Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the first half of the 20th century, one of the most feared complications of general anesthesia was postoperative vomiting (POV), primarily because aspiration of gastric contents into the lungs could lead to death. Early prophylaxis sometimes consisted of advising patients to consume olive oil before general anesthesia to shield the intestinal wall from emetogenic gases. Prevention of POV was one of the primary motivations for developing local/regional anesthesia blocks, first with cocaine and procaine, then with lidocaine. Postoperative nausea, on the other hand, was considered too minor a complication to measure—until the development in the 1950s and 1960s of anesthetic drugs that could be cleared more rapidly (e.g., halothane, barbiturates, and novel opioids), which meant that patients spent more of the immediate postoperative period awake.
While the antiemetic effect of some drugs, such as anticholinergics, were first described more than a century ago, modern understanding of the specific receptor pathways and intracellular processes involved in postoperative nausea and vomiting (PONV) is relatively recent. It was not until the 1950s that interest in antiemetic drugs took off, with the identification of histamine and dopamine receptors in the nausea and vomiting pathway, and hence the clinical utility of H 1 - and D 2 -receptor antagonists like cyclizine, chlorpromazine, and promethazine. This surge in research on antiemetics was largely driven by advances in chemotherapy and a focus on chemotherapy-related outcomes. For example, neuro-oncologists first noticed the antiemetic effect of corticosteroids before the same observation was made for PONV in the 1990s.
The development of 5-hydroxytryptamine type 3 (5-HT 3 )-receptor antagonists marks the greatest advance in antiemetic drug research. Early 5-HT 3 -receptor antagonists were not more effective than other available antiemetics, but they were the first to be specifically designed by the pharmaceutical industry to target chemotherapy-induced nausea and vomiting (CINV) and PONV. This led to an increase in large, well-designed PONV studies, marketing of antiemetic agents, and a focus on PONV as a significant postoperative outcome. The first-generation 5-HT 3 -receptor antagonists are associated with QTc prolongation, but the newest 5-HT 3 -receptor antagonists, palonosetron, appears to have improved efficacy, duration of action, and side effect profile compared with its predecessors. Neurokinin-1 (NK1)-receptor antagonists, such as aprepitant and rolapitant, are the newest class of antiemetic drugs, and they too benefit from a long duration of action and favorable side effect profile. As the current understanding of the nausea and vomiting pathway, pharmacokinetics and pharmacodynamics, and genetics continues to improve, antiemetic drugs are likely to become safer and easier to tailor to individual patients.
Despite thousands of studies, new insights into target receptor function, and the successful development of novel antiemetic agents, the actual mechanisms of nausea and vomiting remain unclear. Most antiemetic drugs act on one of several putative neurotransmitter pathways. 5-HT 3 - receptor antagonists are the most commonly used antiemetic class of drugs ( Table 34.1 ). Other classes include dopamine (D 2 ), histamine (H 1 ), NK1, gamma-aminobutyric acid (GABA) A , opioid, and muscarinic cholinergic receptor antagonists.
| Chemical Name | Empirical Formula | Administration | Daily Dosage (mg) | C max (ng/mL) | AUC (ng•hr/mL) | T max (hr) | Bioavailability (%) | V d (L/kg) | Protein Bound (%) | Metabolism | Plasma Half-Life (hr) | Adverse Effects | Other | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PONV | CINV | ||||||||||||||
| 5-HT 3 Receptor Antagonists | Constipation, headache, QTc prolongation | No sedation | |||||||||||||
| Ondansetron (Zofran) | 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one, monohydrochloride, dihydrate | C 18 H 19 N 3 O | IV | 4 | 32 (0.15 mg/kg × 3) | 0.4 | 56 | 70–76 | CYP 3A4, CYP 1A2, CYP 2D6 | 4 | |||||
| IM | 4 | 8 | 0.7 | ||||||||||||
| Oral | 16 | 8 × 2 | 1.5–2.2 | ||||||||||||
| Sup | 16 | 16 | |||||||||||||
| Granisetron (Kytril) | endo-N-(9-methyl-9-azabicyclo [3.3.1] non-3-yl)-1-methyl-1H-indazole-3-carboxamide hydrochloride | C 18 H 24 N 4 O | IV | 1 | 10 µg/kg | 64 | 60 | 3 | 65 | CYP 3A | 3–14 | ||||
| Oral | 1 | 2 | |||||||||||||
| TD | 3.1 | 3.1 | 527 | 48 | |||||||||||
| Dolasetron (Anzemet) | (2α,6α,8α,9αβ)-octahydro-3-oxo-2,6-methano-2H-quinolizin-8-yl-lH- indole-3-carboxylate monomethanesulfonate, monohydrate | C 19 H 20 N 2 O 3 | IV | 12.5 | 0.6 | 75 | 5.8 | 69–77 | CYP 2D6, CYP 3A, flavin monooxygenase | 8 | HPB black box warning (Canada) | ||||
