Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Isoflurane, desflurane, and sevoflurane decrease myocardial contractility, delay left ventricular (LV) relaxation, and attenuate early LV filling, but do not affect LV compliance.
Volatile anesthetics dilate arteriolar resistance and venous capacitance vessels, but do not alter compliance of the aorta and proximal great vessels or affect characteristic aortic impedance.
Isoflurane, desflurane, and sevoflurane preserve LV-arterial coupling and mechanical efficiency at lower concentrations because systemic vasodilation balances simultaneous depression of contractility.
Volatile anesthetics depress left atrial (LA) contractility, but LA reservoir function is preserved at lower concentrations.
Volatile anesthetics differentially alter autonomic nervous system control of the circulation; these actions contribute to their hemodynamic effects.
Volatile anesthetics are relatively weak coronary vasodilators that do not cause coronary steal, in contrast to potent vasodilators such as adenosine or sodium nitroprusside.
Isoflurane, desflurane, and sevoflurane protect against ischemia-reperfusion injury by activating endogenous signaling pathways associated with pharmacologic pre- and postconditioning, but these laboratory findings do not translate into clinically meaningful improvements in short- or long-term outcome in patients undergoing cardiac surgery who are at risk for perioperative myocardial ischemia.
Nitrous oxide causes modest direct myocardial depression, but this effect is usually offset by simultaneous activation of the sympathetic nervous system.
The hemodynamic effects of nitrous oxide result from mild sympathetic nervous system stimulation, but these actions may be attenuated or abolished by the presence of other anesthetics.
Rapid administration of induction doses of propofol cause transient depression of myocardial contractility that contributes to the hypotension associated with the drug, but infusions of propofol used for conscious sedation or maintenance of anesthesia generate plasma concentrations that are most likely not associated with negative inotropic effects.
The venous and arterial vasodilation produced by propofol is the primary mechanism by which the drug reduces arterial pressure.
Propofol blunts compensatory heart rate responses to hypotension by attenuating baroreceptor reflex–mediated control of the circulation.
Midazolam causes relatively minor changes in systemic and pulmonary hemodynamics as a result of modest myocardial depression and vasodilation.
Hemodynamic stability is the hallmark of anesthetic induction with etomidate, but the drug is capable of producing modest cardiovascular depression in some patients with valvular heart disease.
Ketamine produces cardiovascular stimulation as a result of the drug’s potent central and peripheral sympathomimetic effects.
Ketamine may cause hemodynamic decompensation in some critically ill patients because catecholamine depletion unmasks myocardial depression, adverse effects on LV diastolic function, and direct vasodilation.
Dexmedetomidine produces a unique cardiovascular profile characterized by a biphasic arterial pressor-depressor response.
Direct stimulation of postsynaptic α 2 -adrenoceptors in arterial vascular smooth muscle is responsible for the initial hypertension associated with dexmedetomidine, whereas later declines in arterial pressure occur because of activation of medullary α 2 -adrenergic or imidazoline receptors.
Dexmedetomidine causes persistent decreases in heart rate and cardiac output by inhibiting central sympathetic tone and enhancing parasympathetic activity.
Dexmedetomidine-induced depression of sinus and atrioventricular node function can lead to pronounced bradycardia and sinus pauses in some patients.
Synthetic opioids are relatively devoid of direct cardiovascular effects, but these drugs effectively attenuate central sympathetic nervous system responses to surgical stimulation.
This chapter describes the cardiovascular pharmacology of volatile anesthetics, nitrous oxide, intravenous anesthetics, and synthetic opioids. Comparisons to older volatile (halothane, enflurane) and intravenous (sodium thiopental, diazepam) anesthetics are made only when necessary because these medications are now less commonly used or are no longer available in the United States. It is important for the reader to understand that much of the knowledge about the pharmacology of anesthetic medications was established decades ago. The current chapter underscores this historical perspective by presenting landmark experimental and clinical studies in which anesthetic pharmacology was initially described. A detailed understanding of the cardiovascular pharmacology of these medications is essential for the practice of cardiac anesthesia.
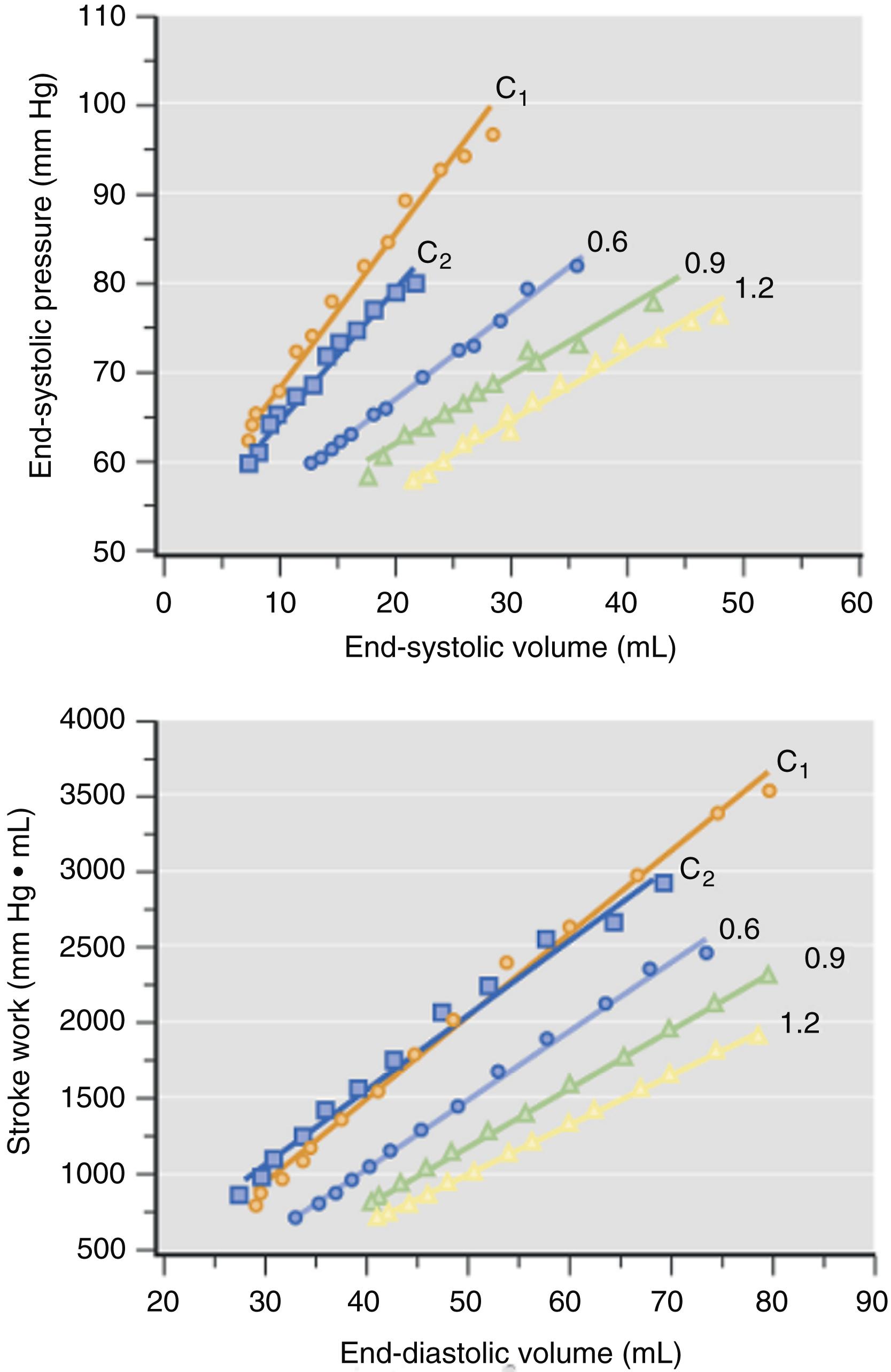
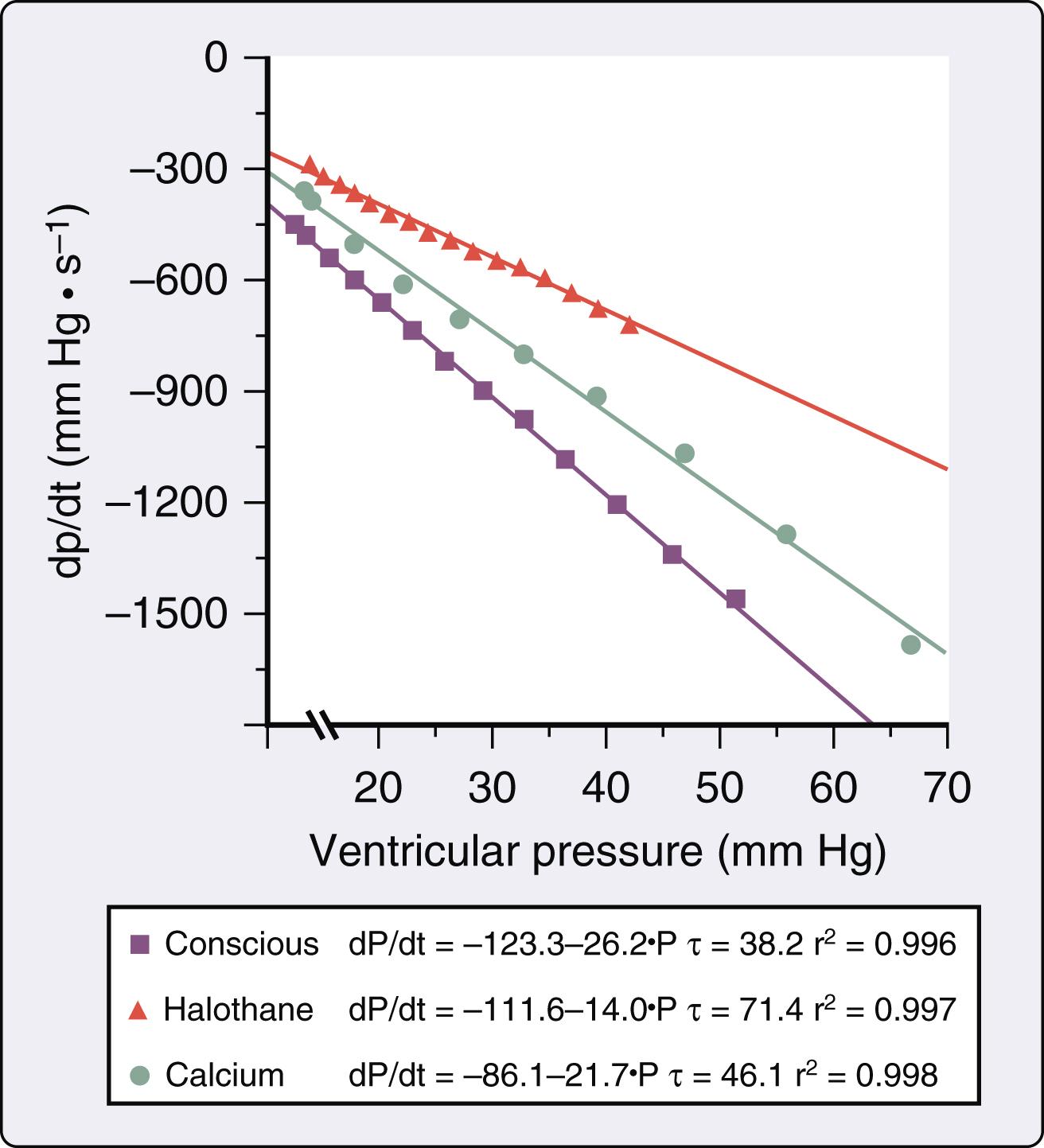
Isoflurane, desflurane, and sevoflurane depress myocardial contractility in vitro and in vivo. Investigations conducted more than 50 years ago showed that halothane causes dose-related depression of force-velocity relations and Frank-Starling curves in isolated cardiac muscle preparations and intact hearts, respectively. These findings accounted for the circulatory depression observed during administration of the volatile anesthetic in humans. Isoflurane also decreased maximal velocity of shortening, peak developed force, and maximal rate of force development during isotonic contraction in isolated papillary muscles consistent with a direct negative inotropic effect. These changes play an important role in determining the anesthetic’s hemodynamic effects in humans. The relative magnitude of myocardial depression produced by volatile anesthetics in vivo is more difficult to establish because simultaneous alterations in systemic and pulmonary hemodynamics and autonomic nervous system activity complicate assessment of contractility. Early studies using isovolumic and ejection phase measures of myocardial contractility (see Chapter 5 ) suggested that halothane and enflurane caused very similar negative inotropic effects, but isoflurane produced less myocardial depression than the older anesthetics. Isoflurane caused smaller reductions in maximum rate of rise of left ventricular pressure (LV +dP/dt max ) than halothane when identical minimum alveolar concentrations (MAC) were directly compared in the presence and absence of autonomic nervous system function, suggesting that differences in myocardial depression caused by these anesthetics occurred independent of autonomic nervous system activity. Differences in the negative inotropic effects of halothane and isoflurane were quantified using relatively heart rate- and load-independent indices of contractility derived from LV pressure-segment length diagrams in a canine model ( Fig. 7.1 ). Contractility was approximately 20% greater during administration of isoflurane compared with halothane at equivalent MAC. Differences in the relative degree of myocardial depression produced by isoflurane and halothane were also inferred in humans using isovolumic and ejection phase measures of contractile state. The negative inotropic actions of volatile anesthetics are exacerbated by hypocalcemia, calcium (Ca 2+ ) channel blockers, and β 1 -adrenoceptor antagonists, and may be reversed by administration of exogenous Ca 2+ , cardiac phosphodiesterase fraction III inhibitors ( Fig. 7.2 ), β 1 -adrenoceptor agonists, Ca 2+ channel agonists, and myofilament Ca 2+ sensitizers. The differential effects of isoflurane and halothane on myocardial contractility are maintained during changes in inotropic state produced by these vasoactive drugs.
