment with radiation or alkylating agents was also positively associated with sarcoma development. Epstein-Barr virus infection in individuals infected with human immunodeficiency virus (HIV) predisposes to leiomyosarcoma.
NRSTS typically presents as a persistent or enlarging, asymptomatic mass. Although NRSTS may arise in any location, extremities constitute the most common sites. However, within the first year of life, soft tissue tumors tend to develop in the trunk and head and neck. A significant fraction of tumors may be associated with pain that likely indicates invasion or compression of surrounding structures.
The evaluation of NRSTS is typically initiated with appropriate imaging then followed by biopsy. For most tumors, plain radiography and magnetic resonance imaging (MRI) are justified. Technetium-99m–labeled methyldiphosphonate ( 99m Tc MDP) nuclear scintigraphy can localize sites of bony metastatic disease. Because as a group these tumors tend to metastasize to the lungs, chest computed tomography (CT) is commonly indicated. Imaging may narrow the differential diagnosis and will aid in assessing resectability by identifying tumor margins, involved tissue planes, and relationship to neurovascular structures. Fluorine-18 ( 18 F)-labeled fluorodeoxyglucose (FDG) or 18 F-labeled fluorothymidine (FLT) positron emission tomography (PET) has been used for sarcoma staging, and combined PET/CT can identify primary tumors as well as metastatic spread and may detect metastatic lesions missed by bone scintigraphy. However, small lesions (<1 cm) are difficult to visualize by PET. FLT-PET has been used to distinguish between low- and high-grade sarcomas.
Accurate tissue diagnosis is critical to establish a treatment plan and to predict prognosis. Because of the diverse range of sarcoma subtypes as well as their rarity, arriving at a definitive diagnosis can be challenging. The complexity of NRSTS diagnosis and grading requires the integration of histopathology with immunohistochemical, ultrastructural, cytogenetic, and molecular studies ( Table 60-3 ). Advance communication between the surgeon or interventional radiologist, pathologist, and pediatric oncologist can help ensure that sufficient material is obtained and suitably processed. Fine-needle aspiration often generates an inadequate specimen to yield a specific diagnosis. Multiple core needle biopsies may be sufficient to initiate the evaluation, with additional tissue resulting from eventual resection. Although sentinel lymph node biopsy is not a standard component of NRSTS evaluation, it could be considered for high-grade sarcomas such as clear cell sarcoma, synovial sarcoma, vascular sarcomas, and undifferentiated sarcomas.
| Tumor | Immunohistochemical Markers | RECURRENT CYTOGENETIC CHANGES | |
|---|---|---|---|
| Associated Karyotype | Genes Implicated | ||
| Alveolar soft part sarcoma | TFE3, MyoD1 (cytoplasmic), desmin (50%), S100 protein (25%), MSA (in some) | der(17)t(x;17)(p11;q25) | ASPSCR1 (ASPL, TUG, RCC17), TFE3 |
| Angiomatoid fibrous histiocytoma | Vimentin, desmin (50%), CD68 (50%), CD99 (50%), EMA (40%) | t(2;22)(q33;q12) | EWSR1, CREB1 |
| t(12;16)(q13;p11) | FUS, ATF1 | ||
| t(12;22)(q13;12) | EWSR1, ATF1 | ||
| Angiosarcoma | ERG, factor VIII–associated (VWF), CD34, FLI1, CD31 | ||
| Congenital infantile fibrosarcoma | Vimentin, actins (30%), desmin (20%), cytokeratins (20%) | t(12;15)(p13;q25) | ETV6, NTRK3 (TRKC) |
| Clear cell sarcoma | MITF, HMB45, S100, melan A | t(12;22)(q13;q12) t(2;12)(q32.3;q12) |
EWSR1, ATF1 EWSR1, CREB1 |
| Dermatofibrosarcoma protuberans | CD34, apolipoprotein D | t(17;22)(q22;q13) | COL1A1, PDGFB |
| Desmoplastic small round cell tumor | WT1 (carboxy terminus), desmin (dotlike), EMA, cytokeratin, vimentin | t(11;12)(p13;q12) | EWSR1, WT1 |
| Desmoid-type fibromatosis | Vimentin, actins, β-catenin (nuclear) | +8, +20 | |
| Ectomesenchymoma | S100 protein, synaptophysin, GFAP, neurofilament protein, rhabdomyosarcoma markers (MSA, desmin, myogenin, MyoD1) | +2, −6, +11, +20 | |
| Epithelioid sarcoma | Cytokeratins, EMA, vimentin, CD34 (60%), CA-125 | ||
| Epithelioid hemangioendothelioma | VWF, CD31, CD34 | t(1;3)(p36.3;q25) | WWTR1, CAMTA1 |
| Cytokeratins (25% weak) | t(X;11)(p11.23;q22.1) | YAP1,TFE3 | |
| Extraosseous Ewing sarcoma | CD99 (membranous), NSE, vimentin, cytokeratins (25%), FLI1 (nuclear) | t(11;22)(q24;q12) | EWSR1, FLI1 |
| t(21;22)(q21;q12) | EWSR1, ERG1 | ||
| t(7;22)(p22;q12) | EWSR1, ETV1 | ||
| t(17;22)(q12;q22) | EWSR1, ETV4 (E1AF) | ||
| t(2;22)(q36;q12) | EWSR1, FEV | ||
| Extrarenal rhabdoid tumor | Vimentin, cytokeratins, EMA, CD99, NSE, synaptophysin | Abnormalities of 22q11 | SMARCB1 (hSNF5, INI1, BAF47) |
| Gastrointestinal stromal tumor | CD117 (KIT), CD34 | ||
| Extraskeletal myxoid chondrosarcoma | S100 protein (50%), CD117 (KIT, 30%), cytokeratin (focal), EMA (30%) Neuroendocrine markers (chromogranin, NSE, synaptophysin) in t(9;17) tumors |
t(9;22)(q22;q12) t(9;17)(q22;q11) t(9;15)(q22;q21) |
EWSR1, NR4A3 (TEC, CHN, CMSF, NOR1) |
| TAF15 (RPB56, TAF2N), NR4A3 | |||
| TCF12 (HTF4), NR4A3 (TEC) | |||
| Giant cell angioblastoma | Actins, vimentin | ||
| Inflammatory myofibroblastic tumor | ALK, vimentin, actins, desmin (50%), cytokeratins (focal), CD68 (25%) | Multiple translocations involving 2p23 | ALK, multiple partners |
| Kaposiform hemangioendothelioma | D2-40, CD31, CD34, FLI1, SMA, D2-40 | ||
| Kaposi sarcoma | D2-40, CD31, CD34, FLI1, HHV-8 (KSHV) (in situ hybridization) | ||
| Leiomyosarcoma | SMA, desmin, h-caldesmon | Complex | |
| Lipoblastoma | S100 protein, desmin | del(8)(q12;q24),r(8) | HAS2, PLAG1 |
| t(7;8)(p22;q13) | COL1A2, RAD51L1 | ||
| Low-grade fibromyxoid sarcoma | Vimentin, actins (focal) | t(7;16)(p33;p11) | FUS, CREB3L1, CREB3L2 (BBF2H7) |
