Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Electroencephalography can detect both cerebral ischemia or hypoxia and seizures and can measure hypnotic effect.
Middle-latency auditory-evoked potentials objectively document inadequate hypnosis.
Somatosensory-evoked potentials may detect developing injury in cortical and subcortical brain structures and peripheral nerves.
Transcranial electric motor–evoked potentials monitor function of the descending motor pathways.
Transcranial Doppler ultrasound examination assesses the direction and character of blood flow through large intracranial arteries and identifies microemboli.
Cerebral oximetry, using spatially resolved transcranial near-infrared spectroscopy, provides a continuous measure of change in the balance of cerebral oxygen supply and demand.
Used in concert, these technologies can reduce the incidence of brain injury and ensure the adequacy of hypnosis.
Yearly, nearly one-half of the 1 million patients undergoing cardiac surgical procedures worldwide will likely experience transient neurologic, cognitive, or neuropsychological dysfunction; in one-quarter of these patients, the changes will be persistent. The direct annual cost to US insurers for brain injury from just one type of cardiac operation, myocardial revascularization, is estimated at $2 billion. Furthermore, the same processes that injure the central nervous system (CNS) also appear to influence dysfunction of other vital organs. Thus, enormous clinical and economic incentives exist to improve CNS protection during cardiac surgical procedures.
Historically, neurophysiologic monitoring during cardiac surgical procedures has elicited little enthusiasm because of the presumed key role of macroembolization. It is widely assumed that most brain injuries during cardiac operations in adults result from cerebral embolization of atheromatous or calcified material dislodged from sclerotic blood vessels during the manipulation of these vessels. Until the introduction of myocardial revascularization without cardiopulmonary bypass (CPB) or aortic clamp application, these injuries often were viewed as unavoidable and untreatable.
Technical developments are altering this perception. First, CNS injuries still occur despite reductions in aortic manipulation with the newer approaches to coronary artery bypass grafting (CABG) and aortic surgical procedures. Second, neurophysiologic studies have implicated hypoperfusion and dysoxygenation as major causative factors in CNS injury ( Box 12.1 ). Because these functional disturbances are often detectable and correctable, the impetus is to examine the role of neurophysiologic monitoring in organ protection.
Atheromatous emboli from aorta manipulation
Lipid microemboli from recirculation of unwashed cardiotomy suction
Gaseous microemboli from air leakage and cavitation
Cerebral hypoperfusion or hyperperfusion
Cerebral hyperthermia
Cerebral dysoxygenation
Electroencephalographic (EEG) monitoring for ischemia detection has been performed since the first CPB procedures. However, its widespread use has previously been limited by a number of factors.
First, small, practical, and affordable EEG monitors have only recently become available.
Second, the traditional diagnostic approach to EEG analysis depended on complex pattern recognition of 21-channel analog waveforms to identify focal ischemic changes. This analytic format necessitated extensive training and constant vigilance. Therefore, EEG monitoring during cardiac operations by anesthesia providers was viewed as impractical. However, reduced electrode array perioperative EEG recordings that include bilateral activity appear to be effective in identifying cortical ischemia and seizure activity in the perioperative and critical care settings. In addition, computerized processing of EEG signals provides simplified trend displays that have helped to overcome many of the earlier interpretational complexities.
Third, EEG analysis during cardiac surgical procedures was often confounded by anesthetic agents, hypothermia, and roller pump artifacts. Fortunately, these technical problems have now been overcome by: (1) elimination or replacement of the troublesome roller pumps with centrifugal pumps, (2) routine use of mild hypothermic or normothermic bypass, and (3) adoption of fast-track anesthesia protocols that avoid marked EEG suppression.
Electroencephalographically directed interventions designed to correct cerebral hypoperfusion during cardiac surgical procedures require an appreciation of the underlying neurophysiologic substrate. Scalp-recorded EEG signals reflect the temporal and spatial summation of long-lasting (10–100 milliseconds) postsynaptic potentials that arise from columnar cortical pyramidal neurons ( Fig. 12.1 ).