| Oral | 100 | 100 | 1 | ||||||||||||
| Tropisetron (Navoban) | 1αH,5αH-Tropan-3α-yl indole-3-carboxylate | C 17 H 20 N 2 O 2 | IV | 2 | 5 | 15.1 | 20.7 | 60–80 | 71 | CYP 3A4, CYP 1A2, CYP 2D6 | 6–8 | ||||
| Oral | 5 | 5 | 3.46 | 32.9 | 2.6 | ||||||||||
| Palonosetron (Aloxi) | (3aS)-2-[(S)-1-Azabicyclo [2.2.2]oct-3-yl]-2,3,3α,4,5,6-hexahydro-1- oxo-1 H benz[ de ]isoquinoline hydrochloride | C 19 H 24 N 2 O | IV | 0.075 | 0.25 | 5.6 | 35.8 | 97 | 8.3 | 62 | CYP 2D6, CYP 3A, CYP 1A2 | 40 | No QTc prolongation | ||
| Oral | 0.075 | 0.5 | |||||||||||||
| D 2 Receptor Antagonists | EPS, QTc prolongation | ||||||||||||||
| Droperidol | 1-[1-[3-(p-Fluorobenzoyl) propyl]-1,2,3,6-tetrahydro-4-pyridyl]-2-benzimidazolinone | C 22 H 22 FN 3 O 2 | IV | 0.625–1.25 × 6–8 | 25 | 3.2 | 69 | 17.8 | 1.5 | >90 | 2–3 | FDA black box warning | |||
| IM | 0.625–1.25 × 6–8 | ||||||||||||||
| Haloperidol (Haldol) | 4-[4-(p-chloro-phenyl)-4-hydroxypiperidino]-4′—fluorobutyrophenone | C 21 H 23 ClFNO 2 | IV | 1–2 | 50–60 | 18 | 92 | 12–36 | |||||||
| IM | 0.2–0.3 | ||||||||||||||
| Oral | 3–6 | ||||||||||||||
| Metoclopramide (Reglan) | 4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxybenzamide monohydrochloride monohydrate | C 14 H 22 ClN 3 O 2 | IV | 25–50 | 100 (1–2 mg/kg) × 8–12 | 1–2 | 80 | 3.5 | 30 | 5–6 | Cumulative CINV doses associated with significant EPS | 10 mg PONV dose insufficient EPS <1% at 25–50 mg | |||
| IM | 25–50 | ||||||||||||||
| Oral | |||||||||||||||
| Corticosteroids | |||||||||||||||
| Dexamethasone | 9-fluoro-11β, 17,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione | C 22 H 29 FO 5 | IV | 4 | 4 × 4 | 80–90 | 70 | CYP 3A4 | 36–54 a | Hyperglycemia | Most dose response studies suggest that 4 mg is sufficient | ||||
| IM | 4 × 4 | ||||||||||||||
| SC | |||||||||||||||
| Oral | 4 × 4 | ||||||||||||||
| NK1 Receptor Antagonists | No sedation | ||||||||||||||
| Aprepitant (Emend) | 5-([(2 R ,3 S )-2-(( R )-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy)-3-(4-fluorophenyl)morpholino]methyl)-1 H -1,2,4-triazol-3(2 H )-one | C 23 H 21 F 7 N 4 O 3 | Oral | 40 | 125 D1/80 D2-3 | 0.7 (40 mg); 1.6 (125 mg); 1.4 (80 mg) | 7.8 (40 mg); 19.6 (125 mg); 21.2 (80 mg) | 3 (40 mg); 4 (80–125 mg) | 60–65 (80–125 mg) | >95 | CYP 3A4, CYP 1A2, CYP 2C19 | 9–13 | |||
| Anticholinergics | |||||||||||||||
| Transdermal scopolamine (Transderm Scop) | α-(hydroxymethyl) benzeneacetic acid 9-methyl-3-oxa-9-azatricyclo [3.3.1.0 ] non-7-yl ester | C 17 H 21 NO 4 | TD | 0.5 | <24 | 10–50 | 72 a | ||||||||
| Opioid Receptor Antagonists | |||||||||||||||
| Alvimopan (Entereg) | [[2(S)-[[4(R)-(3-hydroxyphenyl)-3(R),4-dimethyl-1-piperidinyl]methyl]-1-oxo-3-phenylpropyl]amino]acetic acid dihydrate | C 25 H 32 N 2 O 4 | Oral | 2 | 6 | 80–90 | Intestinal flora | Sedation | Limited evidence | ||||||
| GABA Agonists | |||||||||||||||
| Diazepam (Diastat, Valium) | 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one | C 16 H 13 ClN 2 O | IV | 0.5–2 | 90–100 | 0.8–1.0 | 95–98 | CYP 2C19, CYP 3A4 | 20 | ||||||
| IM | 1–1.5 | ||||||||||||||
| Oral | |||||||||||||||
| Sup | |||||||||||||||
| Lorazepam (Ativan) | 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1,4-benzodiazepin-2-one | C 15 H 10 Cl 2 N 2 O 2 | IV | 1.5 mg/m 2 | 20 | 2 | 90 | 85 | 9–16 | ||||||
| IM | |||||||||||||||
| Oral | 2.5 × 2 | ||||||||||||||
| TD | |||||||||||||||
| Midazolam | 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo [1,5-a][1,4]benzodiazepine hydrochloride | C 18 H 13 ClFN 3 | IV | >90 | 1–3.1 | 97 | CYP 3A4 | 2–6 | |||||||
| IM | 90 | 0.5 | |||||||||||||
| Oral | |||||||||||||||
| H 1 Receptor Antagonists | |||||||||||||||
| Dimenhydrinate (Dramamine) | 2- (diphenylmethoxy)-N,N -dimethylethylamine hydrochloride | C 17 H 21 NO | IV | 50–100 × 4–6 | 61 | 78 | CYP 2D6 | 2–9 | |||||||
| IM | 50–100 × 4–6 | ||||||||||||||
| Oral | 50–100 × 4–6 | 80–110 | 2–3 | ||||||||||||
| Sup | |||||||||||||||
| Promethazine (Phenergan) | 10-[2- (Dimethylamino)propyl] phenothiazine monohydrochloride | C 17 H 20 N 2 S | IV | 12.5–25 × 4–6 | 25 | 93 | 16–19 | Necrosis that may require amputation | FDA black box warning | ||||||
| IM | 12.5–25 × 4–6 | ||||||||||||||
| Oral | 25 × 2 | ||||||||||||||
| Sup | 25 × 2 | ||||||||||||||
The receptors on which antiemetics act certainly play a role in nausea and vomiting. However, given that only 20% to 30% of patients respond to any one agent, nausea and vomiting cannot be solely attributed to activity of one—or several—of these receptor classes. It is also likely that individual variability plays a larger role than previously acknowledged. Although it is essential to understand and investigate the drug-receptor relationship, the therapeutic potential of targeting specific receptor classes is limited.