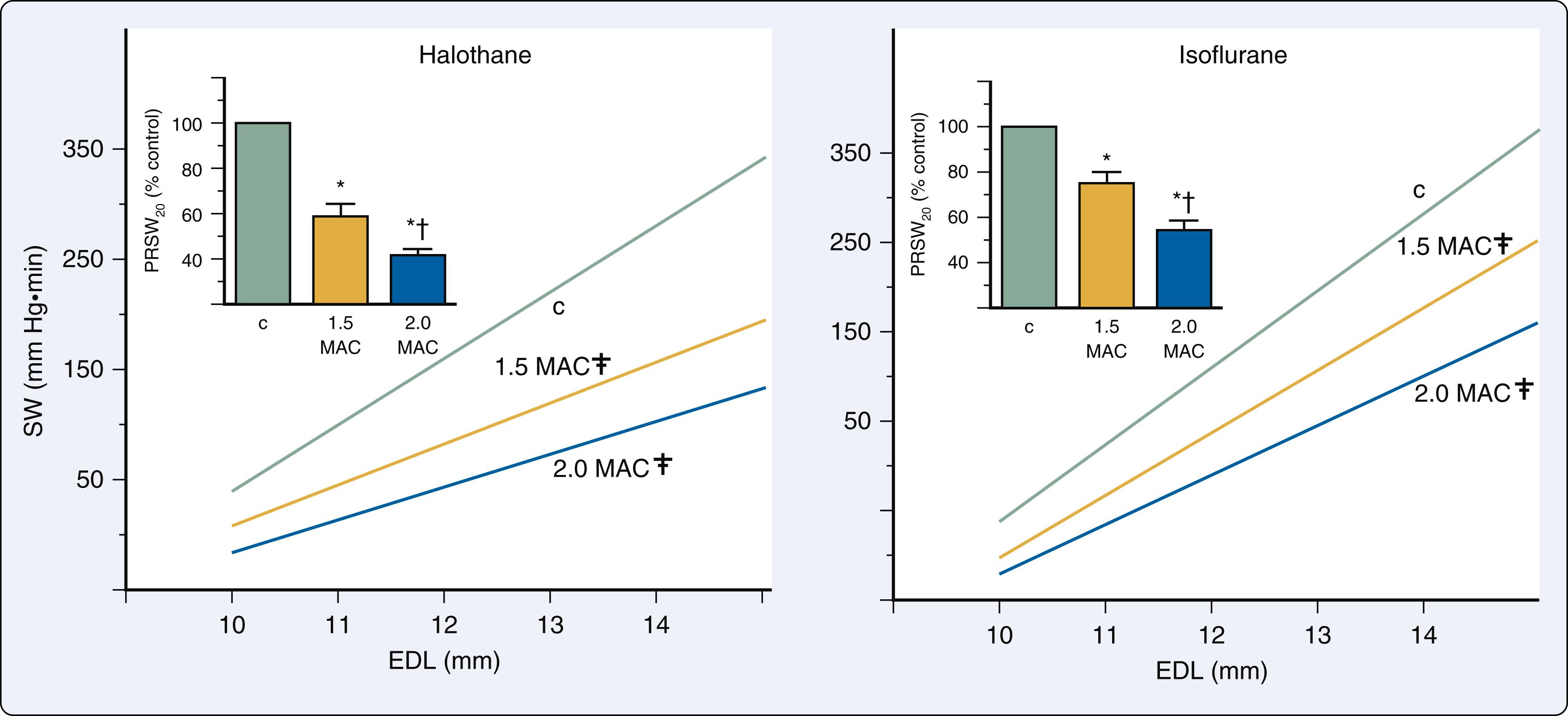
Desflurane causes systemic and coronary hemodynamic effects that are similar to those of isoflurane. Desflurane and isoflurane depressed myocardial function to equivalent degrees using isovolumic and ejection phase measures of contractility. These observations were confirmed using both LV end-systolic pressure-volume relations and preload recruitable stroke work ( Fig. 7.3 ). It is noteworthy that the unique cardiovascular stimulation associated with rapid increases in inspired desflurane concentration in humans may lead to transient increases in myocardial contractility resulting from stimulation of the sympathetic nervous system. The effects of sevoflurane on myocardial contractility are virtually indistinguishable from those produced by isoflurane. Sevoflurane caused less myocardial depression than equivalent MAC concentrations of halothane in pigs, and also maintained contractility to a greater degree than enflurane in humans. Sevoflurane decreased contractile function to approximately 40% to 45% of control values at 1.75 MAC measured using preload recruitable stroke work. This magnitude of myocardial depression is similar to that produced by isoflurane and desflurane using the same canine model. Thus isoflurane, desflurane, and sevoflurane depress contractile state to similar degrees in normal LV myocardium , ( Table 7.1 ).
| Myocardial contractility | Decreased |
| Diastolic function | |
| Isovolumic relaxation | Delayed |
| Early LV filling | Attenuated |
| LV compliance | Unchanged |
| Afterload | |
| Total arterial resistance | Decreased |
| Characteristic aortic impedance | Unchanged |
| Total arterial compliance | Unchanged |
| LV-arterial coupling and mechanical efficiency | Preserved <1 MAC; reduced> 1 MAC |
| LA function | |
| Contractility | Depressed (equal to LV) |
| Reservoir | Preserved <0.9 MAC; reduced> 0.9 MAC |
| Conduit | More important at higher concentrations |
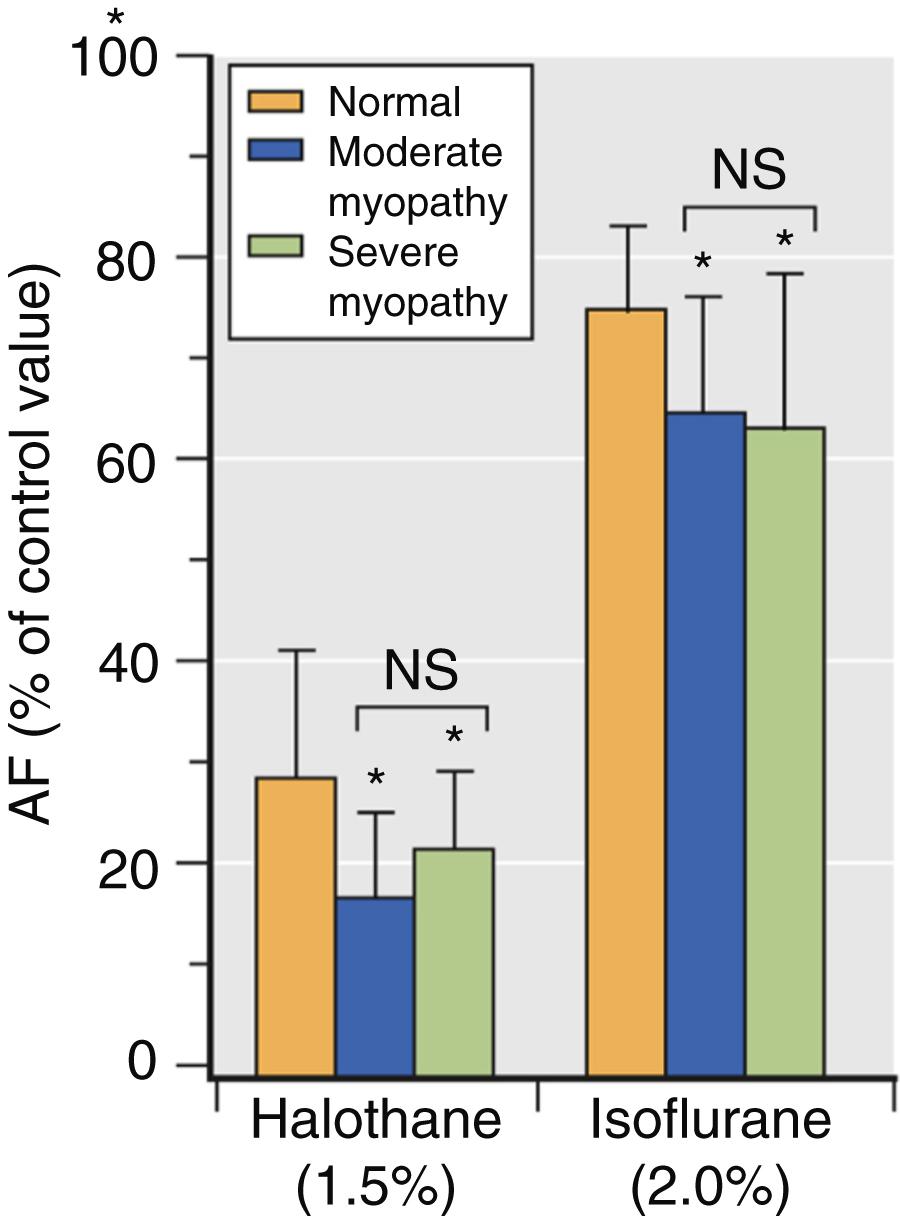
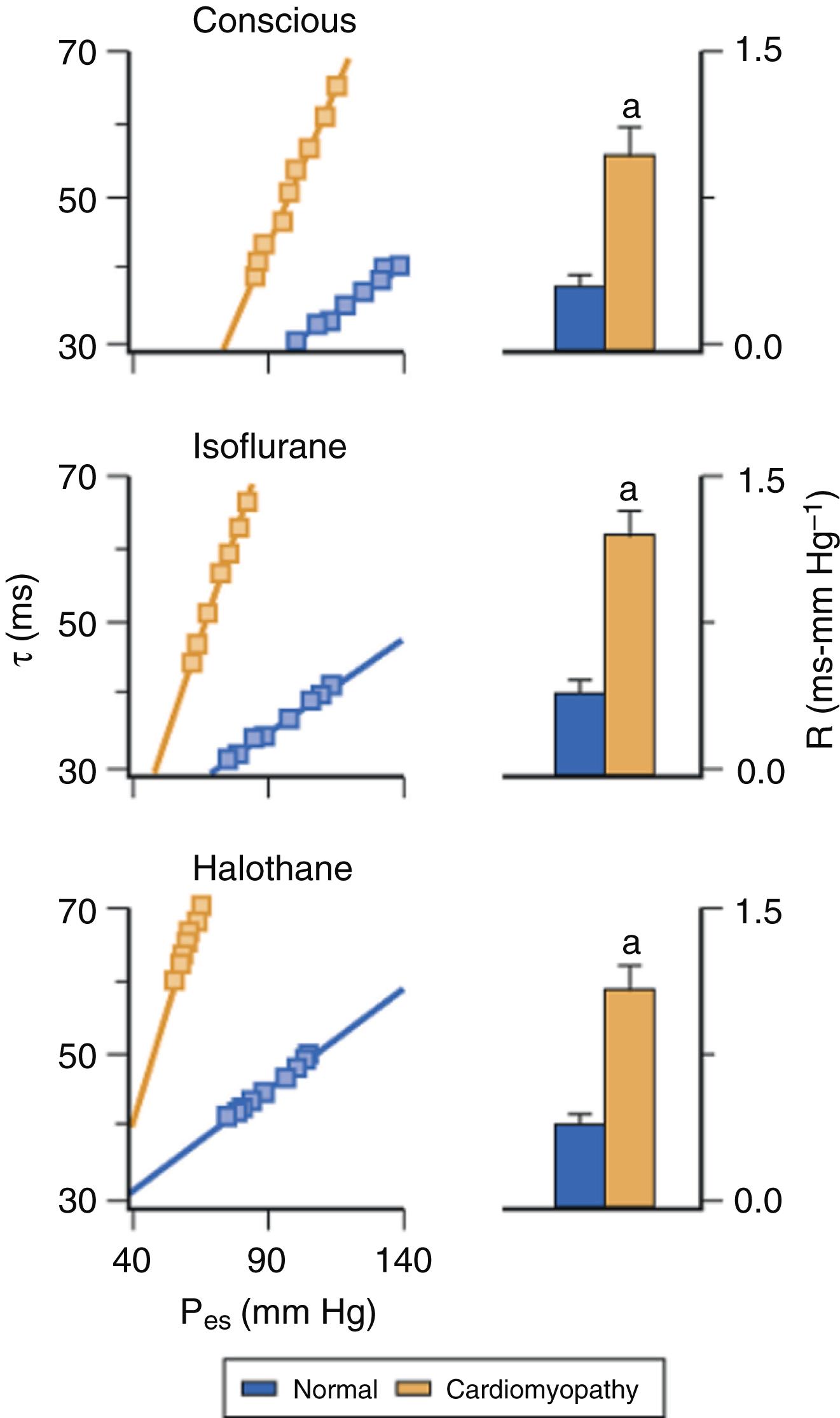
The effects of volatile anesthetics on myocardial contractility in the presence of LV dysfunction have been less extensively studied. An early in vitro study demonstrated that isoflurane causes greater reductions in maximum shortening velocity and the peak rate of force development in papillary muscles from failing compared with normal hearts. Halothane also caused more profound myocardial depression in ischemic compared with normal myocardium. Isoflurane and halothane caused relatively greater negative inotropic effects in models of cardiomyopathy ( Fig. 7.4 ) and pressure-overload hypertrophy. These findings suggested that volatile anesthetic–induced depression of contractility is accentuated in heart failure, and provided indirect evidence that patients with LV systolic dysfunction may be more sensitive to the negative inotropic effects of volatile anesthetics. In contrast, isoflurane, sevoflurane, and desflurane caused similar declines in contractility in ventricular myocytes obtained from rats in the presence and absence of streptozotocin-induced chronic hyperglycemia, an experimental model of diabetic cardiomyopathy.