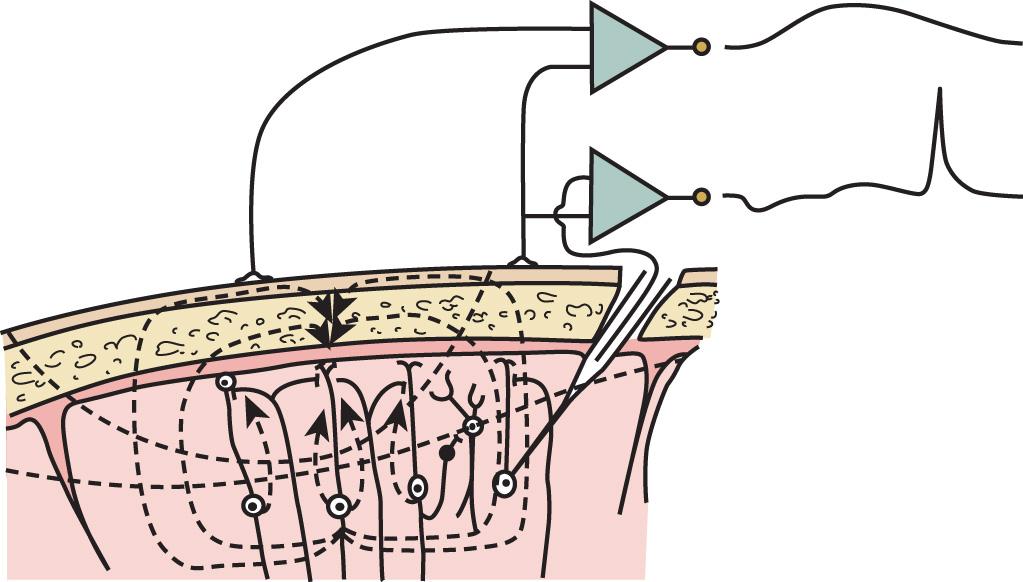
EEG rhythms represent regularly recurring waveforms of similar shape and duration. These signal oscillations depend on the synchronous excitation of a neuronal population. The descriptive nature of conventional EEG findings characterizes the oscillations (measured in cycles per second [cps] or Hertz [Hz]) as sinusoids that were classified according to their amplitude and frequency. The terminology used to describe the frequency bands of the most common oscillatory patterns is illustrated in Fig. 12.2 . In addition, a high-frequency (25–55 Hz) gamma band is recognized ( Table 12.1 ).