Nausea and vomiting can be triggered by a variety of stimuli, including toxins, anxiety, adverse drug reactions, pregnancy, radiation, chemotherapy, and motion. These stimuli are integrated by the vomiting center in the nucleus tractus solitarius (NTS), located primarily in the medulla as well as in the lower pons. The vomiting center receives input from the adjacent chemoreceptor trigger zone (CTZ), the GI tract, the vestibular system, and the cerebral cortex ( Fig. 34.1 ).
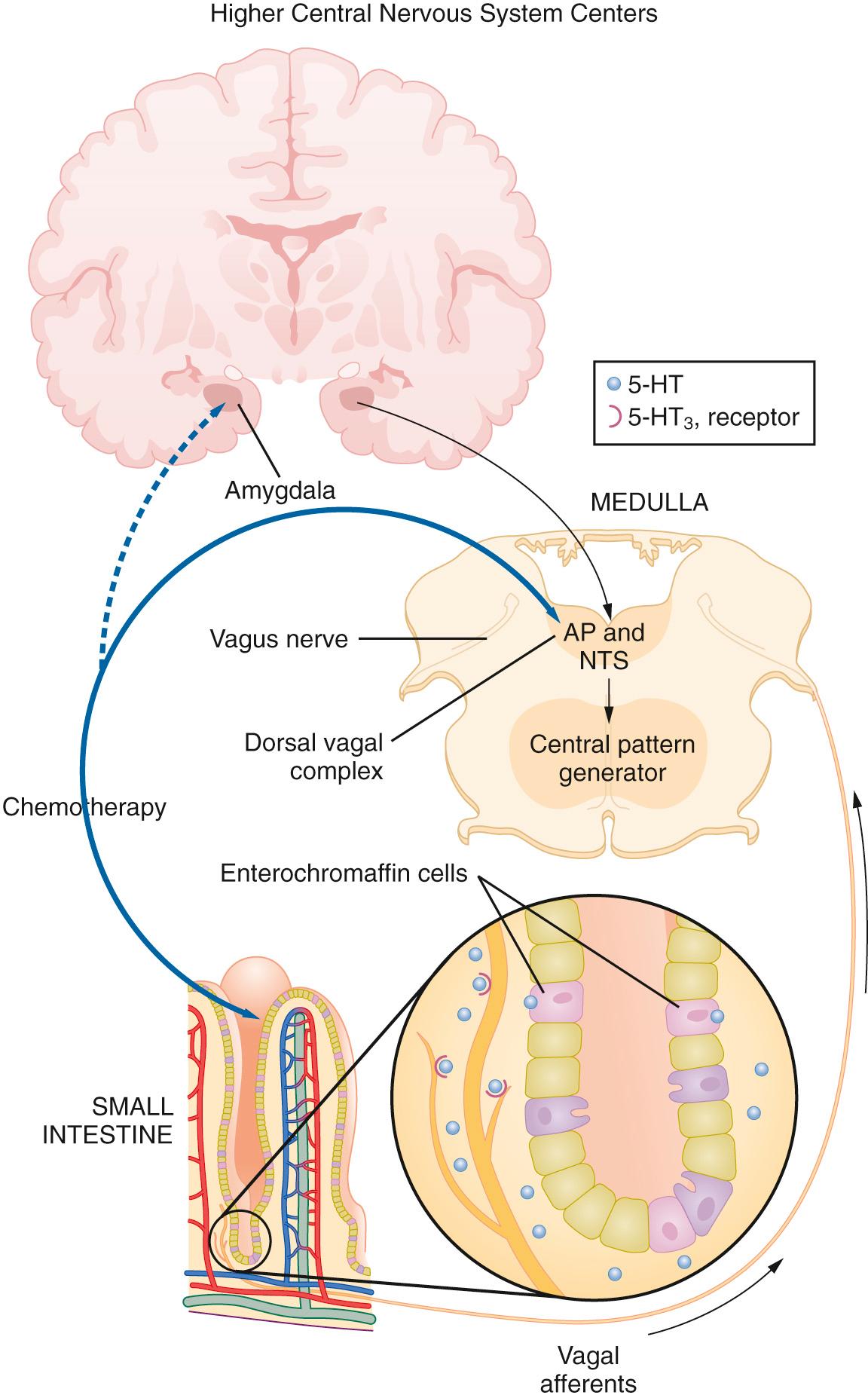
The CTZ is located at the caudal end of the fourth ventricle in the area postrema, a highly vascularized structure that lacks a true blood-brain barrier. Therefore chemosensitive receptors in the CTZ can be directly stimulated by toxins, metabolites, and drugs that circulate in the blood and cerebrospinal fluid. The CTZ communicates with the vomiting center primarily via D 2 receptors as well as 5-HT 3 receptors. Enterochromaffin cells in the GI tract release serotonin, which stimulates vagal afferents that terminate in the CTZ and communicate information regarding intestinal luminal compounds and gastric tone. The vestibular system, located in the bony labyrinth of the temporal lobe, detects changes in equilibrium, which can cause motion sickness. Histamine (H 1 receptor) and acetylcholine (muscarinic acetylcholine receptors) are the neurotransmitters that communicate between the vestibular system and the vomiting center. Anticipatory or anxiety-induced nausea and vomiting probably originates in the cerebral cortex. The cortex has direct input to the vomiting center via several types of neuroreceptors.