| Malignant peripheral nerve sheath tumor | S100 (50-90%), GFAP (50%-90%), CD57 (50%), myelin basic protein (40%), TP53 | Numerical and structural abnormalities of chromosomes 1, 4, 7, 11, 12, 14, 16, 17, and 22 | |
| Mesenchymal chondrosarcoma | S100 protein (cartilaginous component) NSE, CD57, CD99 (membranous) (undifferentiated component) |
Complex cytogenetics alterations del(8)(q13.3;q21.1) |
HEY1, NCOA2 |
| Myxoid liposarcoma | S100 protein | t(12;16)(q13;p11) t(12;22)(q13;q12) |
FUS (TLS), DDIT3 (CHOP) EWSR1, DDIT3 (CHOP) |
| Plexiform fibrohistiocytic tumor | CD68, actins (70%), EMA (30%), BCL2 (30%) | ||
| Solitary fibrous tumor | STAT6 (nuclear), CD34, CD99 (cytoplasmic) | NAB2, STAT6 | |
| Synovial sarcoma | EMA, cytokeratins, S100 protein (30%), CD99 (cytoplasmic), BCL2, TLE1 | t(x;18)(p11;q11) | SS18 (SYT), SSX1 SS18 (SYT), SSX2 |
| Undifferentiated round cell and spindle cell sarcomas | EWSR1, NFATC2, PATZ1, POU5F1, SMARCA5, SP3 CIC, DUX4 |
||
In general, sarcomas can be classified as those with a recognizable recurrent chromosomal rearrangement (typically translocation) and those without. The identification of cytogenetic abnormalities, many of which are highly specific of certain pediatric and young adult NRSTS, is helpful in arriving at or confirming a diagnosis (see Table 60-3 ). However, the same gene can be translocated in different tumor types. For example, the EWSR1 gene is rearranged in DSRT, clear cell sarcoma, myxoid liposarcoma, and a subset of undifferentiated sarcomas in addition to Ewing sarcoma. In contrast, the rearrangement of certain genes is highly restricted, such as SS18 ( SYT ) rearrangement in synovial sarcoma. Molecular studies (e.g., polymerase chain reaction [PCR] assay and interphase fluorescence in situ hybridization [FISH]) may be required to evaluate for specific cytogenetic abnormalities, especially if there a high index of suspicion and standard cytogenetics are unavailable or unremarkable. High-throughput DNA sequencing and other comprehensive genomic approaches have rapidly emerged as strategies to identify genetic alterations in sarcomas. Ongoing efforts to systematically identify mutations in select STS include the Pediatric Cancer Genome Project and the Cancer Genome Atlas. Although translocations can be detected relatively easily when sequencing the whole genome, owing to significant expense and technical considerations, most current high-throughput sequencing applications focus on exonic sequence variation and consequently are not designed to detect intronic breakpoints. However, RNA sequencing as well as whole exome and targeted sequencing of cancer-associated genes offers the potential to identify individuals with specific and therapeutically actionable mutations. To take advantage of emerging techniques for molecular analyses and biomarker identification, additional tumor material, if possible, should be preserved at −70° C for DNA or ribonucleic acid (RNA) analysis. For those sarcomas that may metastasize to the bone marrow, aspiration and biopsy may be performed to complete the evaluation for distant spread.
As with all cancers, appropriate recommendations for therapy depend on accurate predictions of clinical course and prognosis. Clinically informative classification schemes will accurately predict tumor outcome and will aid in the development and interpretation of multicenter trials necessary to study these uncommon cancers. Studies of adult extremity STS suggest that grade followed by tumor depth and size are of independent prognostic value for the rates of distant metastases and tumor mortality. However, the clinicopathologic classification of pediatric NRSTS poses unique challenges. The low incidence of these tumors results in grouping potentially disparate tumors in clinical trials. Patient age may influence outcome. For some tumors, high grade does not predict clinically aggressive behavior, whereas other tumors behave aggressively regardless of histologic appearance. To address these issues, the Pediatric Oncology Group (POG) grading system incorporates histology, cytologic features, and age to classify NRSTS as low, intermediate, or high grade ( Table 60-4 ). Low grade is defined by histologic type, whereas intermediate grade takes into account necrosis and mitosis with secondary consideration of nuclear atypia and tumor cellularity. High-grade tumors include those of specific histologies as well as tumors with more than 15% necrosis or five or more mitoses/10 high-power field. The National Cancer Institute (NCI) and Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading specifications are alternative methodologies validated on sarcomas in adults. Similar to the POG system, they take into consideration mitotic index, necrosis, and histologic type or tumor differentiation. The POG and FNCLCC perform similarly in their ability to correlate with outcome. Gene expression signatures have also been used to predict outcomes in non–translocation-associated sarcomas in adults.
| Grade | Description |
|---|---|
| 1—Low | Myxoid and well-differentiated liposarcoma |
| Well-differentiated or infantile (≤4 years old) fibrosarcoma | |
| Well-differentiated or infantile (≤4 years old) hemangiopericytoma | |
| Well-differentiated malignant peripheral nerve sheath tumor | |
| Extraskeletal myxoid chondrosarcoma | |
| 2—Intermediate | Sarcomas not specifically included in grade 1 and 3 and in which: |
| 3—High | Pleomorphic or round cell liposarcoma |
| Mesenchymal chondrosarcoma | |
| Extraskeletal osteosarcoma | |
| Malignant triton tumor | |
| Alveolar soft part sarcoma | |
| Sarcomas not included in grade 1 with >15% of surface area with necrosis or ≥5 mitoses/10 high-power fields using a 40× objective |
* Secondary criteria. Tumor diagnoses specified in grade 1 or 3 are excluded from grade 2.