Despite these laboratory findings, volatile anesthetic–induced declines in contractility are well tolerated and do not precipitate circulatory collapse when heart failure with reduced ejection fraction (HFrEF; see Chapter 5 ) is present. Isoflurane caused dose-related depression of myocardial contractility in a chronic rapid pacing model of LV dysfunction ( Fig. 7.5 ), but profound hypotension was avoided because simultaneous improvements in LV loading conditions and filling dynamics also occurred that contributed to relative maintenance of cardiac output. Similarly, beneficial decreases in LV preload and afterload were observed during administration of isoflurane to patients with heart failure and coronary artery disease. These changes in loading conditions serve to offset the direct negative inotropic effects of volatile anesthetics and preserve cardiac output by improving the operating range of the heart on the Frank-Starling curve and by enhancing LV diastolic function. The effects of isoflurane, desflurane, and sevoflurane on contractility in heart failure with preserved ejection fraction (HFpEF) are unknown, in large part because suitable models of HFpEF have not been developed. Based on the known pathophysiology of HFpEF, it appears likely that administration of a volatile agent may not be as well tolerated in this setting compared with HFrEF because the arterial and LV stiffening that are characteristic features of HFpEF render the circulation substantially more vulnerable to the acute decreases in load and contractility caused by vasodilating negative inotropic drugs.
Volatile anesthetics depress myocardial contractility by altering intracellular Ca 2+ homeostasis at several subcellular targets within the cardiac myocyte ( Box 7.1 ). Volatile anesthetics inhibit Ca 2+ influx through the sarcolemmal membrane by affecting the function of L- and T-type Ca 2+ channels. Halothane caused greater reductions in this intracellular Ca 2+ transient than isoflurane. The structural conformation and functional integrity of the voltage-dependent Ca 2+ channels are directly altered by volatile anesthetics, as indicated by attenuated binding of Ca 2+ channel blockers. The partial inhibition of Ca 2+ influx has three main consequences: (1) less Ca 2+ is available for contractile activation; (2) Ca 2+ -dependent Ca 2+ release from the sarcoplasmic reticulum (SR) is depressed; and (3) the amount of Ca 2+ that can be subsequently stored in the SR is reduced. In contrast to halothane, isoflurane does not directly stimulate Ca 2+ release from SR, activate ryanodine-sensitive SR Ca 2+ release channels, reduce SR Ca 2+ storage, or cause a nonspecific leak of Ca 2+ from the SR. When combined with decreases in Ca 2+ influx, these alterations in SR function represent the main mechanisms by which isoflurane, desflurane, and sevoflurane cause less depression of contractility than halothane. Isoflurane and sevoflurane also inhibit Ca 2+ transport through the sarcolemmal Ca 2+ -adenosine triphosphatase (ATPase), an action that partially offsets any decrease in SR Ca 2+ stores.
Reduction in Ca 2+ availability for contractile activation
Inhibition of sarcolemmal Ca 2+ influx
Alteration of structure and function of voltage-dependent Ca 2+ channels
Attenuation of Ca 2+ -induced Ca 2+ release from sarcoplasmic reticulum
Decrease in sarcoplasmic reticulum storage of Ca 2+
Inhibition of Na + -Ca 2+ exchange
Reduction in myofibrillar ATPase activity (minor)
Decrease in myofilament Ca 2+ sensitivity (minor)
Volatile anesthetics also depress contractility by inhibiting sodium Na + -Ca 2+ exchange and reducing intracellular Ca 2+ concentration independent of the voltage-dependent Ca 2+ channel. This effect may be particularly important in neonatal myocardium, which may be more sensitive to the negative inotropic actions of volatile anesthetics than adult myocardium. The relative contribution of Na + -Ca 2+ exchanger inhibition to anesthetic-induced depression of myocardial contractility in the intact heart is somewhat controversial, but a role for Na + -Ca 2+ exchange inhibition in anesthetic-induced preconditioning was documented. Volatile anesthetics may also exert direct effects on the contractile apparatus and reduce myofilament Ca 2+ sensitivity. Volatile anesthetics decreased tension development of skinned cardiac myofibrils and reduced myofibrillar ATPase activity. These actions contribute to declines in actin-myosin cross-bridge kinetics during contraction, but not through a direct interaction with cardiac cross-bridge mechanics. In addition, volatile anesthetics may modestly reduce myofilament Ca 2+ sensitivity, but this mechanism probably plays a relatively minor role in the negative inotropic effects of these drugs at clinically relevant concentrations in vivo. The cellular mechanisms of volatile anesthetic–induced depression of contractility in failing myocardium have not been thoroughly studied. Isoflurane and sevoflurane cause more pronounced reductions in peak intracellular Ca 2+ concentration and exaggerated decreases in myofilament Ca 2+ sensitivity in ventricular myocytes obtained from hypertrophied compared with normal hearts. Whether similar alterations in intracellular Ca 2+ regulation are produced by volatile anesthetics in other forms of HFrEF or HFpEF remain to be determined. Profound abnormalities in Ca 2+ homeostasis are characteristic features of failing myocardium, and volatile anesthetics may cause further reductions in contractile function by producing additive or synergistic effects on Ca 2+ metabolism.
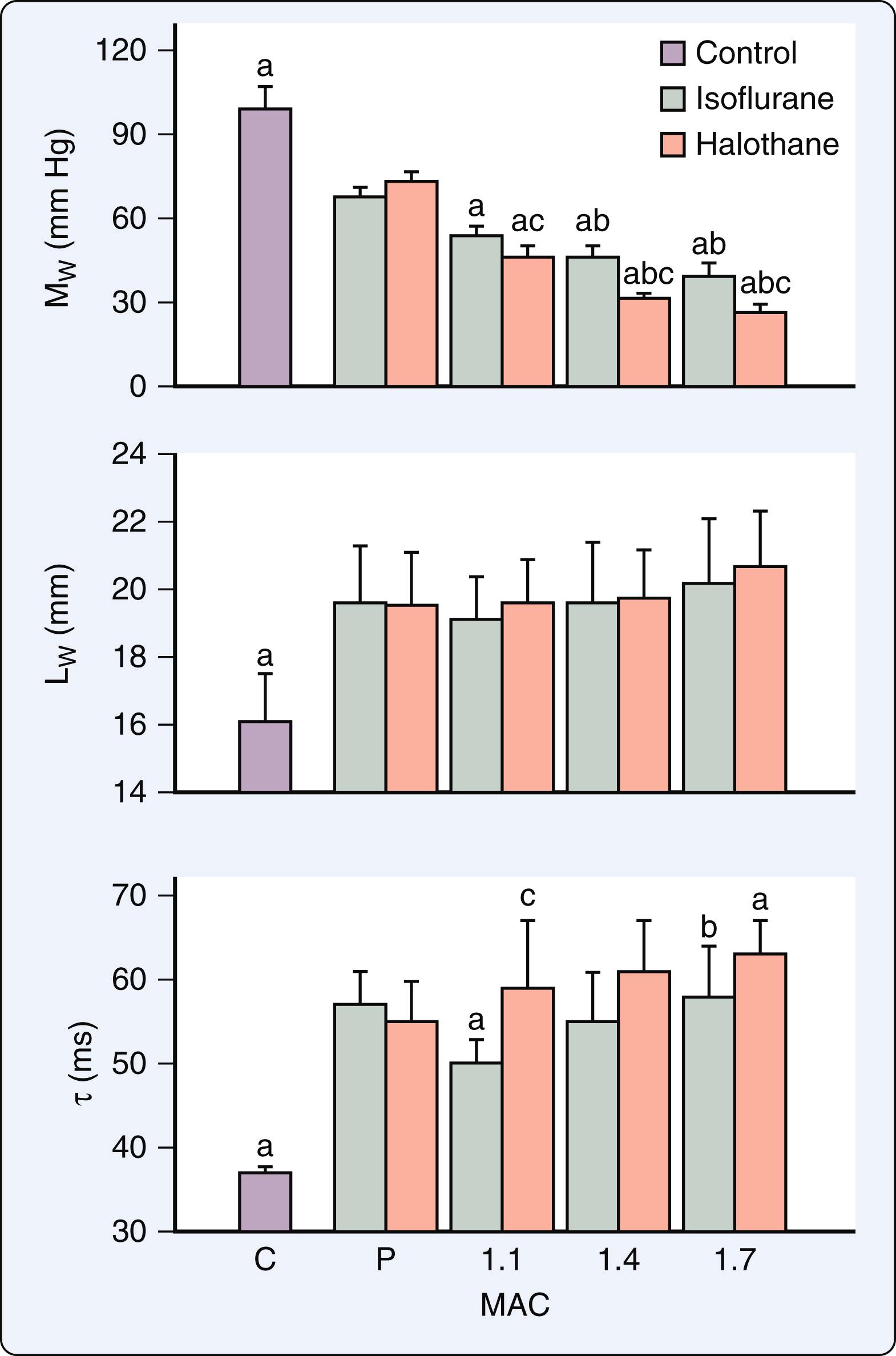
Volatile anesthetics produce dose-related prolongation of LV isovolumic relaxation in vivo ( Fig. 7.6 ). This delay of isovolumic relaxation is associated with declines in early LV filling, but it is not of sufficient magnitude to alter LV chamber stiffness. The delay in LV relaxation may contribute to impaired coronary blood flow during early diastole. Prolonged LV relaxation most likely occurs as a consequence of depressed contractility and not because of a direct negative lusitropic effect, as volatile anesthetics modestly enhance the rate of relaxation of isolated papillary muscle and interventions that increase contractility during anesthesia also simultaneously hasten relaxation ( Fig. 7.7 ). Volatile anesthetics also cause concentration-related decreases in the rate and extent of early LV filling that correlate with their negative inotropic effects. Furthermore, volatile anesthetics reduce LV filling during left atrial (LA) systole by reducing LA contractility. Similar findings were reported in healthy young adults measured with echocardiography. Isoflurane, desflurane, and sevoflurane do not alter invasively derived indices of regional myocardial or chamber stiffness, indicating that the passive mechanical behavior of the LV remains unaffected by these volatile anesthetics.