Serotonin (5-HT 3 ) receptors are ligand-gated sodium ion (Na + ) and potassium ion (K + ) channels found throughout the central and peripheral nervous systems, notably in the CTZ and afferent fibers of the vagus nerve in both the gut and central nervous system (CNS; see Fig. 34.1 ). Serotonin activation of the CTZ and vagal afferents can both trigger the vomiting reflex. Serotonin plays an important role in anesthesia-, chemotherapy-, and radiation-induced nausea and vomiting. Serotonin receptor antagonists can be used as antiemetic treatment because they inhibit both central and peripheral stimulation of 5-HT 3 receptors, and they are effective, nonsedative, and generally well tolerated. Thus 5-HT 3 -receptor antagonists are currently the most commonly used antiemetic agents for PONV, CINV, and rescue treatment.
Ondansetron was the first 5-HT 3 -receptor antagonist approved by the U.S. Food and Drug Administration (FDA), and at the time of its development, was the safest and most effective treatment for early CINV. Its reputation for superior CINV prophylaxis carried over to PONV, but a factorial trial in more than 5000 patients showed that 4 mg ondansetron was only as effective as 4 mg dexamethasone and 1.25 mg droperidol for PONV. Contrary to the common clinical impression that ondansetron is less effective against nausea than against vomiting, the relative risk reduction (RRR, risk ratio) of ondansetron is the same for nausea and for vomiting. However, ondansetron's plasma half-life is only about 4 hours, which is probably why several studies found it to be more efficacious when administered toward the end rather than at the beginning of anesthesia. Like other 5-HT 3 -receptor antagonists, ondansetron's side effects are generally mild to moderate and include constipation and headache, the latter of which is increased by about 3%. First-generation 5-HT 3 -receptor antagonists like ondansetron have also been associated with QTc prolongation, which potentially increases the risk of cardiac arrhythmia and cardiac arrest. The QTc prolongation associated with ondansetron use is similar to that caused by droperidol.
Even though 5-HT 3 -receptor antagonists are among the most effective antiemetic treatments for CINV, 20% to 30% of patients do not respond to 5-HT 3 -receptor antagonism in the early phase of CINV. Furthermore, 50% to 60% of high-risk patients do not respond to these drugs in the late phase of CINV. Several studies have shown that responsiveness to ondansetron appears to be modulated by variations in cytochrome P450 enzyme 2D6 (CYP 2D6) activity and the ABCB1 gene. The ability to predict patient responsiveness to 5-HT 3 -receptor antagonists based on genetic testing for known polymorphisms could prove to be an important breakthrough in individualizing antiemetic therapy.