Pediatric multicenter studies also make use of the surgical-pathologic grouping system of the Intergroup Rhabdomyosarcoma Study (IRS) ( Table 60-5 ), which incorporates tumor spread and outcome of surgical resection, or the International Society of Pediatric Oncology (SIOP)-International Union Against Cancer TNM classification system for pediatric tumors ( Table 60-6 ). STS may also be classified according to the American Joint Commission on Cancer (AJCC) or Enneking staging systems, which integrate tumor size, location relative to the superficial fascia, regional lymph node status, presence of distant metastases, and histologic grade ( Table 60-7 ).
| Group | Definition |
|---|---|
| Low-Stage | |
| I | Localized disease, completely resected |
| II | Total gross resection with evidence of regional spread |
| IIa | Grossly resected tumor with microscopic residual disease |
| IIb | Regional disease with involved nodes, completely resected with no microscopic residual disease |
| IIc | Regional disease with involved nodes, grossly resected but with evidence of microscopic residual disease and/or histologic involvement of the most distal regional lymph node from the primary site in the dissection. |
| High-Stage | |
| III | Incomplete resection with gross residual disease |
| IV | Distant metastatic disease present at onset |
| Stage | Description |
|---|---|
| I | T1—Tumor confined to organ or tissue of origin |
| N0—No evidence of regional lymph node involvement | |
| M0—No evidence of distant metastasis | |
| II | T2—Tumor involving one or more contiguous organs or tissues or with adjacent malignant effusion |
| N0 | |
| M0 | |
| III | Any T |
| N1—Evidence of regional lymph node involvement | |
| M0 | |
| IV | Any T |
| Any N | |
| M1—Evidence of distant metastases |
| Primary Tumor (T) | Regional Lymph Nodes (N) | |||
|
||||
| Distant Metastasis (M) | Histologic Grade (G) | |||
|
|
|||
| Anatomic Stage/Prognostic Groups | ||||
| Stage IA | T1a | N0 | M0 | G1, GX |
| T1b | N0 | M0 | G1, GX | |
| Stage IB | T2a | N0 | M0 | G1, GX |
| T2b | N0 | M0 | G1, GX | |
| Stage IIA | T1a | N0 | M0 | G2, G3 |
| T1b | N0 | M0 | G2, G3 | |
| Stage IIB | T2a | N0 | M0 | G2 |
| T2b | N0 | M0 | G2 | |
| Stage III | T2a, T2b | N0 | M0 | G3 |
| Any T | N1 | M0 | Any G | |
| Stage IV | Any T | Any N | M1 | Any G |
* Note: Superficial tumor is located exclusively above the superficial fascia without invasion of the fascia; deep tumor is located either exclusively beneath the superficial fascia, superficial to the fascia with invasion of or through the fascia, or both superficial yet beneath the fascia.
† Note: presence of positive nodes (N1) in M0 tumors is considered Stage III.
NRSTS most commonly spread locally and hematogenously but may exhibit regional lymph node metastases. The lung is the most common site for distant metastases, but with less frequency these tumors can metastasize to bone or brain. Metastatic spread at the time of diagnosis likely occurs in alveolar soft part sarcoma, high-grade MPNST, clear cell sarcoma, high-grade angiosarcoma, and epithelioid sarcoma.
Several factors have been shown to be prognostic for tumor-associated mortality. In both univariate and multivariate analyses of patients with grossly resected tumors, tumor invasion into contiguous structures and size (>5 cm) are the most highly predictive of decreased 5-year event free survival (EFS) and overall survival (OS). Other factors that correlate with poor outcome including female sex, older age, high-grade tumors, high-grade MPNST histology, and tumors in nonextremity locations.
Surgical resection constitutes the mainstay of treatment of localized tumors. Complete resection for large tumors may result in significant functional compromise, and amputation should be considered for those patients in whom limb-sparing surgery would leave residual tumor. Radiation therapy is typically reserved for those tumors for which complete resection is not possible. However, the high radiation dose required for sarcoma therapy is associated with significant growth-associated side effects in children. Although surgery with or without radiation results in good outcome for localized and low-grade disease, high-grade disease is associated with significant morbidity and mortality. With the exception of synovial sarcoma and extraskeletal Ewing sarcoma, the role of conventional chemotherapy remains ill defined. However, in pediatric NRSTS, responses can be observed and may reduce tumor burden prior to surgical resection. Biologically targeted therapies have altered the management of individual tumor types (e.g., GISTs in adults), and their roles are evolving as they enter clinical trial.
Surgical resection is typically the initial therapy for operable nonmetastatic NRSTS. Complete resection of small (<5 cm) NRSTS results in a 5-year OS in excess of 80%. However, large, high-grade or incompletely resected tumors have a significant chance of local recurrence. Tumor location (e.g., abdomen vs. extremity) and invasion may limit the ability to perform a wide local excision necessary to achieve tumor-free margins. For these patients, external-beam radiation therapy has been used to reduce the risk for local recurrence based on adult studies. Pediatric studies seem to confirm that radiation therapy reduces the risk for local recurrence associated with an inability to achieve a wide surgical margin (>1 cm) for high-grade disease. The impact of postoperative radiation therapy may be most significant for IRS group II patients, although grade and size may mitigate this effect. Higher radiation doses are associated with increased rates of local control in NRSTS. However, these doses are associated with abnormalities of bone development, complicating the delivery of this modality to growing children.
Brachytherapy—interstitial, intercavitary or surface placement of radioisotopes—constitutes an alternative approach to deliver radiotherapy. Brachytherapy can precisely deliver high doses of radiation to the tumor or resection site while sparing normal tissue. Brachytherapy can also be administered concurrently with external-beam radiation, and a study of adult STS suggests that the combination may result in increased rates of local control when surgical margins are positive. Other techniques to augment local control include radiofrequency ablation, cryoablation, and embolization. Strategies for local control that incorporate site-directed chemotherapy include intralesional chemotherapy followed by electroporation at accessible sites and intraperitoneal chemotherapy infusion.