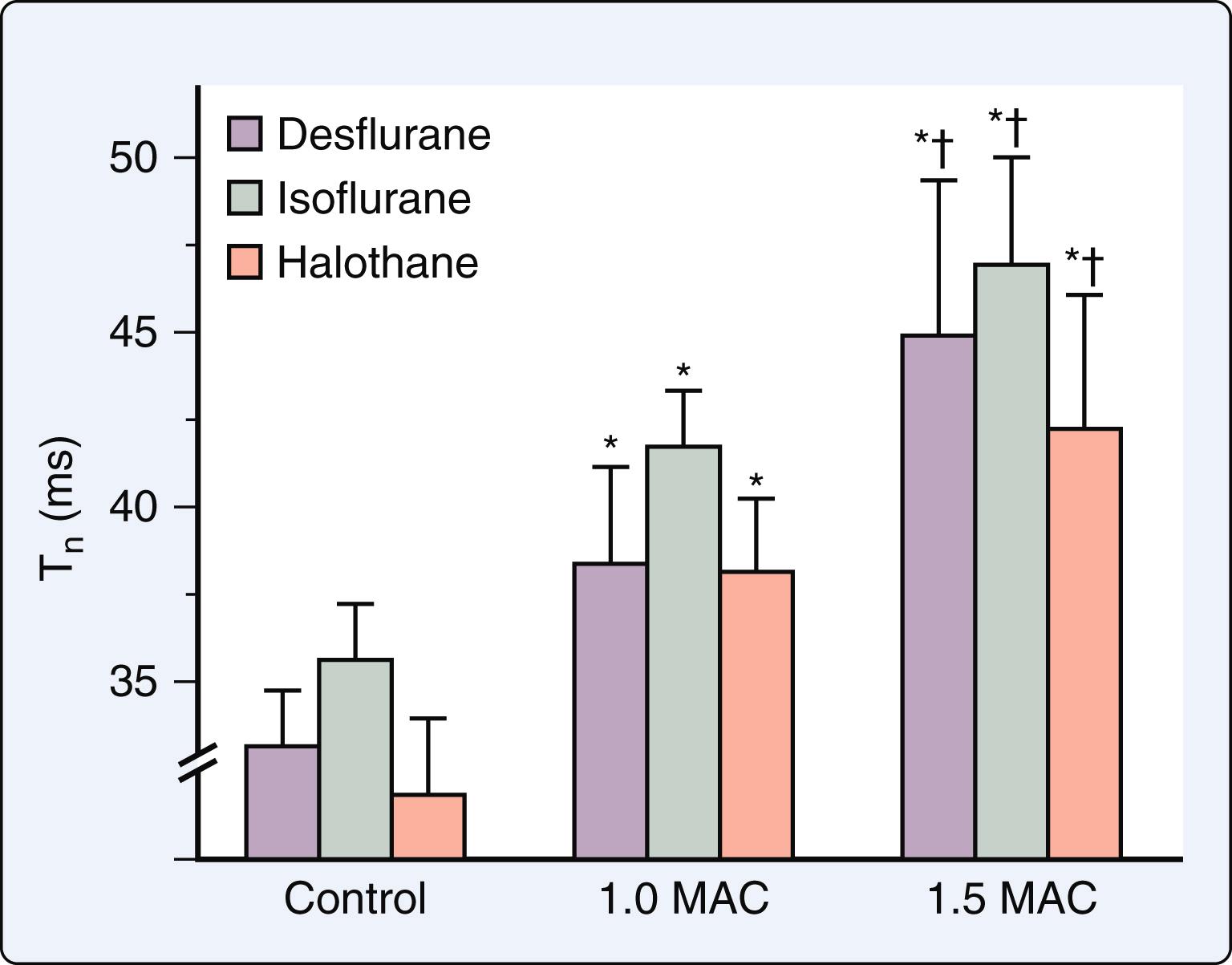
The effects of volatile anesthetics on LV diastolic function were characterized in a canine model of HFrEF. In contrast to the findings in the normal heart, isoflurane enhanced, and halothane did not further impair, indices of LV relaxation and filling in this model despite producing simultaneous negative inotropic effects ( Fig. 7.5 ). Favorable reductions in LV preload were most likely responsible for these results. Isoflurane-induced improvements of LV isovolumic relaxation and filling dynamics are important contributing factors to the relative maintenance of cardiac output observed during administration of this volatile anesthetic when LV contractility is compromised. The findings in this model of HFrEF support clinical observations that patients with HFrEF tolerate volatile anesthetics without evidence of overt hemodynamic decompensation. It is noteworthy that the afterload dependence of LV relaxation was not altered by volatile anesthetics in HFrEF ( Fig. 7.8 ). The afterload dependence of LV relaxation is markedly enhanced in failing myocardium. As a result, medications that reduce afterload (such as volatile anesthetics) not only decrease impedance to LV ejection, but also hasten the rate of LV relaxation and contribute to improvements in LV filling and compliance. The data suggested that volatile anesthetics do not exert direct actions on LV isovolumic relaxation in failing myocardium independent of negative inotropic effects. The actions of volatile anesthetics on LV diastolic function in HFpEF have not been studied to date.
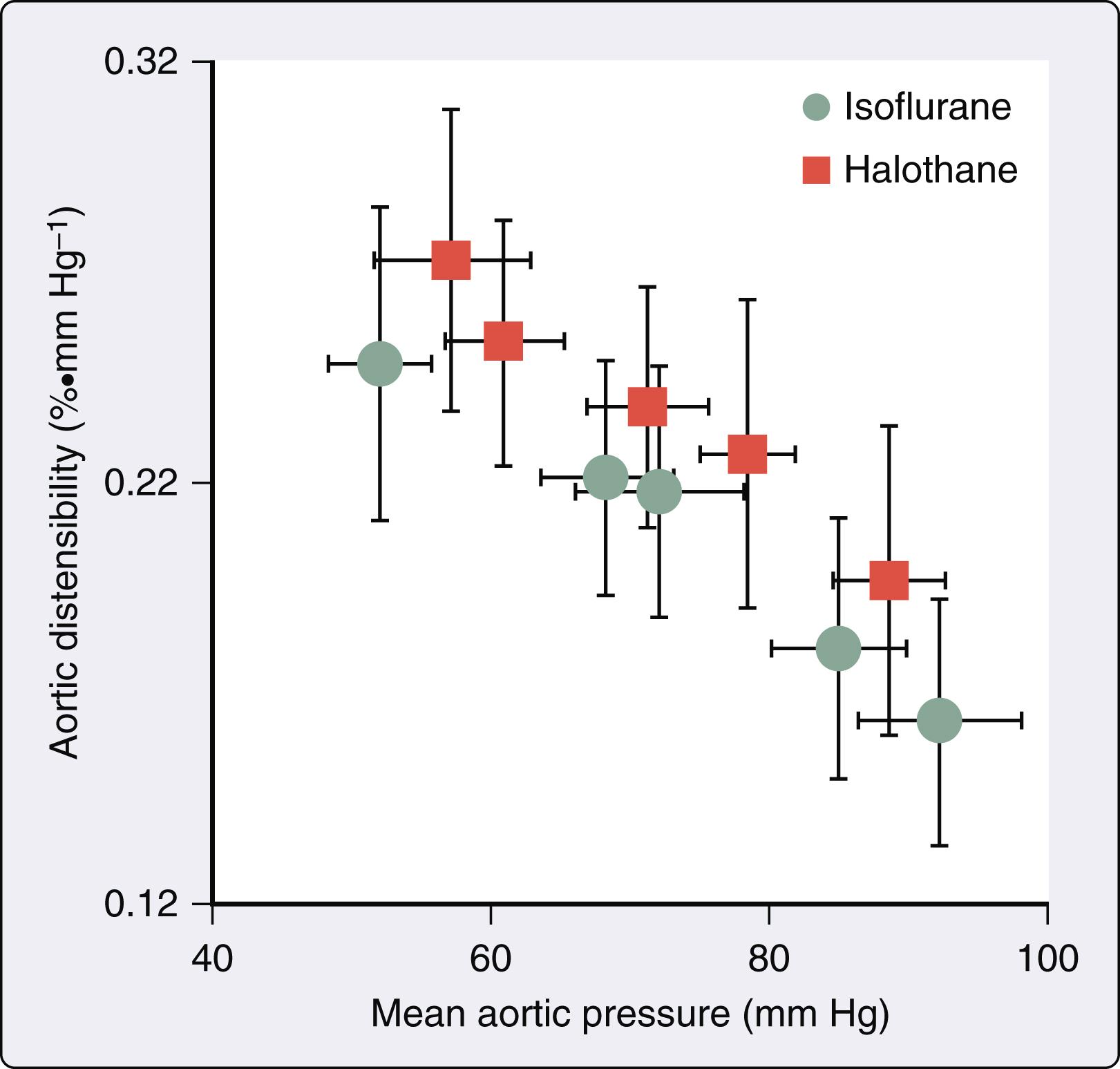
Volatile anesthetics alter aortic input impedance (Z in [ω]; a quantitative index of LV afterload) by affecting the mechanical properties of the arterial vasculature. The Z in (ω) spectrum is frequency-dependent, accounts for the phasic nature of arterial pressure and blood flow, and incorporates arterial wave reflection (see Chapter 5 ). The Z in (ω) spectrum is commonly interpreted using a three-element Windkessel model of the circulation that is composed of total arterial resistance (R), total arterial compliance (C), and characteristic aortic impedance (Z c ). Isoflurane, but not halothane, causes dose-related declines in total arterial resistance, consistent with its known effects on systemic vascular resistance. However, both isoflurane and halothane increased C and Z c concomitant with reductions in mean arterial pressure. An inverse relationship between aortic distensibility and mean arterial pressure was also observed during administration of the volatile anesthetics ( Fig. 7.9 ). Thus the distinction between the action of isoflurane and halothane on LV afterload is related to a differential effect on arteriolar resistance vessels. Desflurane also reduces total arterial resistance to a greater extent than sevoflurane, suggesting that the former anesthetic may be a more potent peripheral arterial vasodilator. The inverse relationship between total arterial compliance and mean arterial pressure remains unchanged in the presence of a volatile anesthetic, unlike the findings with the potent arterial vasodilator sodium nitroprusside. These data emphasize that volatile anesthetics do not fundamentally alter the mechanical characteristics of the aorta and proximal great vessels. Isoflurane and halothane did not produce favorable changes in the determinants of LV afterload in a canine model of HFrEF.