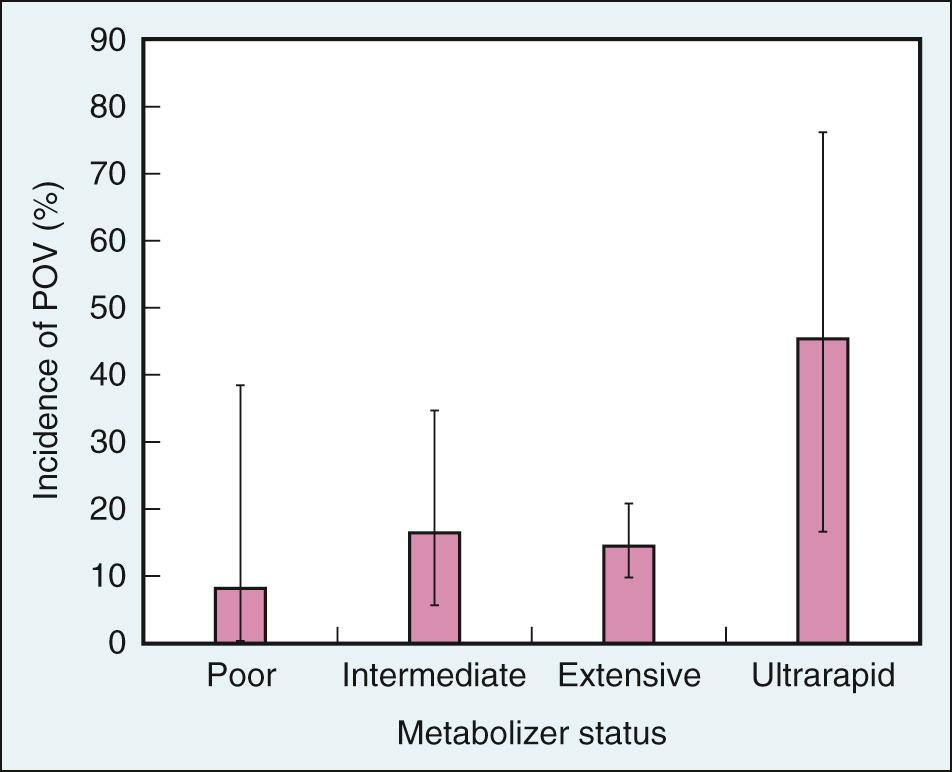
Ondansetron is partially metabolized by hepatic CYP 2D6. There are numerous CYP 2D6 polymorphisms, each associated with one of four metabolic phenotypes: poor (no functional alleles), intermediate (less activity than one functional allele), extensive (two functional alleles, and the most common phenotype), and ultrarapid (three functional alleles). Ultrarapid metabolizers can degrade ondansetron more quickly and are therefore less likely to benefit from prophylaxis with the drug. In fact, several studies have shown that patients with three CYP 2D6 alleles, especially those with three functional alleles, are significantly more likely to experience PONV after prophylaxis with ondansetron than patients with fewer alleles ( Fig. 34.2 ). Ultrarapid metabolism by CYP 2D6 is believed to be partially responsible for prophylactic ondansetron failures in individuals with an ultrarapid metabolic genotype, whereas other enzymes that metabolize ondansetron—namely, CYP 3A4, CYP 2E1, and CYP 1A2—are thought to play a larger role in drug clearance in individuals with poor, intermediate, and extensive metabolism genotypes.
Ondansetron pharmacokinetics also appear to be modulated by polymorphisms of the gene that codes for the drug efflux transporter adenosine triphosphate–binding cassette subfamily B member 1 (ABCB1). The ABCB1 pump transports at least three 5-HT 3 -receptor antagonists, including ondansetron, across the blood-brain barrier, thereby limiting accumulation of these drugs in the CNS. Polymorphisms of ABCB1 that reduce its activity increase the concentration of 5-HT 3 -receptor antagonists in the brain, which enhances efficacy. Indeed, cancer patients with a 3435C>T genetic polymorphism were less likely to experience chemotherapy-induced vomiting (CIV) in the first 24 hours after prophylaxis with ondansetron. Similarly, 3435C>T and/or 2677G>T/A polymorphisms are associated with a lower incidence of PONV in surgery patients, but only within the first 2 postoperative hours.
Other first-generation 5-HT 3 -receptor antagonists include granisetron and dolasetron. Both drugs have a plasma half-life about twice as long as ondansetron. In general, 5-HT 3 -receptor antagonists are considered equally effective at equipotent doses. Compared with 4 mg ondansetron, 12.5 mg dolasetron and 1 mg granisetron appear to be the minimal effective dose for the prevention of PONV. Both drugs are associated with side effects similar to those of ondansetron, including QTc prolongation. CYP 2D6, the enzyme responsible for partial metabolism of ondansetron, is the primary enzyme for dolasetron metabolism. In contrast, granisetron is primarily metabolized by CYP 3A4 and not at all by CYP 2D6. Therefore the efficacy of dolasetron might be modulated by the CYP 2D6 polymorphisms mentioned earlier, whereas ABCB1 polymorphisms might play a larger role in enhancing the efficacy of granisetron.
Palonosetron is the newest and most effective 5-HT 3 -receptor antagonist for preventing acute and delayed emesis associated with chemotherapy and for reducing nausea severity ( Fig. 34.3 ). Palonosetron is characterized by 2500-fold greater affinity than serotonin and 100-fold greater affinity than other 5-HT 3 -receptor antagonists. Palonosetron also has a long half-life of 40 hours. However, palonosetron's high binding affinity and long half-life cannot explain its superiority to other 5-HT 3 -receptor antagonists. High binding affinity does not account for palonosetron's superiority against higher doses of dolasetron or ondansetron (i.e., higher doses of less potent drugs do not overcome the potency difference). Similarly, a long half-life cannot account for palonosetron's superiority against more frequent redosing of ondansetron.
Instead, recent research suggests that palonosetron's high efficacy can be attributed to the manner in which it binds to 5-HT 3 receptors. Whereas other 5-HT 3 -receptor antagonists bind only to the agonist binding site, palonosetron also binds at an allosteric site that increases receptor affinity for the receptor antagonist at the agonist binding site. Given that granisetron and ondansetron induce little to no receptor internalization, this allosteric binding might also be responsible for palonosetron's relatively high rate (50%–60%) of receptor internalization and low receptor exocytosis. Internalized receptor-antagonist complexes are less likely to be dissociated, and, in turn, bound receptors are less likely to be exocytosed and subsequently reactivated by agonists at the cell surface. The low receptor density at the cell surface owing to palonosetron results in prolonged inhibition of receptor function—that is, protection against delayed emesis. Indeed, studies have shown that prophylaxis with palonosetron decreases CIV in a significant proportion of patients in the 5 days following chemotherapy treatment.