Because complete resection of NRSTS is associated with a high rate of cure, chemotherapy is principally employed when tumors are unresectable, incompletely resected, or metastatic. The infrequency of each of the heterogeneous tumors that constitute the class of NRSTS makes clinical trials of chemotherapy particularly challenging. To enable multicenter groups to accrue sufficient subjects, the nonselective inclusion of NRSTS complicates the interpretation and extrapolation of most trials. Nonetheless, several consistent themes emerge. In contrast to the striking successes of chemotherapy for Ewing sarcoma and rhabdomyosarcoma, NRSTS tend to be relatively chemoresistant, with synovial sarcoma and extraosseous Ewing sarcoma as exceptions. For patients with fully resected tumors, the inclusion of chemotherapy fails to increase survival. The impact of chemotherapy may be most significant for patients harboring tumors with the highest risk for metastatic spread. For high grade, grossly resected tumors, chemotherapy was associated with a 5-year metastasis-free survival of 49.5% compared with 0% for untreated patients. Chemotherapy may also be beneficial for patients with unresectable tumors. For both chemotherapy-sensitive and chemotherapy-resistant tumors, neoadjuvant treatment may permit the subsequent surgical resection of initially nonresectable tumors. Patients with unresected or metastatic tumors have a poor outcome, although 40% to 50% of patients with advanced disease demonstrate some response (partial + complete response) to a regimen containing either ifosfamide and doxorubicin or cyclophosphamide and doxorubicin. Treatment with doxorubicin, cyclophosphamide, and vincristine alternating with ifosfamide and etoposide together with aggressive local control for incompletely resected tumors or those metastatic at presentation, resulted in a partial + complete response of 80%. Approximately 40% of children and adolescents with NRSTS responded to ifosfamide and doxorubicin, results similar to those for cyclophosphamide and doxorubicin. Based on the principle that advanced sarcomas may respond to intensive therapies, the feasibility of dose-intensified neoadjuvant chemotherapy has been an end point of several trials using doxorubicin, ifosfamide, and dacarbazine in adults and doxorubicin, cyclophosphamide, ifosfamide, etoposide, and vincristine in children. However, evidence in support of high-dose chemotherapy with autologous stem cell infusion remains limited.
Regarding specific chemotherapeutic agents, topoisomerase 1 inhibitors may also have activity against these tumors. Topotecan with high-dose cyclophosphamide as well as irinotecan demonstrated limited efficacy against recurrent NRSTS, and the effect of irinotecan together with temozolomide is currently being studied. The combination of vinorelbine with low-dose cyclophosphamide may also have activity against these tumors. Gemcitabine and docetaxel, both of which have some activity against adult sarcomas, were more effective together than gemcitabine alone in adult patients with metastatic sarcoma. In adults, advanced disease may also respond to the combination of gemcitabine and vinorelbine. In children, however, the efficacy of gemcitabine or docetaxel administered individually in the setting of relapsed pediatric solid tumors seems small. As previously mentioned, synovial sarcoma is considered an exception to the general chemoresistance of NRSTS. Children and adolescents exhibit a 5-year EFS of about 70% with decreased EFS, progression-free survival (PFS), and OS associated with tumor size (>5 cm), local invasion, and IRS grouping (III and IV). Another exception is extraosseous Ewing sarcoma, which is generally grouped with bony Ewing sarcoma for treatment purposes (see Chapter 61, “Ewing Sarcoma” ).
Insights regarding the biology of STS are beginning to identify novel targetable pathways. The management of GISTs in adults has been altered by treatment with KIT and PDGFR-targeted tyrosine kinase inhibitors. Multitargeted tyrosine kinase inhibitors have demonstrated efficacy in nonadipocytic STS in adults, and sorafenib has activity against desmoid tumors. Insulin-like growth factor 1 (IGF-1) inhibition has demonstrated efficacy in a fraction of patients with DSRT. Targeting the mammalian target of rapamycin (mTOR) pathway demonstrated a significant, although brief, delay in disease progression in a large adult trial of sarcomas previously treated with cytotoxic chemotherapy.
STS can be classified into two groups based on cytogenetics. Tumors that occur in children and young adults tend to reveal relatively simple karyotypes often characterized by an easily recognizable and frequently pathognomonic reciprocal chromosomal translocation. In contrast, STS of adults typically demonstrate complex karyotypes without recurrent cytogenetic abnormalities. Cellular mechanisms for telomere maintenance may correlate with translocation status. Tumors harboring single translocations tend to have normal to short telomeres, suggestive of active telomerase, whereas tumors with complex karyotypes have longer telomeres, suggesting the use of alternative mechanisms of telomere lengthening. Although other cellular changes, in addition to translocations, are likely to be necessary for sarcoma development, the difference between sarcomas of children and young adults and those of older individuals may reflect divergent cellular origins. Experimentally, haploinsufficiency of the nonhomologous end-joining DNA repair pathway in the context of Ink4a/Arf -deficient mice leads to the development of sarcoma with varied histology and karyotype. Although the tumors that develop in these mice demonstrate translocations, amplifications, and deletions, the translocated regions are not recognizably syntenic with known human translocations suggesting that these sarcomas more closely reflect adult sarcomas. This finding also highlights the issue of whether complex cytogenetic abnormalities themselves result in oncogenesis or are a manifestation of genomic instability associated with the loss of cell cycle and DNA repair checkpoints.
Liposarcoma is the most common of the STS in adults. In children, however, approximately 95% of adipose tumors are benign lipomas and lipoblastomas with liposarcomas accounting for the remainder. Lipoblastomas tend to occur in children younger than 3 years of age, whereas liposarcomas predominate in older school-aged children and adolescents. Liposarcoma is classified into three morphologic types: well-differentiated/dedifferentiated, myxoid/round cell, and pleomorphic. Morphologic types correspond to a developmental progression bearing similarities to that seen during induced mesenchymal differentiation into adipocytes. Dedifferentiated/well-differentiated and pleomorphic liposarcomas occur predominantly in the retroperitoneum, whereas myxoid/round cell histology tends to occur in the extremities. High-grade, dedifferentiated liposarcomas carry the highest risk for recurrence and metastasis. In contrast to adults, most children present with the myxoid variant. Myxoid liposarcoma is histopathologically characterized by proliferation of round to spindled primitive mesenchymal cells and scattered lipoblasts in an abundant faintly eosinophilic, homogeneous myxoid matrix ( Fig. 60-2 ). A network of delicate straight and curvilinear capillaries is distinctive. The presence of a round cell component may increase the risk for disease progression. Complete resection is associated with prolonged tumor-free survival. When margins are microscopically positive, the addition of radiation therapy may increase disease-free survival. The histologic variants of liposarcoma are associated with differences in response to chemotherapy. Myxoid liposarcoma demonstrates the highest likelihood of response to a doxorubicin and ifosfamide regimen. Myxoid liposarcoma also exhibits sensitivity to trabectedin (ecteinascidin-743; ET-743), a DNA minor groove–binding small molecule.