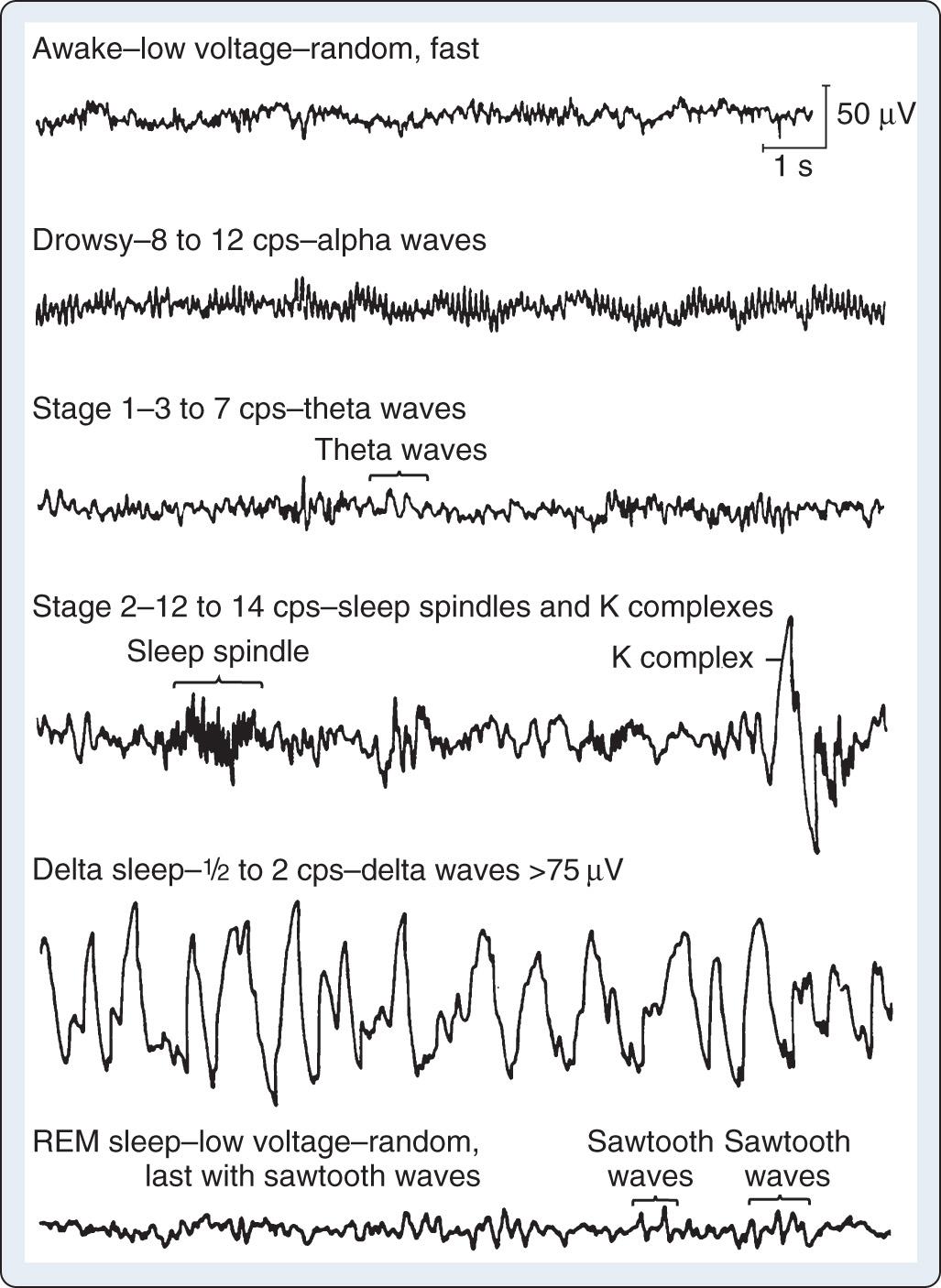
| Delta (δ) | 0.1–4 Hz |
| Theta (θ) | 4–8 Hz |
| Alpha (α) | 8–14 Hz |
| Beta (β) | 14–25 Hz |
| Gamma (γ) | 25–55 Hz |
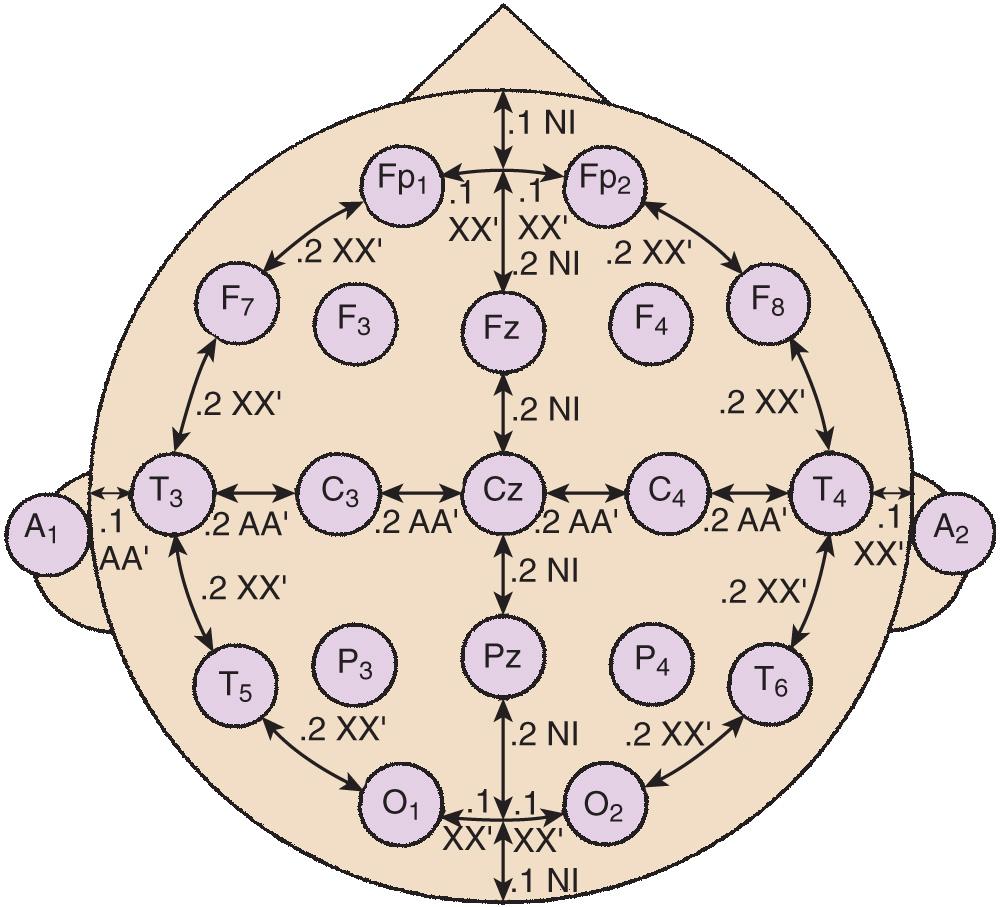
Standardized electrode placement is based on the International 10–20 System ( Fig. 12.3 ). It permits uniform spacing of electrodes, independent of head circumference, in scalp regions known to correlate with specific areas of cerebral cortex. Four anatomic landmarks are used—the nasion, inion, and preauricular points.