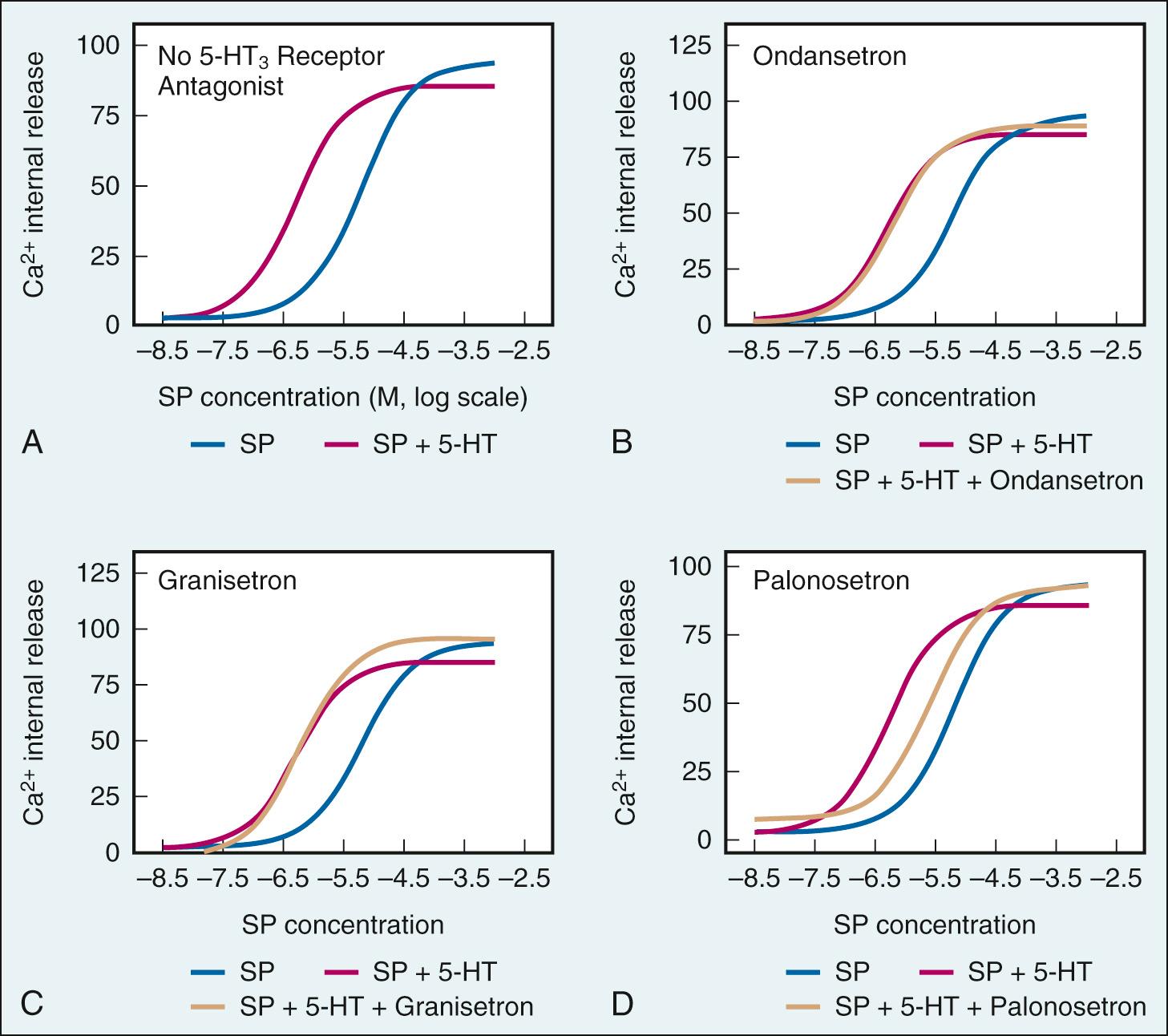
Another mechanism that contributes to palonosetron's high efficacy is its inhibition of cross talk between 5-HT 3 and NK1 receptor signaling pathways. Palonosetron and the NK1 agonist substance P (SP) cannot bind to each other's respective target receptors. However, serotonin and SP enhance each other's potency, and 5-HT 3 -receptor antagonists and NK1-receptor antagonists block activation of vagal afferents by the other agonist. Palonosetron is associated with a sixfold reduction in serotonin enhancement of SP potency in vitro ( Fig. 34.4 ). Granisetron and ondansetron, on the other hand, had no effect on SP potency in vitro. In an in vivo study in rat nodose ganglia, palonosetron reduced cisplatin-induced SP activation for hours after cisplatin administration. Palonosetron thus appears to have a prolonged downstream effect on SP function in vitro and in vivo that might be due to palonosetron's ability to cause 5-HT 3 receptor internalization and reduce receptor density at the cell surface.