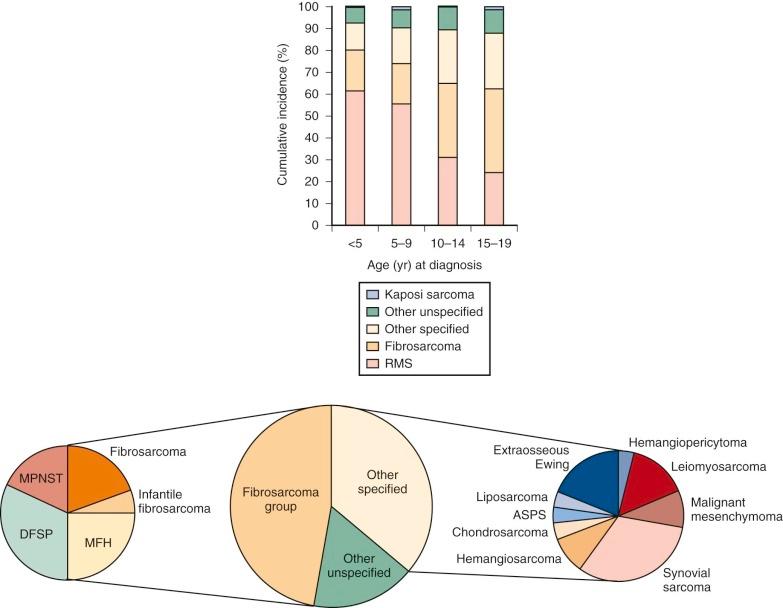
Myxoid and round cell liposarcomas share recurrent chromosomal translocations that distinguish them from other liposarcoma histologies. t(12;16)(q13;p11) results in fusion of FUS (also known as TLS, an abbreviation for translocated in liposarcoma) with the transcription factor DDIT3 (CHOP), and t(12;22)(q13;q11) fuses EWSR1 with DDIT3 . DDIT3 is a member of the b-ZIP family of transcriptional regulatory proteins and seems to function as a negative transcriptional regulator. Chimerism with both EWSR1 and FUS (TLS) suggests that the retained domains of EWSR1 and FUS confer similar activities to the DNA-binding domain of DDIT3. Ectopic expression of either FUS-DDIT3 or EWSR1-DDIT3 in NIH-3T3 cells resulted in characteristics of oncogenic transformation, including growth in soft agar and tumor formation in immunocompromised mice. Furthermore, implantation of mice with primary murine mesenchymal progenitor cells transduced with FUS-DDIT3 led to the development of tumors that morphologically and immunohistochemically resemble human myxoid liposarcoma.
Well-dedifferentiated liposarcomas are associated with complex karyotypes. However, a frequent finding is amplification of 12q, which includes MDM2 and CDK4. MDM2 binds TP53 to inhibit its activity. In the context of wild-type TP53, inhibition of MDM2 by the small molecule nutlin3 results in increased apoptosis in liposarcoma cell lines. Transgenic mice expressing FUS-DDIT3 in mesenchymal progenitor cells developed sarcomas only in the absence of TP53. Interestingly, treatment of these mice with trabectidin resulted in adipocytic differentiation, which was increased by concurrent treatment with the peroxisome proliferator-activated receptor γ (PPARγ) agonist rosiglitazone. Comprehensive genomic analysis of several sarcomas demonstrated TP53 and NF1 mutation in a subset of pleomorphic liposarcomas whereas PIK3CA was mutated in nearly 20% of myxoid/round cell liposarcomas.
Chromosomal rearrangements of 8q12 are found in lipoblastoma, and their detection can aid in diagnosis. These rearrangements have implicated the zinc finger DNA-binding protein PLAG1. In lipoblastoma, translocation places the PLAG1 coding exons under the transcriptional control of the HAS2, COL1A2, or RAD51L1 promoters. PLAG1 was originally identified based on its translocation in pleomorphic adenoma of the salivary gland. In contrast to lipoblastoma, PLAG1 is translocated near CTNNB1, CHCHD7 or TCEA1 in pleomorphic adenoma.
Desmoid tumor, also known as deep musculoaponeurotic fibromatosis, presents from infancy through adulthood with a median age at diagnosis in the early 30s and a twofold higher incidence in women. Although most tumors occur spontaneously, FAP patients, and in particular those with Gardner syndrome, have reported incidences of desmoid tumors ranging from 3.5% to 32%. Desmoid tumors in the pediatric population tend to occur in the second decade and are located in the extremities and head and neck, in contrast to the adult population in whom abdominal and truncal sites are common. Abdominal desmoids are more common in FAP patients, whereas limb and trunk locations predominate in non-FAP patients.
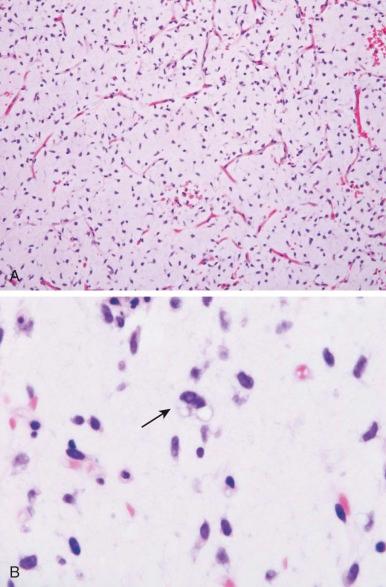
The tumors consist of an infiltrating, uniform, spindled cell proliferation lacking nuclear pleomorphism and mitotic activity ( Fig. 60-3 ). Although desmoid tumors have a high potential for local invasion and recurrence, they never metastasize. Surgery is the primary treatment; however, tumors have a significant chance of local recurrence even if negative margins can be achieved. Achieving negative margins without severe disfigurement or functional impairment may be impossible. In these circumstances, preoperative chemotherapy or partial resection and postoperative chemotherapy should be considered, although, given a highly variable clinical course, close observation may be justified. Multiple drug regimens have been shown to shrink or stabilize desmoid tumor growth. These include methotrexate with vinblastine or tamoxifen with diclofenac. Other potentially active treatments include the combination of doxorubicin, vincristine, dactinomycin, and cyclophosphamide or the combination of doxorubicin and dacarbazine. Responses to imatinib and sorafenib have been observed in about half of desmoid patients.