The frequency range involved in production of the EEG waveform is termed its bandwidth . The upper and lower bandwidth boundaries are controlled by filters that reject frequencies above and below the EEG bandwidth. Both the appearance of the unprocessed EEG waveform and the value of univariate numeric EEG descriptors such as the mean dominant frequency (MDF) may be heavily influenced by signal bandwidth that is often user-controlled by high- and low-frequency filter settings. Similarly, the same cerebral biopotential recorded by different EEG devices may result in dissimilar waveforms and numeric values.
Traditional display of the electroencephalogram is a graph of biopotential voltage ( y -axis) as a function of time and consequently is described as a time-domain process. The objective of a diagnostic electroencephalogram is to identify the most likely cause of a detected abnormality at one moment in time. Typically, a diagnostic electroencephalogram is obtained under controlled conditions, using precisely defined protocols. Recorded EEG appearance is visually compared with reference patterns. Interpretation is based on recognition of unique waveform patterns that are pathognomonic for specific clinical conditions. In contrast, the goal of EEG monitoring is to identify clinically important change from an individualized baseline. Unlike diagnostic EEG interpretation, monitoring requires immediate assessment of continuously fluctuating signals in an electronically hostile, complex, and poorly controlled recording environment. Therefore, of necessity, interpretation relies less on pattern recognition and more on statistical characterization of change. Numeric descriptors thus may appropriately form an integral part of EEG monitoring.
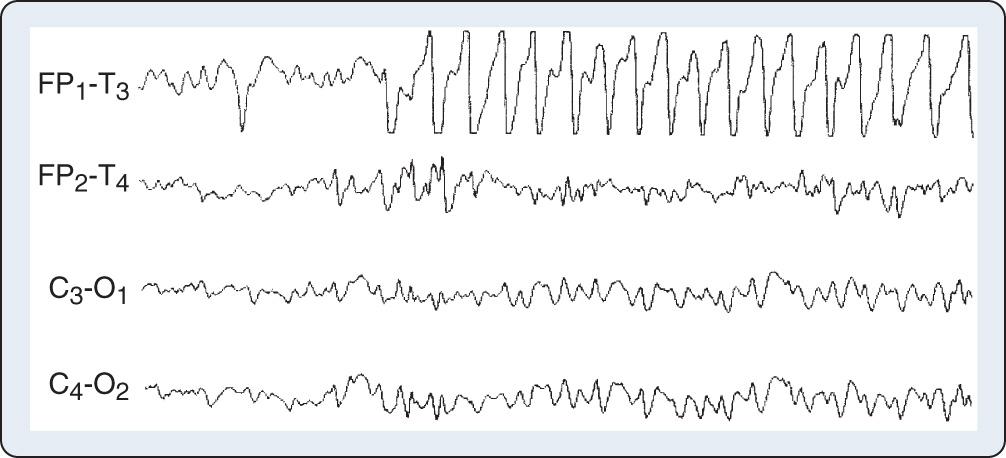
Both EEG diagnostic and monitoring interpretations are based in part on the “law of the electroencephalogram” ( Box 12.2 ). It states that amplitude and dominant frequency are inversely related. The inverse relationship between amplitude and frequency generally is maintained during unchanging cerebral metabolic states. Parallel increases in both may occur in some hypermetabolic states such as seizure activity, whereas decreases may be seen in hypometabolic states such as hypothermia. In the absence of these influences, simultaneous decreases in both amplitude and frequency may indicate ischemia or anoxia ( Fig. 12.4 ); a parallel increase may represent artifact ( Fig. 12.5 ).