Studies have shown that 0.25 mg and 0.075 mg palonosetron are effective doses for preventing CINV and PONV, respectively, and with a half-life of 40 hours, palonosetron provides therapeutic effect for a 72-hour period. Unlike other 5-HT 3 -receptor antagonists, palonosetron is not associated with QTc prolongation. Palonosetron's long half-life makes it a potentially important treatment for postdischarge nausea and vomiting (PDNV), although its relative efficacy in this setting has yet to be demonstrated.
Low-dose (0.625–1.25 mg intravenously [IV]) droperidol is an effective antiemetic for treatment of PONV and opioid-induced nausea and vomiting (OINV), with similar efficacy against nausea (RR [relative risk] = 0.65) and vomiting (RR = 0.65). Droperidol has a short half-life of 3 hours and, if used for the prevention of PONV, should be administered toward the end of anesthesia. At low doses, droperidol is an α-adrenergic receptor blocker that causes increased sedation (RR = 1.32). Therefore for PONV prophylaxis, droperidol should be administered at the minimum effective dose of 0.625 mg IV to reduce the risk of adverse effects. For OINV prophylaxis, although 50 µg of droperidol is the most effective dose to add to a morphine or piritramide patient-controlled analgesia (PCA) infusion pump, 25 µg of droperidol is the safer, recommended dose.
In 2001, reports of arrhythmia and death associated with use of droperidol led the FDA to attach a black box warning to the drug's label, after which droperidol use decreased 10-fold in the United States. According to the new label, droperidol is contraindicated in patients with known or suspected QT prolongation. Therefore absence of QT prolongation must be confirmed by electrocardiogram before droperidol administration, and electrocardiogram monitoring must be continued for 2 to 3 hours after drug administration. Many hospitals have removed droperidol from their formularies or placed restrictions on its use, in addition to drug shortages, so that droperidol usage is less than 2% of cases in the United States, even though more than 90% of anesthesiologists believe that the FDA black box warning is unwarranted.
Those who argue against the black box warning question the clinical relevance of droperidol-induced QT prolongation, particularly because QT prolongation is associated with general anesthesia itself, as well as other drugs commonly administered during surgery (e.g., antibiotics). QTc prolongation after placebo, 0.625 mg droperidol, and 1.25 mg droperidol were 12, 15, and 22 msec, respectively. Similarly, 0.75 mg droperidol and 4 mg ondansetron were associated with 17- and 20-msec QT prolongation, respectively. It is possible, however, that these studies were inadequately powered to include patients with a rare but clinically significant predisposition to QT prolongation. Of note, there are polymorphisms in the ether-à-go-go related gene (hERG) receptor that occur in about 0.5% to 2% of the population, and it is possible that these are the patients who are at high risk when exposed to droperidol. Thus it is not possible to exclude the possibility that adding droperidol to other QT-prolonging interventions, including general anesthesia, can trigger QT prolongations that lead to cardiac arrhythmia.
As a result of the black box warning for droperidol, there is a renewed interest in haloperidol, an older butyrophenone. Haloperidol is an effective treatment for psychiatric disorders at high doses and is an effective antiemetic at low doses. Haloperidol has a longer plasma half-life of 10 to 20 hours after intravenous administration. Like other D 2 -receptor antagonists, haloperidol is associated with extrapyramidal effects, including acute dystonia, pseudoparkinsonism, and akathisia. Haloperidol is metabolized in the liver, where 23% of haloperidol is reduced by a carbonyl reductase into a functional metabolite with high binding affinity to σ-opioid and D 2 and D 3 receptors. However, there is significant interindividual variation in haloperidol pharmacokinetics.
Plasma concentrations of haloperidol correlate with dosage, drug efficacy, and incidence of adverse effects. Although CYP 3A4 is the primary enzyme responsible for haloperidol metabolism, with CYP 2D6 appearing to play only a minor role, several studies have shown that certain CYP 2D6 genotypes are associated with poor metabolism and are correlated with higher haloperidol plasma concentrations and lower drug clearance than genotypes associated with extensive metabolism ( Fig. 34.5A and B ). Specifically, individuals with 0, 1, 2, and more than 2 active CYP 2D6 alleles are considered poor, intermediate, extensive (most common), and ultrafast metabolizers, respectively. Thus poor metabolizers are at higher risk for adverse effects than are intermediate and extensive metabolizers.