Although most desmoid tumor cells are karyotypically diploid, abnormalities of the Y chromosome and 5q and gains in chromosome 8 and 20 may be noted. Abnormalities have been identified in a subset of tumor cells, and the finding of trisomy 8 may suggest an increased risk for recurrence. Gardner syndrome, which is associated with colonic polyposis and abdominal desmoid tumors, is a phenotypic variant of FAP, which is caused by mutation of APC. APC regulates β-catenin, the downstream mediator of WNT signaling. Stabilized β-catenin forms a multiprotein complex with members of the T-cell factor/lymphoid enhancer factor family (TCF-LEF) resulting in transcriptional activation of TCF-LEF target genes. APC mutations resulting in increased β-catenin or stabilizing mutations in the β-catenin gene ( CTNNB1) have been implicated in the proliferation of sporadic desmoid tumors. Eighty-five to 90 percent of sporadic desmoid tumors are associated with somatic mutation of CTNNB1. Mice expressing either mutant APC or stabilized β-catenin develop aggressive fibromatoses.
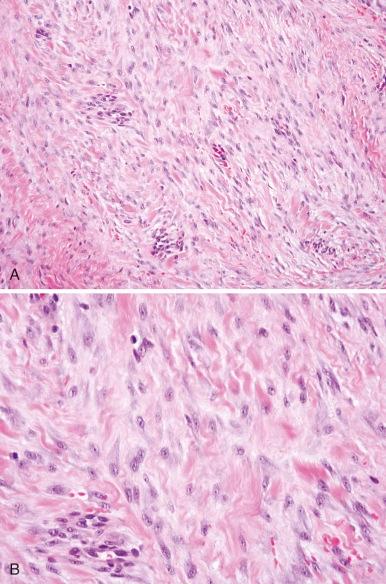
In recent World Health Organization (WHO) classifications of soft tissue tumors, the term hemangiopericytoma is no longer used; instead, a majority of these tumors are currently classified as solitary fibrous tumors. Infantile hemangiopericytoma, recognized as a distinct category of benign hemangiopericytoma by Enzinger, is currently regarded in the category of infantile myofibroma/myofibromatosis. Infantile myofibromatosis is a benign proliferation of myofibroblastic cells affecting almost exclusively young children. The tumors are solitary or multicentric and may involve the skin, muscle, viscera, or bone. Sixty percent are noted at birth, and 88% occur before the age of 2. More commonly found in males, solitary lesions typically develop in the soft tissues of the head and neck and trunk. In contrast, the multicentric form is more common in females and in addition to affecting soft tissues it can develop in bones and viscera. Histopathologically, infantile myofibroma and myofibromatosis have similar features. They are multinodular and biphasic with alternating hemangiopericytoma-like, densely cellular areas and less cellular areas with smooth muscle differentiation immunoreactive to actin ( Fig. 60-4 ). Solitary lesions can rarely recur. Multicentric lesions undergo spontaneous regression; however, multiple visceral lesions may increase in size and number prior to regression, compromising vital structures and, rarely, leading to death. Optimal initial therapy involves complete surgical excision. Life-threatening multifocal disease has been treated successfully with low-dose vincristine and dactinomycin or low-dose vinblastine and methotrexate. Urinary basic fibroblast growth factor has been shown to be elevated in an infant during the proliferative phase, and connective tissue growth factor is expressed in both tumor and stromal cells. An autosomal dominant pattern of inheritance has been associated with mutations in the growth factor receptor PDGFRB.
Infantile myofibroma/myofibromatosis should be distinguished from pericytoma with t(7;12), a distinct tumor with histologic, immunohistochemical, and ultrastructural features of pericytic differentiation and a translocation involving t(7;12)(p22;q13). Although typically developing in soft tissue, this entity can develop in bone. The translocation brings together the β-actin gene (ACTB) with the zinc finger transcription factor GLI1. Originally identified based on amplification in human glioma, GLI1 is activated by Sonic hedgehog signaling through the transmembrane receptor PTCH. In t(7;12) pericytoma the carboxyl terminus of GLI1 is fused with the amino-terminal domain of ACTB. Intriguingly, GLI1 has also been found to be amplified in a subset of rhabdomyosarcoma and osteosarcoma that lack typical markers of differentiation.
Infantile (congenital) fibrosarcoma is one of the most common NRSTS in young children. In contrast to fibrosarcoma in adults, this low-grade tumor displays a benign clinical behavior and is typically cured by surgical excision. However, large tumors for which surgical excision would result in significant morbidity may be treated with preoperative chemotherapy ( Fig. 60-5, A ). Regimens with activity against these tumors include vincristine and dactinomycin alone or together with either cyclophosphamide, ifosfamide, or doxorubicin.
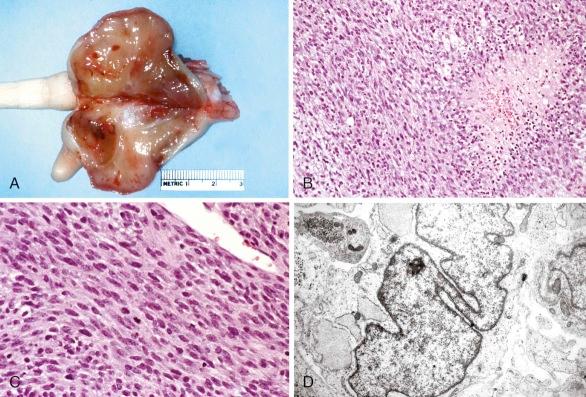
Histopathologically, the tumor is characterized by intersecting bundles with a herringbone pattern composed of cellular spindle to ovoid cells with a high proliferative rate, frequent mitoses, and areas of necrosis (see Fig. 60-5, B and C ). Some tumors may have a hemangiopericytoma-like pattern of growth. Scattered chronic inflammatory cells are frequently present. Tumor cells have a fibroblastic and myofibroblastic ultrastructure (see Fig. 60-5, D ) and immunophenotype with variable positivity for desmin and actin. Aberrant cytokeratin expression is observed in 15% to 20% of the tumors.