In the absence of disease, electroencephalographic amplitude and frequency are inversely related
Simultaneous decrease may indicate ischemia, anoxia, or excessive hypnosis
Simultaneous increase may indicate seizure or artifact
Time-domain analysis of traditional electroencephalography uses linear signal amplitude (ie, voltage) and time scales. The amplitude range of EEG signals is quite large (several hundred microvolts), and univariate statistical measures of its central tendency and dispersion may contain clinically useful information. Furthermore, amplitude variation may show clinically significant changes in reactivity that can be obscured by frequency-domain analysis. Advances in the technology of EEG amplitude integration have prompted a resurgent interest in this attractively simple approach, particularly in pediatrics.
An alternative method, frequency-domain analysis, is exemplified by the prismatic decomposition of white light into its component frequencies (ie, color spectrum). As the basis of spectral analysis, the Fourier theorem states that a periodic function can be represented in part by a sinusoid at the fundamental frequency and an infinite series of integer multiples (ie, harmonics). The Fourier function at a specific frequency equals the amplitude and phase angle of the associated sinusoid. Graphs of amplitude and phase angle as functions of frequency are called Fourier spectra (ie, spectral analysis). The EEG amplitude spectral scale ( Fig. 12.6 ) squares voltage values to eliminate troublesome negative values. Squaring changes the unit of amplitude measure from microvolts to either picowatts (pW) or nanowatts (nW). However, a power amplitude scale tends to overemphasize large-amplitude changes. Clinically important changes in low-amplitude components that are readily discernible in the linearly scaled unprocessed EEG waveform may become invisible in power spectral displays.
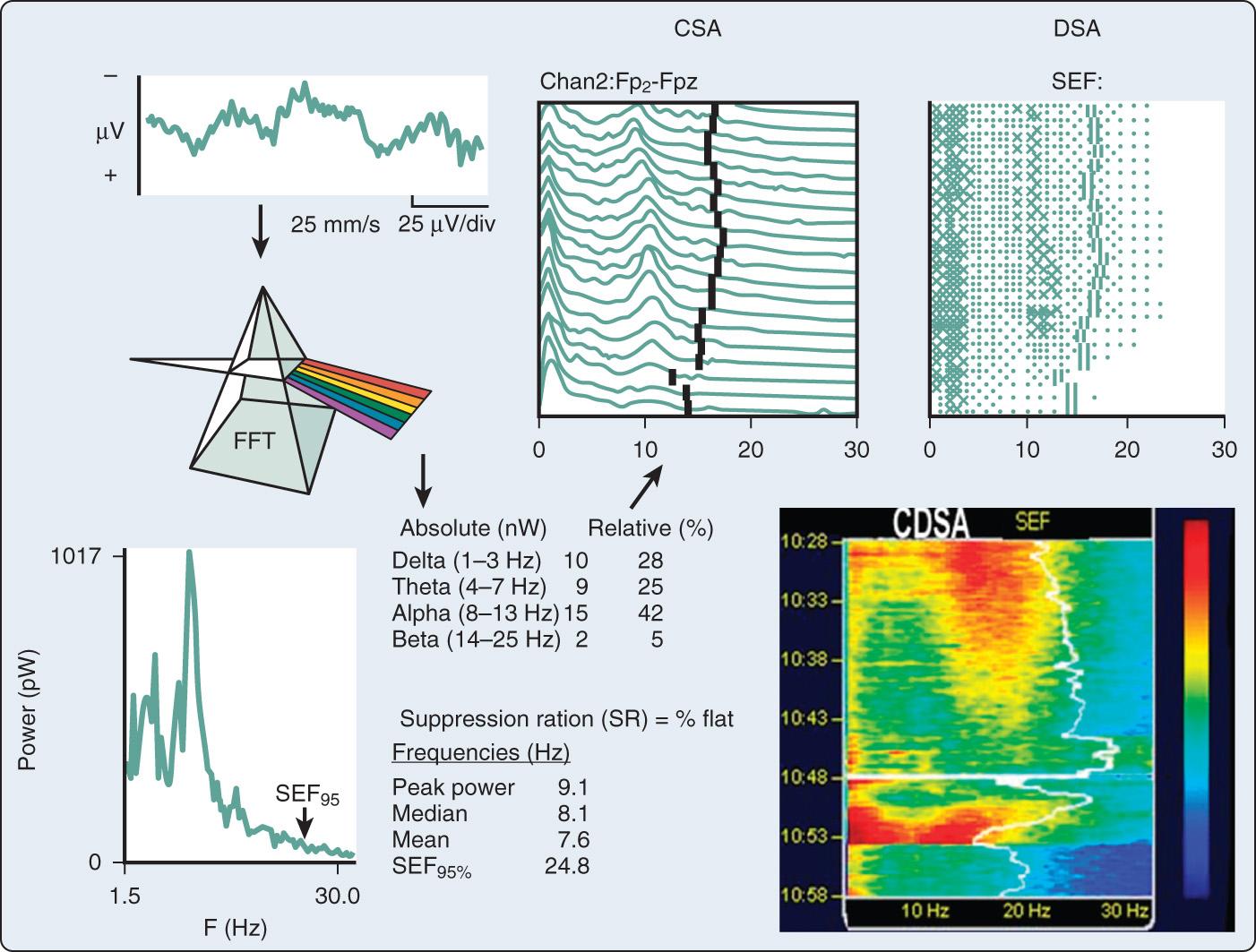
Simplification of the large amount of spectral information generally has been achieved through the use of univariate numeric descriptors. Most commonly, the power contained in a specified traditional EEG frequency band (delta, theta, alpha, or beta) is calculated in absolute, relative, or normalized terms.
The most widely used univariate frequency descriptors ( Box 12.3 ) are as follows:
Total power (TP).
Peak power frequency (PPF), the single frequency of the spectrum that contains the highest amplitude.
Mean dominant frequency (MDF), the sum of power contained at each frequency of the spectrum times its frequency divided by the TP.
Spectral edge frequency (SEF), the frequency below which a predetermined fraction, usually 90% or 95%, of the spectral power occurs.
Suppression ratio (SR), the percentage of flat-line electroencephalogram contained within sampled epochs.