LV-arterial coupling is described using a series elastic chamber model of the cardiovascular system in which the elastances of the LV (E es ) and the arterial circulation (E a ) need to be appropriately matched to assure optimal transfer of stroke volume from the LV to the aorta. LV and arterial elastances are derived from pressure-volume analysis (see Chapter 5 ). LV mechanical efficiency (the ratio of stroke work (SW; kinetic energy) to pressure-volume area [PVA; potential energy]) may also be calculated in pressure-volume phase space. Desflurane, sevoflurane, and isoflurane maintain LV-arterial coupling (ratio of E es to E a ) and mechanical efficiency (ratio of SW to PVA) at lower anesthetic concentrations (<0.9 MAC) by producing nearly proportional declines in contractility and afterload ( Fig. 7.10 ). However, mechanical matching between the LV and the arterial vasculature and the efficiency of total LV energy transfer to external stroke work both degenerate at higher concentrations, indicating that anesthetic-induced reductions in contractility are no longer balanced by declines in afterload. These detrimental alterations in LV-arterial coupling and mechanical efficiency contribute to reductions in overall cardiac performance observed with higher concentrations of the anesthetics.
![Figure 7.10, Histograms depicting LV-arterial coupling (E es /E a ; top) and mechanical efficiency (stroke work [ SW ]/pressure-volume area [ PVA ]); bottom ) before (control 1; C 1 ), during 0.6, 0.9, and 1.2 minimum alveolar concentration (MAC) , and after desflurane (control 2; C 2 ). a Significantly ( P < .05) different from C 1 ; b Significantly ( P < .05) different from 0.6 MAC desflurane. Figure 7.10, Histograms depicting LV-arterial coupling (E es /E a ; top) and mechanical efficiency (stroke work [ SW ]/pressure-volume area [ PVA ]); bottom ) before (control 1; C 1 ), during 0.6, 0.9, and 1.2 minimum alveolar concentration (MAC) , and after desflurane (control 2; C 2 ). a Significantly ( P < .05) different from C 1 ; b Significantly ( P < .05) different from 0.6 MAC desflurane.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/CardiovascularPharmacologyofAnesthetics/9_3s20B9780323829243000077.jpg)
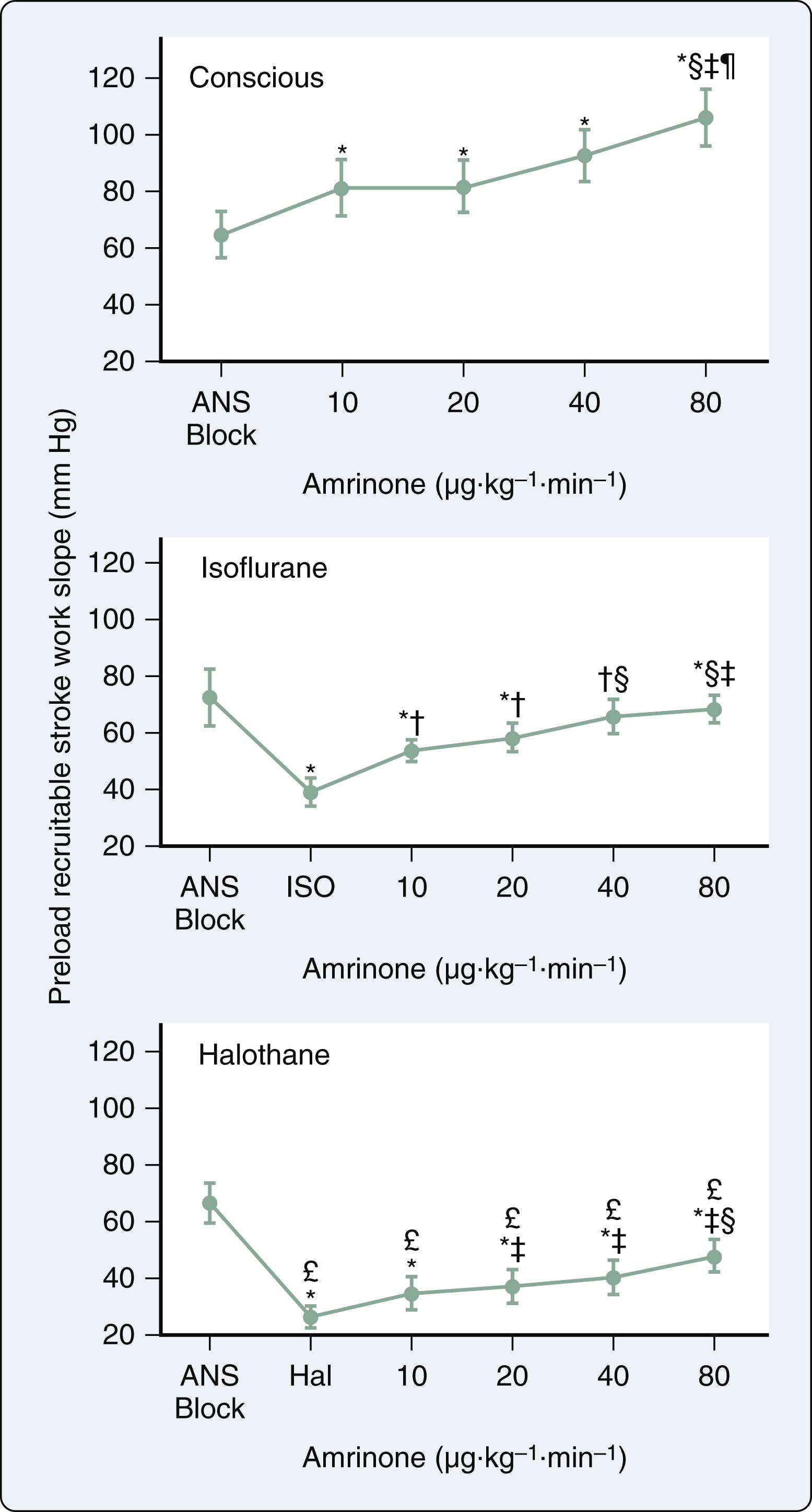
The LA has three major functions: (1) contractile chamber that actively empties immediately before the onset of LV systole and establishes final LV end-diastolic volume; (2) reservoir that stores pulmonary venous return when the mitral valve is closed; and (3) conduit that empties its contents into the LV down a pressure gradient after the mitral valve opens and continues to passively transfer pulmonary venous blood flow throughout LV diastasis. Volatile anesthetics caused direct negative inotropic effects in human atrial myocardium. Not surprisingly, the intracellular mechanisms for anesthetic-induced depression of contractility in atrial myocardium are virtually indistinguishable from those observed in ventricular myocardium. Desflurane, sevoflurane, and isoflurane reduced LA contractility by approximately 50% at an end-tidal concentration of 1.2 MAC when evaluated using pressure-volume analysis in the intact heart ( Fig. 7.11 ). The magnitude of this negative inotropic effect in the LA was very similar to that observed in LV. Desflurane, sevoflurane, and isoflurane also impaired LA and LV relaxation to equivalent degrees. The volatile anesthetics also affect the passive mechanical behavior of the LA. Reservoir function was preserved during the administration of less than 1 MAC of volatile anesthetic, which contributed to the relative maintenance of LV stroke volume by compensating for decreases in LV filling associated with a diminished contribution of LA contraction. In addition, the volatile anesthetics reduce dynamic LA chamber stiffness, which most likely contributes to the preservation of reservoir function because the delays in LA relaxation and declines in LV systolic function that also occur during administration of a volatile anesthetic would be expected to decrease reservoir function. Nevertheless, LA reservoir function is ultimately reduced at higher concentrations because further impairment of LA relaxation and LV contractility occurs.
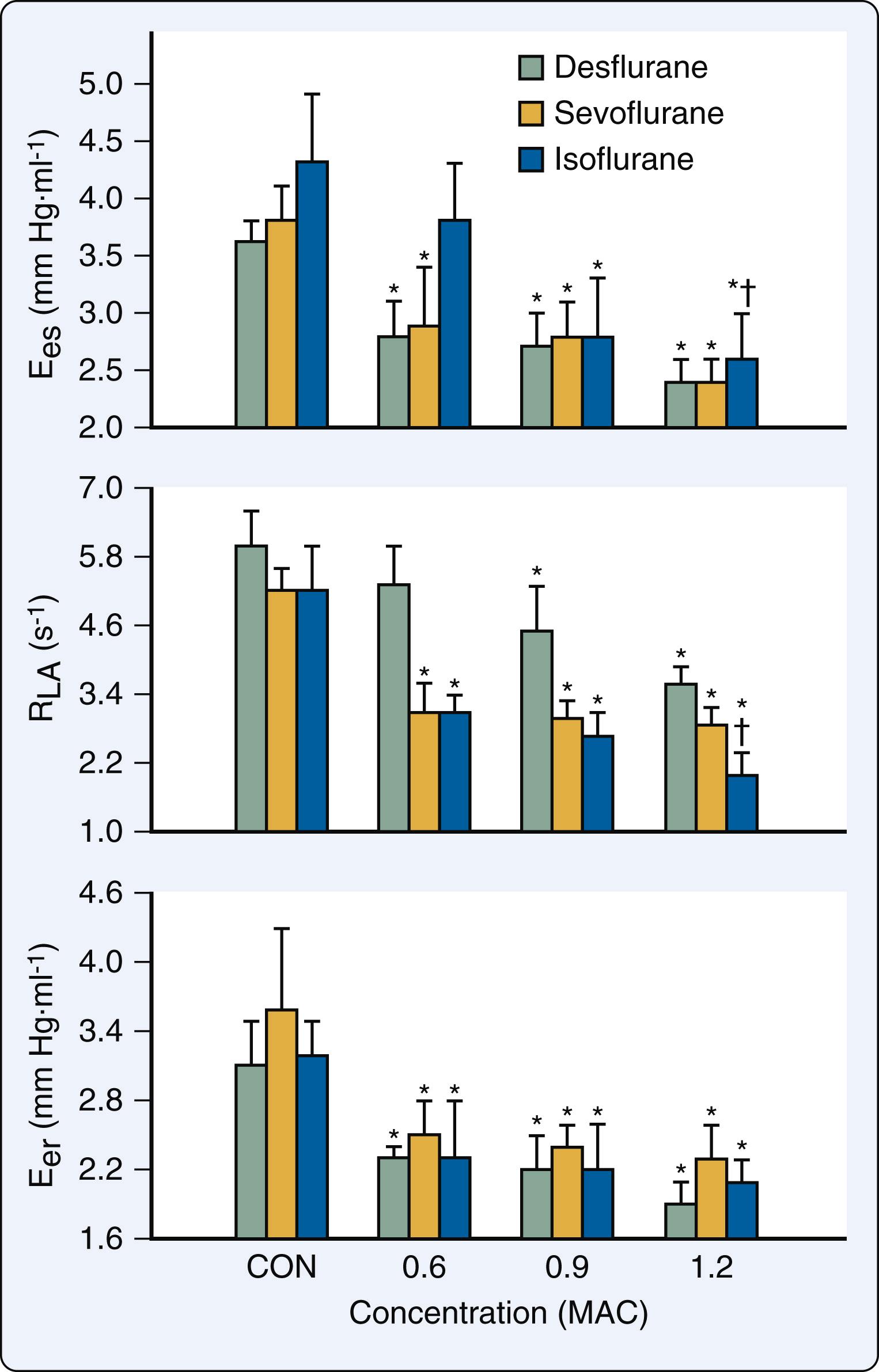
Desflurane, sevoflurane, and isoflurane cause decreases in the ratio of LA SW to PVA (mechanical efficiency) and increases in the ratio of LA conduit to total reservoir volume. These data indicate that the LA contribution to LV filling becomes less active and more passive during the administration of the volatile anesthetics. Furthermore, desflurane, sevoflurane, and isoflurane decrease the ratio of LA to LV elastances (E es /E LV ), consistent with impaired mechanical matching between these chambers. As described earlier, volatile anesthetics delay LV isovolumic relaxation and impair early LV filling concomitant with direct negative inotropic effects. Thus the attenuation of transfer of kinetic energy from the LA to the LV probably results from the combination of LA contractile depression and LV diastolic dysfunction. Volatile anesthetic–induced abnormalities in LA-LV matching are greater than analogous impairment of LV-arterial coupling because the anesthetics produced beneficial alterations in the determinants of LV afterload that partially compensate for simultaneous depression of LV myocardial contractility.