Infantile fibrosarcoma is characterized by t(12;15)(p13;q25). This translocation fuses the amino-terminal helix-loop-helix oligomerization domain of the ETV6 transcription factor (also known as TEL ) with the carboxyl-terminal tyrosine kinase domain of the neurotrophic tyrosine kinase receptor, type 3 ( NTRK3, also known as TRKC ). The detection of ETV6-NTRK3 by reverse-transcriptase PCR is highly specific and can distinguish infantile fibrosarcoma from more aggressive spindle cell sarcomas. Intriguingly, the same translocation also occurs in congenital mesoblastic nephroma, secretory breast carcinoma, and acute myeloid leukemia.
Chimerism results in constitutive activation by autophosphorylation of NTRK3 tyrosine residues. Translocation also results in aberrant NTRK3 expression. ETV6 is widely expressed whereas NTRK3 is normally restricted to neuronal cells and fetal kidney. ETV6-NTRK3 expression can transform NIH3T3 cells as assayed by growth in soft agar and in xenografts in immunocompromised mice. ETV6-NTRK3 is associated with several signaling pathways that may be important in oncogenesis. ETV6-NTRK3 activates both the MAPK and AKT pathways, with AKT activation mediated by direct interaction of ETV6-NTRK3 with SRC. Activation of signaling also leads to phosphorylation of YAP1 and WWTR1, Hippo pathway transcriptional coactivators, and a characteristic transcriptional profile. ETV6-NTRK3–mediated fibroblast transformation also requires the insulin-like growth factor receptor (IGF1R) possibly through directly interacting with the IGF1R substrate IRS-1. The ETV6-NTRK3 fusion may also act by suppressing transforming growth factor-β (TGF-β) signaling through interaction with the type II TGF-β receptor.
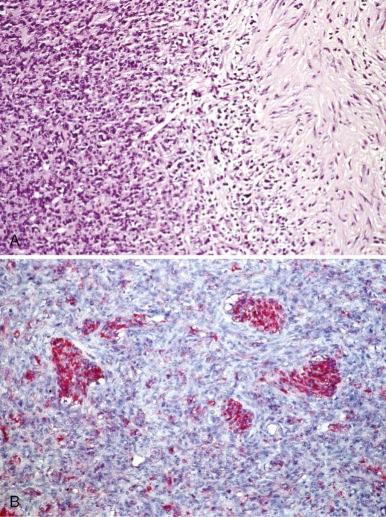
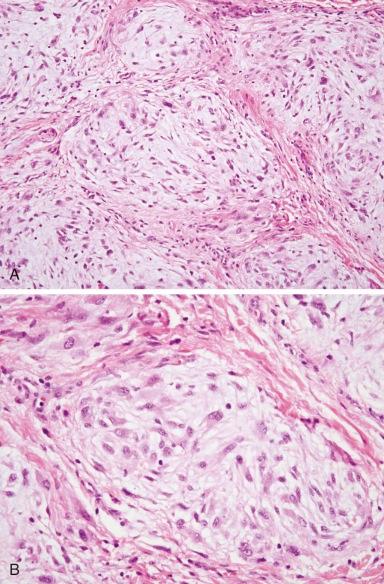
Dermatofibrosarcoma protuberans (DFSP) is a rare cutaneous fibrohistiocytic tumor characterized by locally aggressive behavior. DFSP may begin as a plaquelike, raised red-blue lesion followed by a rapid growth phase that may be associated with the development of one or more nodules. DFSP appears as a cellular, spindle cell proliferation, frequently with a distinct storiform pattern that infiltrates the surrounding dermis and subcutaneous fat ( Fig. 60-6, A ). Tumor cells are typically immunoreactive for apolipoprotein D and CD34 (see Fig. 60-6, B ). The Bednar tumor refers to an uncommon DFSP variant with scattered dendritic cells containing melanin. The majority of the tumors develop on the trunk and upper extremity. Although typically diagnosed during the fourth and fifth decades, DFSP may occur in children and adolescents. Inadequate resection of the tumor is associated with a significant risk for local recurrence. Radiation therapy can be used effectively as an adjuvant for the treatment of resection sites with positive margins. Rarely, DFSP can evolve into a high-grade fibrosarcoma often associated with a loss of CD34 staining and an increased risk for metastasis ( Fig. 60-7, A and B ). Giant cell fibroblastoma, a rare tumor predominantly of males younger than 10 years of age, is in the spectrum of DFSP. Histopathologically, giant cell fibroblastoma is composed of variably cellular spindle or stellate cells often in a myxoid background containing pseudovascular spaces and multinucleated giant cells (see Fig. 60-7, C ).
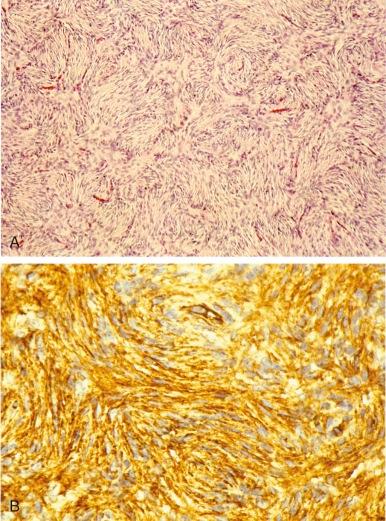
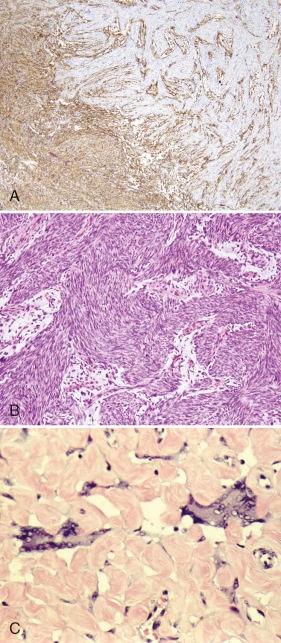
Like DFSP, giant cell fibroblastoma is characteristically immunoreactive for CD34 and apolipoprotein D. Both lesions also harbor a pathognomonic translocation between the collagen, type I, alpha 1, gene (COL1A1) and the platelet-derived growth factor-β gene (PDGFB). In contrast to the other sarcoma-associated translocations, the translocated COL1A1 - PDGFB locus may be present as a low-level amplification on a ring chromosome. Detection of the resulting COL1A1-PDGFB transcript can provide confirmation of the diagnosis. The fusion of PDGFB with COL1A1 preserves the full coding region of PDGFB, and the resulting chimeric polypeptide is subject to intracellular processing that results in the secretion of a native PDGFB molecule that is capable of activating its receptor, PDGFR. Although the mechanisms for autocrine-activated PDGFR signaling are not known, it may function through STAT3 and ERK activation. Presumably through the inhibition of autocrine PDGFR activation, the growth of xenografted primary human DFSP cells can be inhibited with the kinase inhibitor imatinib (Gleevec). Treatment with imatinib has also been shown to be effective in children and in patients with metastatic or unresectable disease. Imatinib-resistant DFSP may respond to sorafenib or sunitinib. Response to vinblastine and methotrexate has also been reported.