Multivariate (ie, composed of several variables) descriptors have been developed to improve simple numeric characterization of clinically important EEG changes. With this approach, algorithms are used to generate a single number that represents the pattern of amplitude-frequency-phase relationships occurring in a single epoch. Several commercially available monitors provide unitless numbers that have been transformed to arbitrary (ie, 0–100) scales. Each monitor provides a different probability estimate of a patient's response to verbal instruction. Current monitors designed for use by anesthesia providers are listed in Table 12.2 . BIS (bispectral index, Covidien, Boulder, CO), NT (NarcoTrend, Monitor Technik, Bad Bramstedt, Germany), PSI (Sedline, Masimo, Irvine, CA), and SNAP II (Stryker Instruments, Kalamazoo, MI) are rule-based proprietary indices empirically derived from patients' data. In contrast, CSI (Danmeter A/S, Odense, Denmark) uses a fuzzy logic–based algorithm, whereas SE applies standard entropy equations to EEG analysis. Each product is designed to require the use of proprietary self-adhesive forehead sensors. Collectively, these products are now in widespread use as objective measures of hypnotic effect.
Total power (TP)
Peak power frequency (PPF)
Mean dominant frequency (MDF)
95% spectral edge frequency (SEF)
Suppression ratio (SR)
| Acronym | Index Name | Mode | Manufacturer |
|---|---|---|---|
| BIS | Bispectral | Bilateral | Covidien, Boulder, CO |
| CSI | Cerebral state | Unilateral | Danmeter A/S, Odense, Denmark |
| NT | Narcotrend | Bilateral | MonitorTechnik, Bad Bramstedt, Germany |
| PSI | Patient state | Bilateral | Masimo, Irvine, CA |
| SE | State entropy | Unilateral | GE Healthcare/Datex-Ohmeda, Helsinki, Finland |
| SNAP II | SNAP II | Unilateral | Stryker Instruments, Kalamazoo, MI |
Scalp-recorded cerebral biopotentials are complex physiologic signals. They represent the algebraic summation of voltage changes produced from cortical synaptic activity (ie, electroencephalogram), upper facial muscle activity (ie, facial electromyogram [fEMG]), and eye movement (ie, electro-oculogram [EOG]). During consciousness and light sedation, high-frequency gamma power (ie, 25–55 Hz) is a mixture of electroencephalography and subcortically influenced facial electromyography. Muscle activity makes a larger contribution because of the closer proximity of signal generators to the recording electrodes. Hypnotic and analgesic agents typically suppress both cerebral and muscle activities, with resulting reduced gamma power. Because the upper facial muscles are relatively insensitive to moderate neuromuscular blockade, they may remain reactive to noxious stimuli. Nociception results in sudden gamma power increase, independent of activity in the lower-frequency classic EEG bands.