Isoflurane depressed LA contractility, impaired LA-LV coupling, and reduced the active LA contribution to LV filling in a model of HRrEF, findings that were similar in magnitude to those observed in normal hearts. In contrast, LA reservoir function was reduced in HFrEF at lower isoflurane concentrations (0.6 and 0.9 MAC), suggesting that the ability of the LA to act as a reservoir for pulmonary venous return is attenuated in heart failure. This isoflurane-induced reduction in LA storage ability suggests that quantity of blood transferred from the LA to the LV upon mitral valve opening may be reduced. These observations implicate another potential mechanism (in addition to delayed LV relaxation and reduced LV compliance) by which volatile anesthetics reduce early LV filling in HFrEF. Profound LA dysfunction is a characteristic feature of HFpEF, and it is highly likely that the consequences of volatile anesthetics on LA contractile and reservoir function will exacerbate this condition. This hypothesis has yet to be formally tested.
Volatile anesthetics slow the rate of sinoatrial node discharge by affecting sinoatrial node automaticity ( Box 7.2 ). These actions may be altered by vasoactive drugs or autonomic nervous system activity. Halothane and, to a lesser extent, isoflurane shorten cardiac action potential and the effective refractory period duration in normal Purkinje fibers, but these medications also prolong His-Purkinje and ventricular conduction times. In addition, halothane and isoflurane prolong atrioventricular (AV) conduction time and refractory period. Despite these findings, primary disturbances in AV conduction leading to second- or third-degree AV block do not occur with volatile anesthetics without preexisting conduction disease or concurrent administration of drugs that directly prolong AV conduction time. Volatile anesthetics may exert pro- or anti-arrhythmogenic actions against abnormal electrophysiology resulting from myocardial ischemia or infarction by opposing subsidiary pacemaker activity. For example, halothane and isoflurane protect against ventricular fibrillation produced by coronary artery occlusion and reperfusion through this mechanism. Conversely, volatile anesthetics may be proarrhythmogenic in Purkinje fibers in ischemic myocardium by facilitating reentrant activity or increasing temporal dispersion of refractory period recovery. Inhibition of the slow Na + current in false tendon fibers and induction of reentry of premature impulses into more refractory Purkinje fibers in the border zone of an ischemic area are proposed mechanisms for this effect. Halothane and isoflurane prolong the QT c interval, suggesting that patients with idiopathic or acquired long QT syndrome may be at greater risk of developing torsade de pointes ventricular tachycardia during administration of a volatile anesthetic.
Slow rate of SA node discharge
Prolong AV nodal conduction time and refractory period
Prolong QT c interval
Sensitize myocardium to pro-arrhythmic actions of epinephrine (isoflurane, desflurane, and sevoflurane < halothane)
Sensitization is defined as the interaction between a catecholamine and a volatile anesthetic that reduces the threshold for atrial or ventricular arrhythmias to occur. Epinephrine is known to stimulate premature ventricular contractions and facilitate the development of sustained ventricular tachyarrhythmias in a dose-related manner during halothane anesthesia. A synergistic interaction between α 1 - and β 1 -adrenoceptors has been strongly implicated in the pathogenesis of these epinephrine-induced ventricular arrhythmias. Stimulation of the α 1A -adrenoceptor in the His-Purkinje system by epinephrine transiently slows Purkinje fiber conduction during administration of halothane to facilitate an arrhythmogenic substrate. Halothane, and to a lesser extent, other volatile anesthetics, sensitize myocardium to the proarrhythmogenic effects of epinephrine. The dose of epinephrine required to produce ventricular arrhythmias during desflurane or sevoflurane anesthesia is substantially greater than that required with halothane. Catecholamine sensitization also promotes abnormal automaticity of dominant and latent atrial pacemakers during administration of volatile anesthetics. These effects may produce premature ventricular contractions and arrhythmias originating from the His bundle, but intact sinoatrial node function substantially attenuates this arrhythmogenic effect.
Studies conducted in animal models demonstrated that volatile anesthetics depress baroreceptor reflex control of arterial pressure to varying degrees. This inhibition occurs at multiple sites in the reflex pathway: (1) depression of central nervous system integration of afferent baroreceptor input; (2) attenuation of efferent autonomic nervous system activity; and (3) reductions in ganglionic transmission and end-organ response. Volatile anesthetics increased resting afferent nerve traffic and enhanced the sensitivity of arterial baroreceptors by a Ca 2+ -dependent mechanism. These increases in baroreceptor sensitivity and discharge frequency tonically reduce overall sympathetic nervous system activity and attenuate sympathetic responses to hypotension. Isoflurane and halothane were shown to inhibit pre- and postganglionic sympathetic nerve activity at clinically relevant concentrations, suggesting that attenuation of ganglionic transmission represents a major mechanism by which these anesthetics depress sympathetic nerve traffic. A reduction of sympathetic outflow caused by volatile anesthetics is also demonstrated when endogenous plasma norepinephrine kinetics are considered. Isoflurane and halothane decrease plasma norepinephrine concentrations to varying degrees by causing a more pronounced decline in norepinephrine spill over than clearance. Volatile anesthetics may also attenuate parasympathetic nervous system function. Halothane depressed vagal nerve efferent activity by direct measurement of parasympathetic nerve activity. Volatile anesthetics also inhibit reflex bradycardia in response to increases in arterial pressure. Parasympathetic and sympathetic nervous system outflows appear to be depressed to equivalent degrees during halothane or isoflurane anesthesia.
The effects of volatile anesthetics on neural control of the cardiovascular system have been incompletely examined in healthy humans and have not been described in detail in patients with autonomic nervous system dysfunction. Halothane produced greater attenuation of baroreceptor reflex regulation of heart rate than did isoflurane. Depression of baroreceptor function by halothane may be greater than that produced by fentanyl, diazepam, and nitrous oxide. Baroreceptor-mediated control of peripheral arteriolar tone was also attenuated in young volunteers during halothane anesthesia. Sevoflurane was shown to cause greater depression of sympathetic nerve activity (measured directly with microneurography) in response to hypotension compared with desflurane. These findings were consistent with those demonstrating pronounced sympathetic hyperactivity during rapid increases in inspired desflurane or isoflurane concentration in humans. Importantly, the actions of volatile anesthetics on baroreceptor reflex control of the circulation may be profoundly altered during autonomic dysfunction in elderly patients or those with essential hypertension, diabetes mellitus, or heart failure.
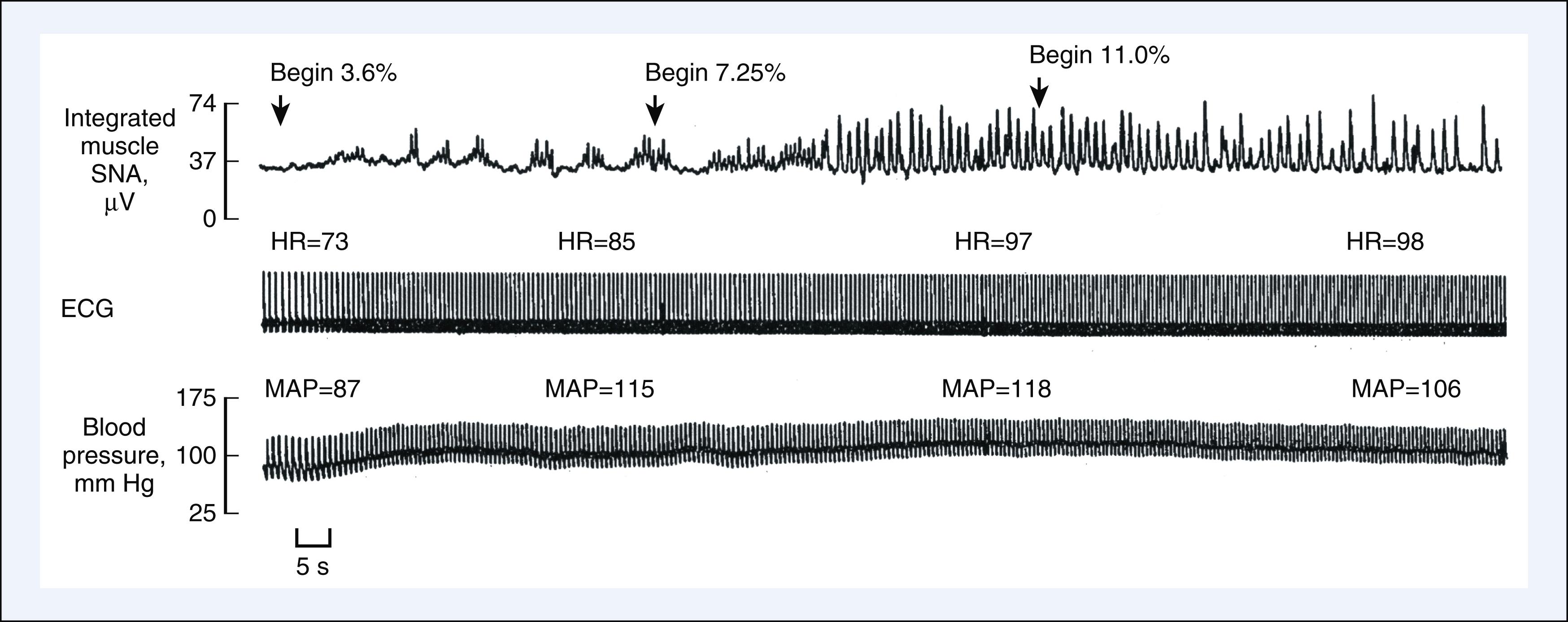
Volatile anesthetics cause direct negative chronotropic actions in vitro by depressing sinoatrial node activity, but alterations in heart rate in the intact circulation are primarily determined by their interaction with baroreceptor reflexes ( Box 7.3 ). Halothane did not appreciably change heart rate in humans because this anesthetic attenuated baroreceptor reflex responses to hypotension. In contrast, isoflurane and desflurane increase heart rate in response to hypotension because baroreceptor reflexes are relatively preserved. Desflurane- and isoflurane-induced tachycardia may be especially prominent in children and young adults or in the presence of vagolytic drugs and, conversely, may be attenuated in neonates and geriatric patients or by the concomitant administration of opioids. Rapid increases in the inspired desflurane concentration above 1 MAC may cause marked transient increases in heart rate and arterial pressure resulting from sympathetic nervous system activation ( Fig. 7.12 ). Tachycardia and hypertension may also be observed when the inspired isoflurane concentration is rapidly increased. The cardiovascular stimulation induced by rapid increases in desflurane or isoflurane concentration in humans results from activation of tracheopulmonary receptors and is attenuated by pretreatment with β 1 -adrenoceptor antagonists, α 2 -adrenoceptor agonists, or opioids. In contrast to desflurane and isoflurane, sevoflurane does not alter heart rate or cause cardiovascular stimulation during rapid increases in anesthetic concentration.
Tachycardia (I, D > S)
Preservation of baroreceptor reflexes and autonomic nervous system regulation of the circulation (I, D > S)
Arterial vasodilation and reduced systemic vascular resistance
Hypotension
Stable cardiac output
Modest decrease in stroke volume
Modest decrease in myocardial contractility
Temporal recovery from circulatory depression
Cardiovascular stimulation during rapid increases in inspired I or D concentration
Volatile anesthetics cause dose-dependent decreases in arterial pressure, the mechanism of which differs between anesthetics. Hypotension produced by halothane was attributed primarily to reductions in myocardial contractility and cardiac output, but isoflurane, desflurane, and sevoflurane reduce arterial pressure mostly as a consequence of reductions in LV afterload. These anesthetics generally maintain cardiac output because they produce less myocardial depression and greater decreases in systemic vascular resistance. Isoflurane and desflurane also preserve autonomic nervous system regulation of the circulation to a greater degree than other volatile anesthetics. The baroreceptor reflex–mediated tachycardia that occurs during isoflurane and desflurane anesthesia serves to maintain cardiac output despite modest decreases in contractility and stroke volume. The decreases in arterial pressure produced by volatile anesthetics may be attenuated by surgical stimulation or concomitant administration of nitrous oxide. Volatile anesthetics also cause modest, dose-related increases in right atrial pressure. The cardiovascular effects of volatile anesthetics vary with the duration of anesthesia. Increases in contractility and cardiac output and decreases in LV preload and afterload occur after several hours of constant MAC anesthesia. Temporal recovery from circulatory depression was greatest during halothane anesthesia, but this phenomenon also occurs to a lesser extent during prolonged administration of isoflurane and desflurane.