Low-grade fibromyxoid sarcoma (LGFMS) typically develops in the lower extremities of young adults and occasionally in adolescents ( Fig. 60-8 ). Described by Evans in 1987, it is characterized by bland, uniform spindle cells with a short fascicular or whorly pattern of growth in a dense collagenous or variably myxoid stroma ( Fig. 60-9 ). An abrupt transition between cellular myxoid and hypocellular collagenous areas is frequently present. Dense collagenous areas mimic desmoid tumor. Myxoid areas contain characteristic arcades of curvilinear vessels that may be sclerotic or be surrounded by more densely cellular spindle cells. Mitotic count is characteristically low. Occasionally there are giant collagenous rosettes (hyalinizing spindle cell tumor with giant rosettes). Vimentin is the only constant marker present in tumor cells. Some tumors may be focally immunoreactive for actin, CD34, cytokeratins, or epithelial membrane antigen (EMA). MUC4 reactivity is common in LGFMS and can distinguish it from other histologically similar tumors. A superficial location occurs more frequently in children. Although primary resection typically results in disease control, local recurrences and pulmonary metastases can develop in 5% to 10% of patients after many years. However, resection of recurrent disease can result in long-term remission. LGFMS seems to be chemoresistant, although limited responses to trabectedin have been reported.
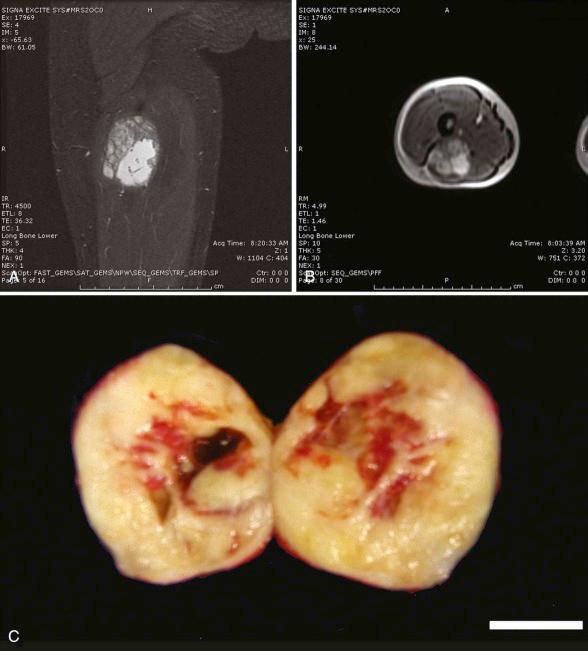
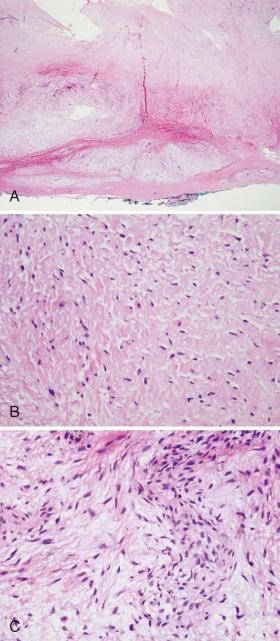
LGFMS is associated with t(7;16)(q34;p11) or t(11;16)(p11;p11), which results in the fusion of FUS (TLS) with CREB3L2 or CREB3L1. These translocations are also found in sclerosing epithelioid fibrosarcoma, suggesting that these tumors may be related. EWSR1 - CREB3L1 has also been noted. Native CREB3L2 is a member of the CREB basic leucine zipper family of transcription factors and acts as a transducer of endoplasmic reticulum (ER) stress signals. Normally tethered through a transmembrane domain to the ER, ER stress results in cleavage of the cytoplasmic amino terminal b-ZIP domain, enabling it to translocate to the nucleus and to activate transcription from cyclic adenosine monophosphate (AMP)–responsive elements. Chimerism with FUS, a translocation partner with CHOP in myxoid liposarcoma, likely deregulates CREB3L2 transcriptional activity. PCR-based identification of the fusion gene product from paraffin sections can be used to confirm the diagnosis. LGFMS is associated with a gene expression profile that distinguishes it from similar tumors and is marked by the expression of FOXL1.
Myxofibrosarcoma (MFS) is one of the most common sarcomas of the elderly but has been reported in adolescents and young adults. Typically developing in the sixth and seventh decades of life, MFS exhibits a predilection for the dermal or subcutaneous tissues of the extremities. MFS may be alternatively referred to as low-grade myxoid malignant fibrous histiocytoma or fibrosarcoma, myxoid type. Histopathologically, it is characterized by a multinodular growth of spindled or stellate cells in a myxoid stroma ( Fig. 60-10 ). MFS grading has prognostic value and is based on cellularity, nuclear pleomorphism, and mitotic activity. Resection is typically curative for low- or intermediate-grade tumors; however, recurrent tumors may show morphologic and molecular multistep tumor progression. Large tumor size, necrosis, and decreased myxoid stroma in low-grade tumors are associated with a significant risk for local and distant recurrence. Intermediate- and high-grade tumors have a significant risk for recurrence and metastasis. In a study of adults, 23% of patients with intermediate- or high-grade tumors developed metastases, most commonly to the lung and lymph nodes and rarely to the brain. A subset of intermediate- or high-grade tumors exhibiting an epithelioid morphology seems to behave more aggressively than other high-grade histologies. In addition to grade, an initial incomplete resection and infiltrative MRI T2-weighted signal correlates with an increased risk for local recurrence. Combined chemotherapy and irradiation has efficacy for MFS. Cytogenetically, MFS may be diploid but is typically complex. Numerous chromosomal gains and losses as well as ring chromosomes have been observed. Large-scale 7q gains were associated with increased expression of the hepatocyte growth factor receptor MET and CDK6, and increased expressed of these proteins was associated with lower metastasis-free survival. Ezrin expression also correlates with decreased metastasis-free survival. Isolated chromosomal translocations have been noted.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here