The EEG analyzers just described either provide separate quantitative estimates of the high-frequency information or incorporate this information into the hypnotic index. For example, the Datex-Ohmeda Entropy Module (GE Healthcare/Datex-Ohmeda, Helsinki, Finland) separately analyzes the 32- to 47-Hz band and terms the signal Response Entropy (RE). Addition of RE to the lower-frequency state entropy (SE) is claimed by the manufacturer to facilitate distinction between changes in hypnosis and analgesia, although supporting evidence for this proposition awaits carefully designed and adequately powered randomized, prospective studies. EEG suppression decreases both entropy indices because noise-free flat-line EEG segments are generally thought to have near-zero entropy. However, during cardiac surgical procedures, EEG signals that appear to be totally suppressed may be associated with paradoxically very high entropy values. To minimize this problem, SE uses a special algorithm that assigns zero entropy to totally suppressed EEG epochs.
In addition to the quantitative EEG numeric indices, many monitors also display pseudo-three-dimensional plots of successive power spectra as a function of time. This frequency-domain approach was originated by Joy and was popularized by Bickford, who coined the term “compressed spectral array” (CSA). Its popularity stems in part from enormous data compression. For example, the essential information contained in a 4-hour traditional EEG recording consuming more than 1000 pages of unprocessed waveforms can be displayed in CSA format on a single page.
With CSA (see Fig. 12.6 ), successive power spectra of brief (2- to 60-second) EEG epochs are displayed as smoothed histograms of amplitude as a function of frequency. Spectral compression is achieved by partially overlaying successive spectra, with time represented on the z -axis. Hidden-line suppression improves clarity by avoiding overlap of successive traces. Although the display is aesthetically attractive, it has limitations. The extent of data loss resulting from spectral overlapping depends on the nonstandard axial rotation that varies among EEG monitors.
An alternative to the CSA display to reduce data loss is the diversity-modulated spectral array (DSA) that uses a two-dimensional monochrome dot matrix plot of time as a function of frequency (see Fig. 12.6 ). The density of dots indicates the amplitude at a particular time-frequency intersection (eg, an intense large spot indicates high amplitude). Clinically significant shifts in frequency may be detected earlier and more easily than with CSA. However, the resolution of amplitude changes is reduced. Therefore color DSA (CDSA) was developed to enhance amplitude resolution (see Fig. 12.6 ). The CSA, DSA, and CDSA displays are not well suited for the detection of nonstationary or transient phenomena such as burst suppression or epileptiform activity.
In summary, a quick assessment of EEG change in either the time- or frequency-domain focuses on (1) maximal peak-to-peak amplitude, (2) relation of maximal amplitude to dominant frequency, (3) amplitude and frequency variability, and (4) new or growing asymmetry between homotopic (ie, same position on each cerebral hemisphere) EEG derivations. These objectives are generally best achieved through the viewing of both unprocessed and processed displays with a clear understanding of the characteristics and limitations of each ( Box 12.4 ).
Maximum peak-to-peak amplitude (or total power)
Relation of maximum amplitude to dominant frequency
Amplitude and frequency variability
Right-to-left symmetry
Auditory-evoked potentials (AEPs) assess specific areas of the brainstem, midbrain, and auditory cortices. Because of their simplicity and reproducibility, AEPs are suitable for monitoring patients during cardiovascular surgical procedures. Specific applications of AEP monitoring in this environment are the assessment of temperature effects on brainstem function and the evaluation of hypnotic effect. Direct involvement of cardiac anesthesia providers with AEP monitoring is likely to increase following the introduction of EEG/AEP modules designed for use with available operating room physiologic monitors.