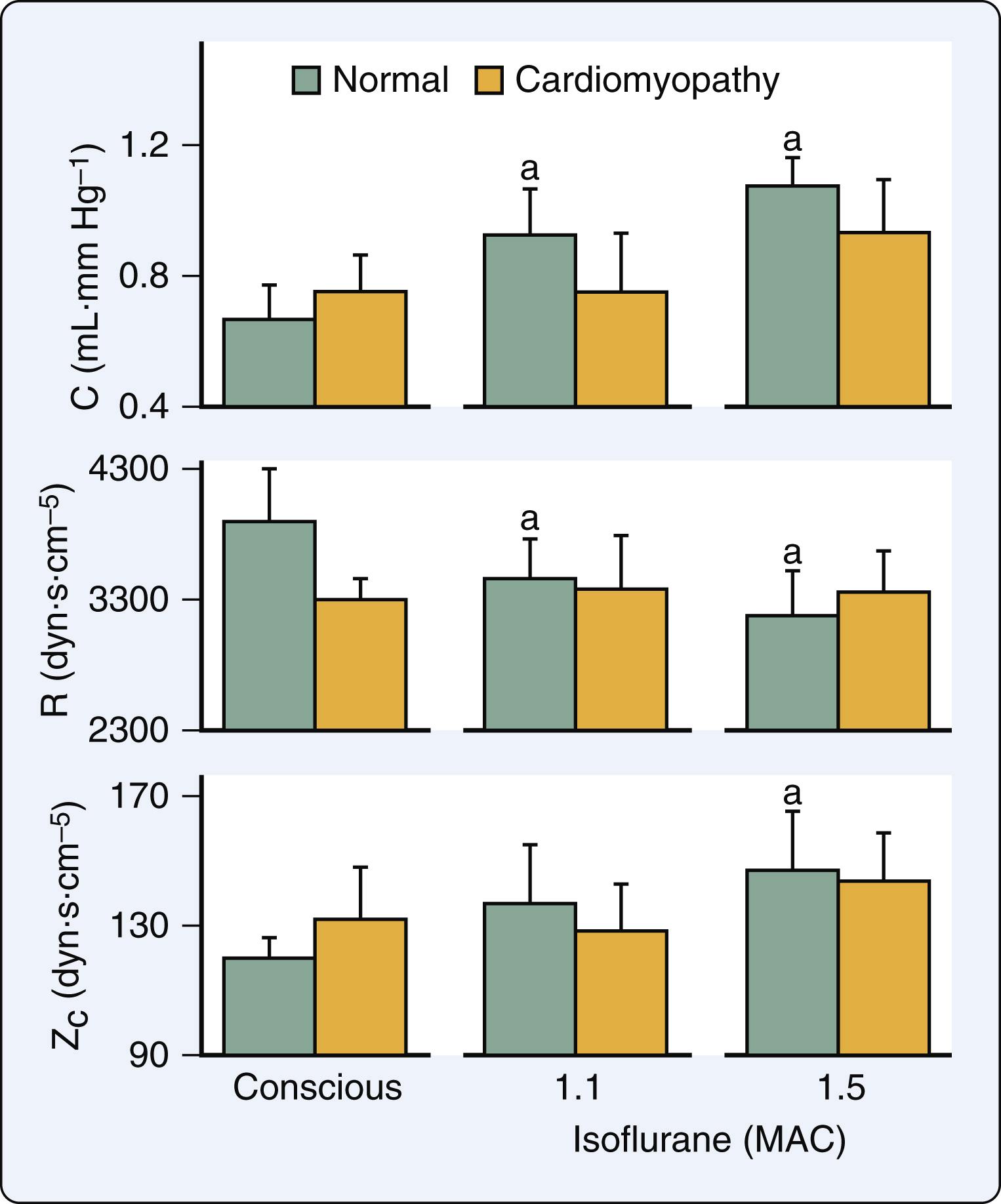
The systemic hemodynamic effects of volatile anesthetics in the presence of LV systolic dysfunction are similar but not identical to those observed in the normal heart. Volatile anesthetics, including isoflurane, modestly increase or do not affect the heart rate in chronic rapid pacing- or doxorubicin-induced models of HFrEF and in patients with coronary artery disease and LV dysfunction. These findings may be attributed to the alterations in baroreceptor reflex activity, β 1 -adrenoceptor downregulation, increases in central sympathetic nervous system activity, and withdrawal of parasympathetic nervous system tone that are associated with heart failure. Isoflurane markedly reduced LV end-diastolic pressure and chamber dimensions in an experimental model of HFrEF. Similar declines in pulmonary artery pressures were observed during isoflurane anesthesia in patients with coronary artery disease and HFrEF. These findings emphasize that venodilation is a predominant hemodynamic feature of isoflurane in HFrEF. In contrast to the findings in normal hearts, isoflurane did not cause favorable reductions in total arterial resistance quantified using aortic input impedance in a model of HFrEF ( Fig. 7.13 ). As a result of these actions and the simultaneous declines in LV preload and contractility, cardiac output may be reduced to a greater extent during isoflurane anesthesia when preexisting LV dysfunction is present.
Volatile anesthetics are direct coronary artery vasodilators, but the effects of these medications on the intact coronary circulation are substantially more complex. Volatile anesthetics reduce myocardial oxygen demand as a result of their hemodynamic consequences. These actions decrease coronary blood flow through metabolic autoregulation. Volatile anesthetics also reduce coronary perfusion pressure, which is another essential determinant of coronary blood flow. These observations should make it abundantly clear to the reader that a combination of direct and indirect actions determine the net effect of volatile anesthetics on coronary vascular tone. All volatile anesthetics cause vasodilation of isolated coronary arteries. Halothane produced greater coronary artery dilation than did isoflurane at equivalent MAC concentrations in isolated coronary arteries larger than 2000 μm, whereas isoflurane preferentially causes vasodilation of predominantly small (<900 μm) epicardial coronary arteries. Differential suppression of voltage-dependent Ca 2+ current by halothane compared with isoflurane may account for these disparities in vasodilation on the basis of vessel diameter.
The direct negative inotropic effects of volatile anesthetics caused a decline in coronary blood flow in isolated, contracting hearts during precise control of loading conditions through flow-metabolism coupling. As a result, the decrease in myocardial oxygen demand associated with depression of myocardial contractility was accompanied by an apparent increase in coronary vascular resistance (the ratio of pressure to flow) in this model. Such an observation might be incorrectly perceived as coronary vasoconstriction. However, examination of the actions of volatile anesthetics on myocardial oxygen extraction and the ratio of myocardial oxygen delivery to consumption indicated that these medications are coronary vasodilators. Halothane and isoflurane decreased myocardial oxygen extraction and increased the ratio of oxygen delivery to consumption in isolated hearts. These findings indicated that volatile anesthetics produce direct coronary vasodilation because myocardial oxygen delivery exceeds consumption and coronary sinus oxygen tension increases (luxuriant perfusion). Halothane, isoflurane, and sevoflurane also caused equivalent reductions in adenosine-induced coronary flow reserve in tetrodotoxin-arrested, isolated hearts. Since mechanical work was not performed in this preparation, these findings supported the hypothesis that volatile anesthetics cause direct coronary vasodilation of similar magnitude.
Halothane has variable effects on coronary blood flow and coronary vascular resistance in vivo that occur concomitant with changes in myocardial oxygen demand. Halothane-induced decreases in demand caused declines in coronary blood flow with relative maintenance or modest increases in coronary vascular resistance. Despite these absolute decreases in coronary blood flow, halothane also increased coronary sinus oxygen tension and reduced oxygen extraction by the myocardium, indicating that halothane was a relatively weak coronary vasodilator in the intact heart. Isoflurane also variably alters coronary blood flow. Isoflurane reduced myocardial oxygen consumption and simultaneously decreased oxygen extraction, indicating direct coronary vasodilation. Isoflurane also produced mild, transient increases in blood flow independent of changes in myocardial oxygen consumption and autonomic nervous system activity during inhalation induction. Perfusion of the left anterior descending coronary artery with blood previously equilibrated with isoflurane dramatically increased coronary blood flow, but only mild coronary vasodilation occurred after a period of anesthetic equilibration in this model. The increases in coronary blood flow produced by isoflurane were not accompanied by epicardial coronary artery dilation, confirming that isoflurane dilates predominantly small coronary arteries. In contrast, the potent coronary vasodilator adenosine caused considerably greater vasodilation in coronary microvessels than isoflurane. Desflurane and isoflurane caused similar increases in the ratio of oxygen delivery to consumption and decreases in oxygen extraction, consistent with coronary vasodilation. However, the increases in coronary blood flow produced by desflurane, but not isoflurane, were attenuated by pharmacological blockade of the autonomic nervous system, suggesting that isoflurane causes relatively greater direct coronary vasodilation in vivo than desflurane. In contrast to the findings with isoflurane and desflurane, sevoflurane did not cause significant coronary vasodilation.
Coronary vasodilator reserve is defined as the ratio of peak coronary blood flow after brief coronary artery occlusion (reactive hyperemia) to baseline flow. Volatile anesthetics differentially affect this phenomenon. Coronary vasodilator reserve was greater during isoflurane compared with halothane anesthesia. This observation suggested the erroneous interpretation that halothane may be a more potent coronary vasodilator than isoflurane because greater baseline coronary vasodilation should be accompanied by a reduced ability to further increase coronary blood flow in response to a brief ischemic episode. However, halothane also reduced the determinants of myocardial oxygen demand to a greater degree than isoflurane. Peak coronary blood flow during reactive hyperemia and percent flow debt repayment are directly related to the intensity of the ischemic stimulus and the magnitude of oxygen debt accumulated during coronary artery occlusion. Thus differences in coronary vasodilator reserve produced by isoflurane and halothane most likely reflected differences in ischemic burden during coronary occlusion and not the relative magnitude of vasodilation caused by these two volatile anesthetics.
Pressure-flow autoregulation refers to the intrinsic ability of the coronary vascular bed to maintain constant blood flow during variations in perfusion pressure. Dilation of coronary arteriolar resistance vessels by volatile anesthetics alters pressure-flow autoregulation in the coronary vasculature. Changes in autoregulation produced by vasoactive drugs are determined by the slope of the pressure-flow curve generated by progressive constriction of a coronary artery. Volatile anesthetics differentially affect the pressure-flow relationship based on their relative potency as vasodilators ( Fig. 7.14 ). Thus isoflurane produced more profound alterations in autoregulation because it causes greater vasodilation than the older volatile anesthetics as indicated by a greater increase in the slope of the pressure-flow relationship. Nevertheless, the impairment of pressure-flow autoregulation produced by isoflurane and other volatile anesthetics was small compared with that caused by adenosine. In contrast to volatile anesthetics, adenosine caused maximal coronary vasodilation and inhibited pressure autoregulation to such a degree that coronary blood flow became a direct function of coronary perfusion pressure. Thus volatile anesthetics are relatively weak coronary vasodilators.