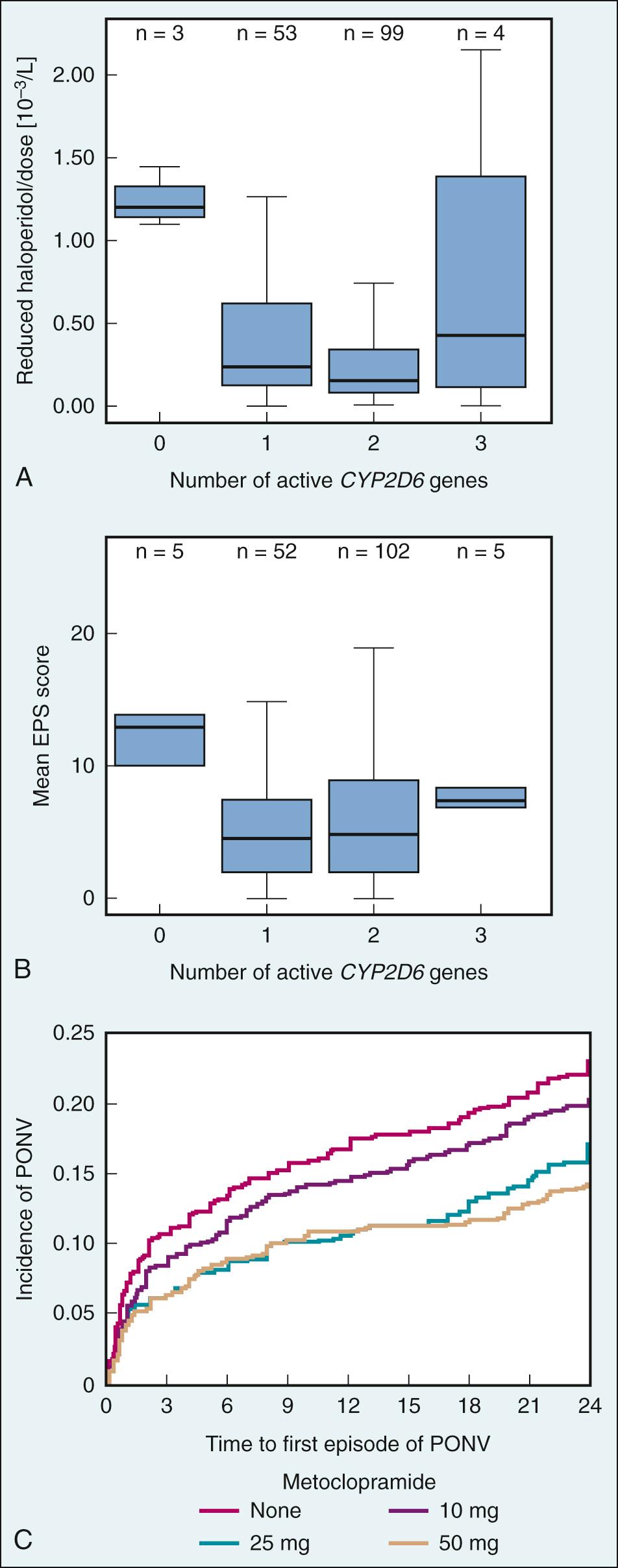
Reports of QT prolongation, torsades de pointes, and sudden death associated with use of haloperidol similar to those associated with droperidol led the FDA to issue an FDA alert for haloperidol in 2007. It has not received a black box label because these severe adverse effects occurred in patients who had received off-label intravenous administration of haloperidol at doses greater than 35 mg/day, whereas only intramuscular administration has been approved by the FDA.
Metoclopramide, a procainamide derivative and a benzamide prokinetic agent, is the most commonly used D 2 -receptor antagonist for antiemetic prophylaxis, primarily for PONV and chemotherapy associated with low emetogenic risk. It is assumed that both the central D 2 -receptor antagonist activity at the CTZ and vomiting center and peripheral activity in the GI tract contribute to the antiemetic effect. Metoclopramide acts on peripheral D 2 , muscarinic, and 5-HT 4 receptors to induce prokinetic activity. Opioids can cause delayed gastric emptying, but metoclopramide enhances gastric motility and increases intestinal peristalsis, which reduces reflux of stomach contents and the urge to vomit. Because of its short half-life of 5 to 6 hours, metoclopramide is likely to have greatest efficacy if administered at the end of surgery.
Metoclopramide was first prescribed for CINV in high doses (e.g., 200 mg every 4–6 hours), which cause extrapyramidal symptoms in more than 10% of patients. To reduce the incidence of adverse effects, metoclopramide is available in vials of just 10 mg. However, extensive studies and a meta-analysis have demonstrated that 10 mg metoclopramide has no clinically relevant antiemetic effect. In fact, a large and well-designed dose-response study in more than 3000 patients demonstrated that doses of 25 and 50 mg metoclopramide are effective in reducing PONV by about 37% (RR = 0.63, a similar efficacy as other commonly used antiemetics), whereas the rate for extrapyramidal symptoms was less than 1% (see Fig. 34.5C ).
Like haloperidol, metoclopramide is metabolized primarily by CYP 2D6. Although several studies have shown CYP 2D6 polymorphisms that result in reduced CYP 2D6 activity are associated with a higher incidence of metoclopramide adverse effects, no studies have investigated yet whether CYP 2D6 polymorphisms influence the antiemetic efficacy of the drug. Given that nearly 25% of metoclopramide is excreted unchanged, however, the effect of CYP 2D6 polymorphisms might be relatively small, at least in patients with normal renal function.
Like other D 2 -receptor antagonists, metoclopramide is associated with severe cardiac adverse effects. High doses are associated with a high incidence of extrapyramidal symptoms, but lower doses (25–50 mg) are associated with a less than 1% incidence of dyskinetic and/or extrapyramidal symptoms. It is important to note that the FDA issued a black box warning for metoclopramide, given the high risk of developing tardive dyskinesia if metoclopramide use extends beyond 12 weeks. However, this concern likely does not apply to a short-term course of metoclopramide in the perioperative setting.
Other D 2 -receptor antagonists such as alizapride, perphenazine, and prochlorperazine might be as effective as other commonly used antiemetics, but they are rarely used, and their side effect profiles are unclear compared with that of other antiemetics.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here