Acoustic stimuli trigger a neural response integrated by a synchronized neuronal depolarization that travels from the auditory nerve to the cerebral cortex. Scalp-recorded signals, obtained from electrodes located at the vertex and earlobe, contain both the AEPs and other unrelated EEG and electromyographic activity. Extraction of the relatively low-amplitude AEP from the larger-amplitude background activity requires signal-averaging techniques. Because the AEP character remains constant for each stimulus repetition, averaging of many repetitions increases the signal amplitude linearly. Thus increases in the signal-to-noise ratio of 10-fold to 30-fold are commonly achieved. For the AEP sensory stimulus, acoustic clicks are the most commonly used. These broadband signals are generated by unidirectional rectangular short pulses (40–500 microseconds) with frequency spectra lower than 10 kHz.
Brainstem auditory-evoked potentials (BAEPs) are useful in assessing brainstem and subcortical function during surgical procedures, in part because of their relative resistance to the suppressant effects of most anesthetic agents.
The middle-latency AEPs (MLAEPs), with poststimulus latencies between 10 and 100 milliseconds, are generated in the midbrain and primary auditory cortex. Latency and amplitude changes allow reliable detection of consciousness and nociception during cardiac surgical procedures. In addition, parallel monitoring of MLAEP and quantitative EEG descriptors (ie, BIS) may permit distinction between the hypnotic and antinociceptive anesthetic components. This approach has also been used successfully in pediatric cardiac surgical patients to assess postoperative sedation objectively.
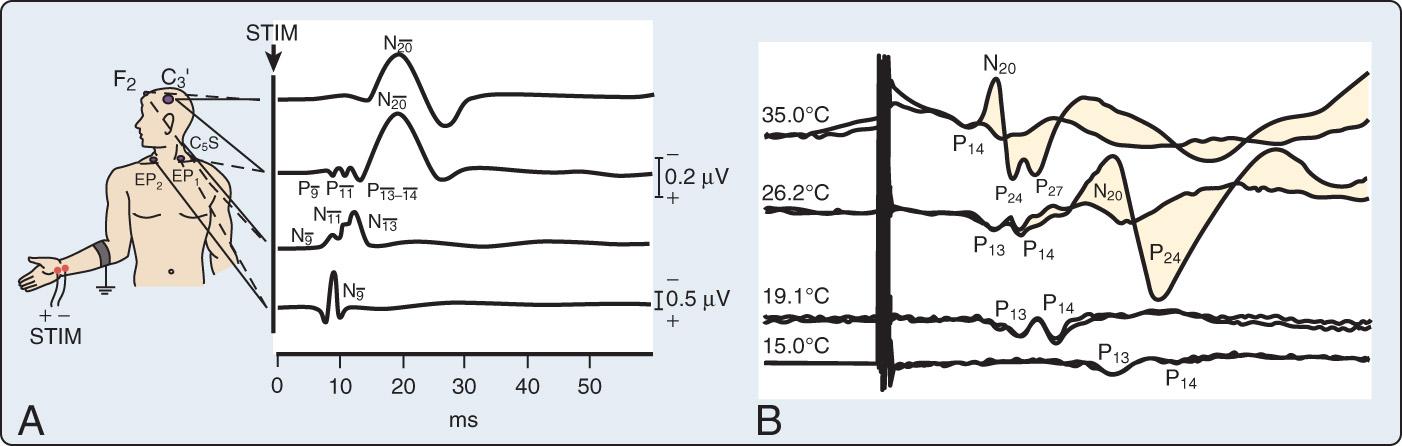
In many ways, the somatosensory-evoked potential (SSEP) is similar to the AEP. An electrical stimulus is applied peripherally to the arms or legs, or both, and the progression of the neuronal transmission through the spinal cord and subcortical structures is tracked, with various neurogenerators producing specific positive or negative deflection of the recorded signal at various times. In this way, SSEPs provide an objective measure of ascending sensory pathway function. Like AEPs, they are recorded by signal averaging over a large number of stimuli, with the duration of recording after each stimulus being somewhat longer and thus the frequency of stimulation somewhat lower. SSEPs are moderately sensitive to depression from inhaled anesthetic agents, but they do not generally preclude the use of potent agents in a balanced technique or as supplement to a high-dose narcotic approach. Fig. 12.7 illustrates the key neural structures involved in a prominent upper limb sensory pathway suitable for cardiac surgical neuromonitoring.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here