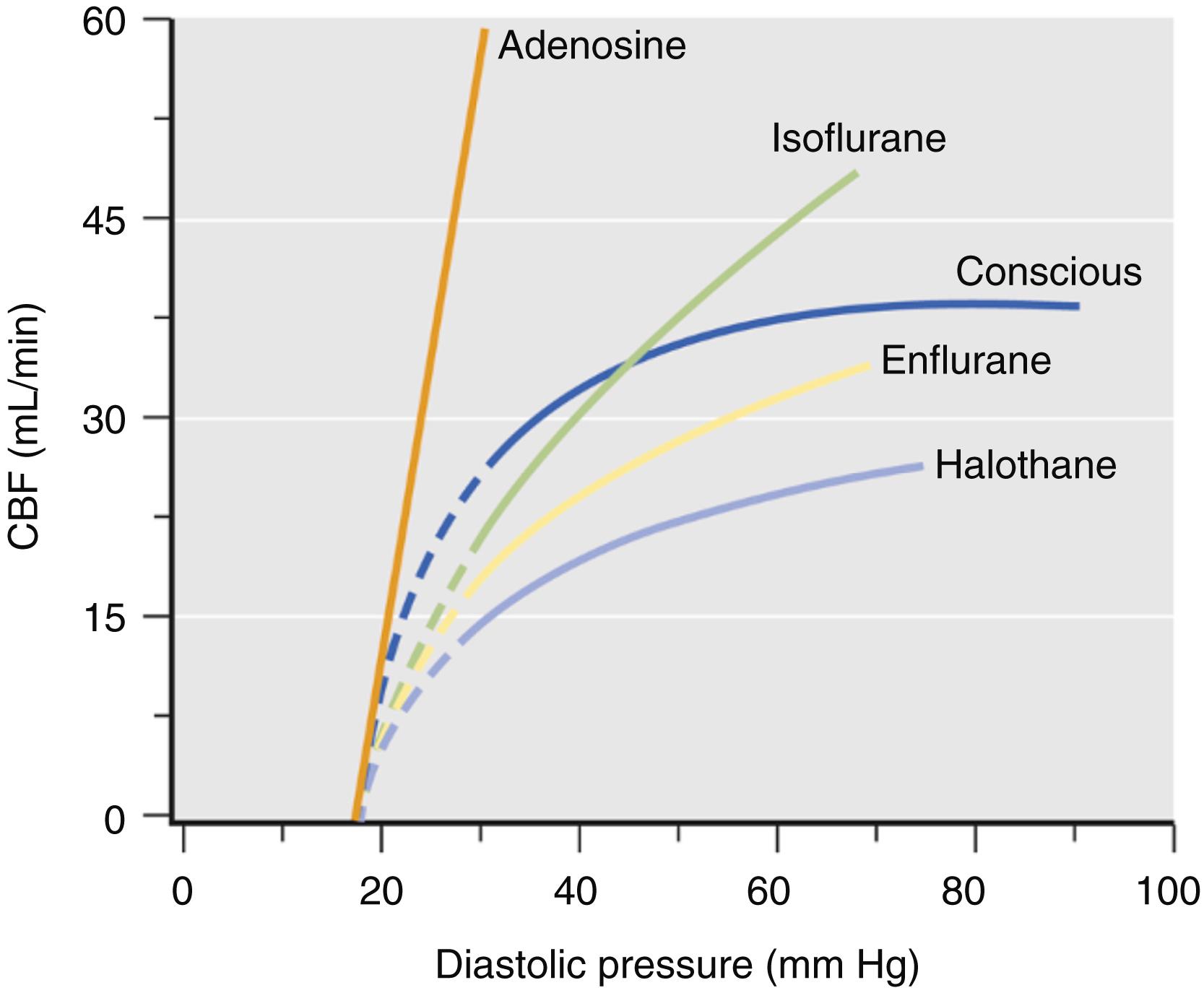
Volatile anesthetics produce direct coronary artery relaxation by affecting intracellular Ca 2+ regulation at several locations in the coronary vascular smooth muscle cell through four major mechanisms: (1) inhibition of Ca 2+ influx through voltage- and receptor-operated Ca 2+ channels; (2) reduction of Ca 2+ accumulation in and release by SR; (3) inhibition of G proteins linked to phospholipase C; and (4) decreases in formation of the second messenger inositol triphosphate. The coronary vasodilating actions of volatile anesthetics were initially thought to involve nitric oxide metabolism; nitric oxide was not responsible for volatile anesthetic–induced coronary vasodilation because endothelial denudation or treatment with nitric oxide synthase inhibitors failed to alter this effect in animal models ( Fig. 7.15 ). Instead, volatile anesthetics cause coronary vasodilation by activation of adenosine triphosphate (ATP)-sensitive potassium (K ATP ) channels. Identification of K ATP channels in coronary vascular smooth muscle 6 years after they were first characterized in ventricular myocytes in 1983 stimulated investigations into their role in the regulation of coronary vasomotor tone. Membrane hyperpolarization produced by K ATP channel opening attenuates Ca 2+ entry into the vascular smooth muscle cell through voltage-dependent Ca 2+ channels, resulting in vasodilation. K ATP channels were shown to play an essential role in establishing basal coronary arterial tone and coronary vasodilation during hypoxia, autoregulation, reactive hyperemia, and exposure to β 1 -adrenoceptor agonists, acetylcholine, and prostacyclin. Adenosine also contributed to hypoxia-induced and flow-mediated coronary vasodilation through K ATP channel opening.
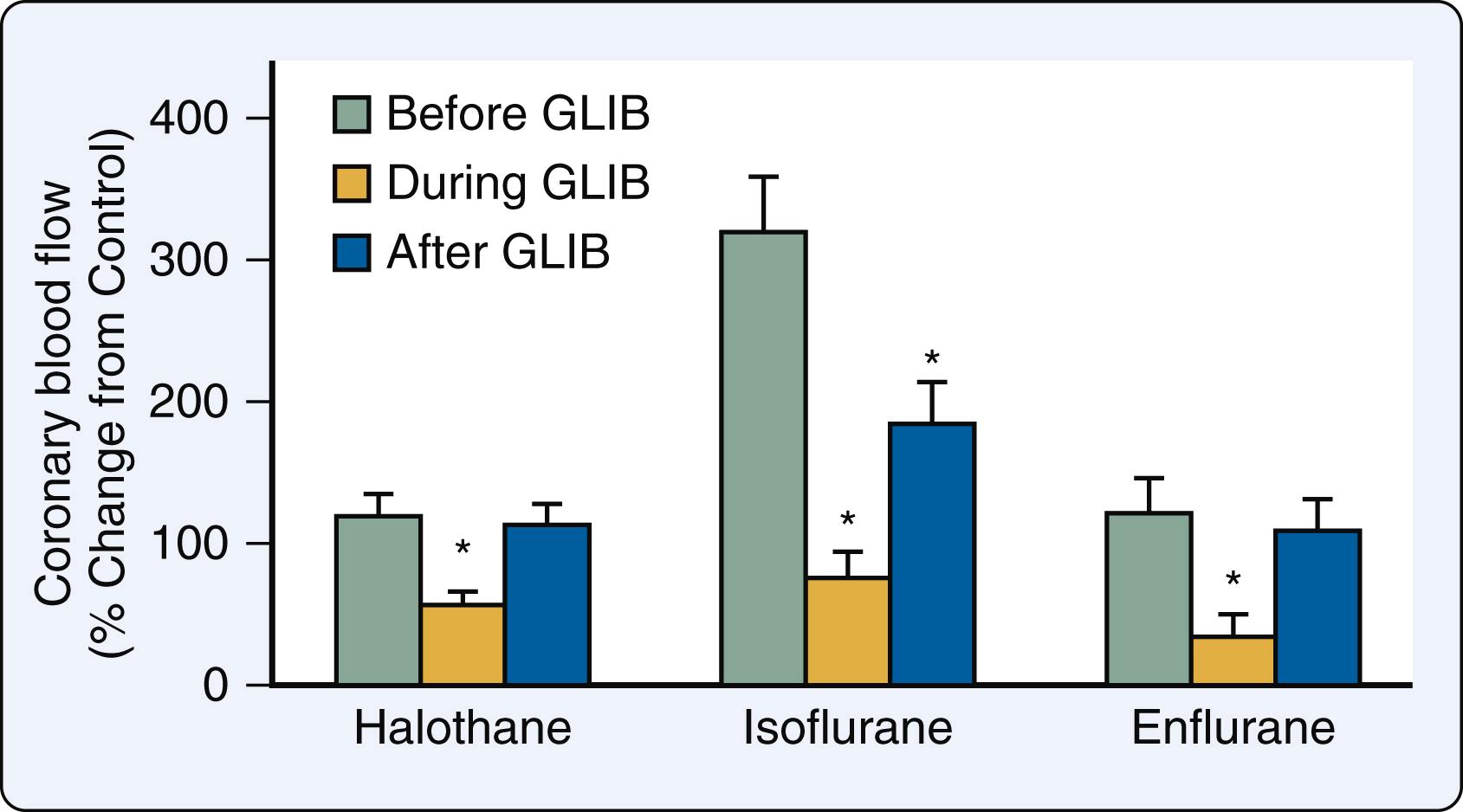
The growing recognition of the critical role of K ATP channels in coronary vasodilation led to the hypothesis that these channels also mediate volatile anesthetic–induced coronary vasodilation. Halothane caused marked coronary vasodilation in tetrodotoxin-arrested isolated rat hearts and preconstricted porcine epicardial coronary artery rings. Pretreatment with the K ATP channel blocker glyburide (previously known as glibenclamide) attenuated halothane-induced increases in coronary blood flow while administration of a high K + buffer almost completely abolished this response by overwhelming the blocker’s effects and restoring intracellular K + concentration. Glyburide also potentiated the relaxation of epicardial coronary artery rings produced by halothane in the presence but not the absence of an intact endothelium. These findings were confirmed and extended in open-chest pigs and dogs. , After cannulating a coronary artery, the vessel was perfused with blood directly from a membrane oxygenator, thereby allowing selective regional administration of isoflurane and other drugs with minimal hemodynamic effects. Isoflurane caused a dose-related increase in regional coronary blood flow, similar to the results obtained in a similar model of selective coronary artery perfusion. Glyburide substantially blunted these isoflurane-mediated actions ( Fig. 7.16 ) and also attenuated adenosine-induced vasodilation, but the K ATP channel blocker did not alter the vasodilator response to the nitric oxide donor sodium nitroprusside. Similar findings were observed with other volatile anesthetics. The results provided strong evidence that the K ATP channel is the major mediator of coronary vasodilation by volatile anesthetics.
Isoflurane and halothane decreased subendocardial blood flow and myocardial lactate extraction, produced contractile dysfunction, and caused electrocardiographic changes in experimental models of coronary artery stenosis concomitant with declines in coronary perfusion pressure. Regional ischemia during isoflurane- or halothane-induced reductions in perfusion pressure was functionally indicated by the appearance of paradoxical systolic lengthening and postsystolic shortening. Contractile dysfunction in the region distal to a critical coronary stenosis was more severe during isoflurane compared with halothane anesthesia coincident with higher flows in the normal zone and lower flows in the ischemic zone. These findings suggested that coronary vasodilation produced by isoflurane may cause a detrimental redistribution of coronary blood flow away from ischemic myocardium (termed “coronary steal”) if hypotension were present.
A controversial study in 1983 supported these experimental findings and postulated that coronary steal may occur during isoflurane anesthesia in patients with coronary artery disease. This study reported that signs of acute myocardial ischemia including ST-segment depression, T-wave inversion, and decreased lactate extraction were associated with administration of the volatile anesthetic. Since the results occurred in the presence of marked decreases in coronary vascular resistance and myocardial oxygen extraction (indicative of luxuriant perfusion), the investigators concluded that isoflurane was a potent direct coronary vasodilator that had caused coronary steal. The findings suggested that administration of isoflurane may be potentially dangerous to patients with coronary artery disease in whom vulnerable anatomy was present and threatened the widespread use of the volatile anesthetic in this setting. However, no specific evidence of blood flow redistribution between normal and collateral-dependent zones was presented in this study and subsequent investigations failed to support its conclusion. For example, coronary blood flow remained unchanged during isoflurane anesthesia in patients undergoing coronary artery bypass graft (CABG) surgery, but coronary sinus oxygen content increased consistent with modest coronary vasodilation. Not only did isoflurane fail to produce electrocardiographic or metabolic evidence of ischemia, but the volatile anesthetic also increased the tolerance to pacing-induced (demand) ischemia in patients with coronary artery disease. In fact, myocardial ischemia occurring during isoflurane anesthesia was observed only if tachycardia or hypotension were present. Indeed, no compelling evidence demonstrating redistribution of coronary blood flow away from ischemic myocardium in patients anesthetized with isoflurane has been reported.
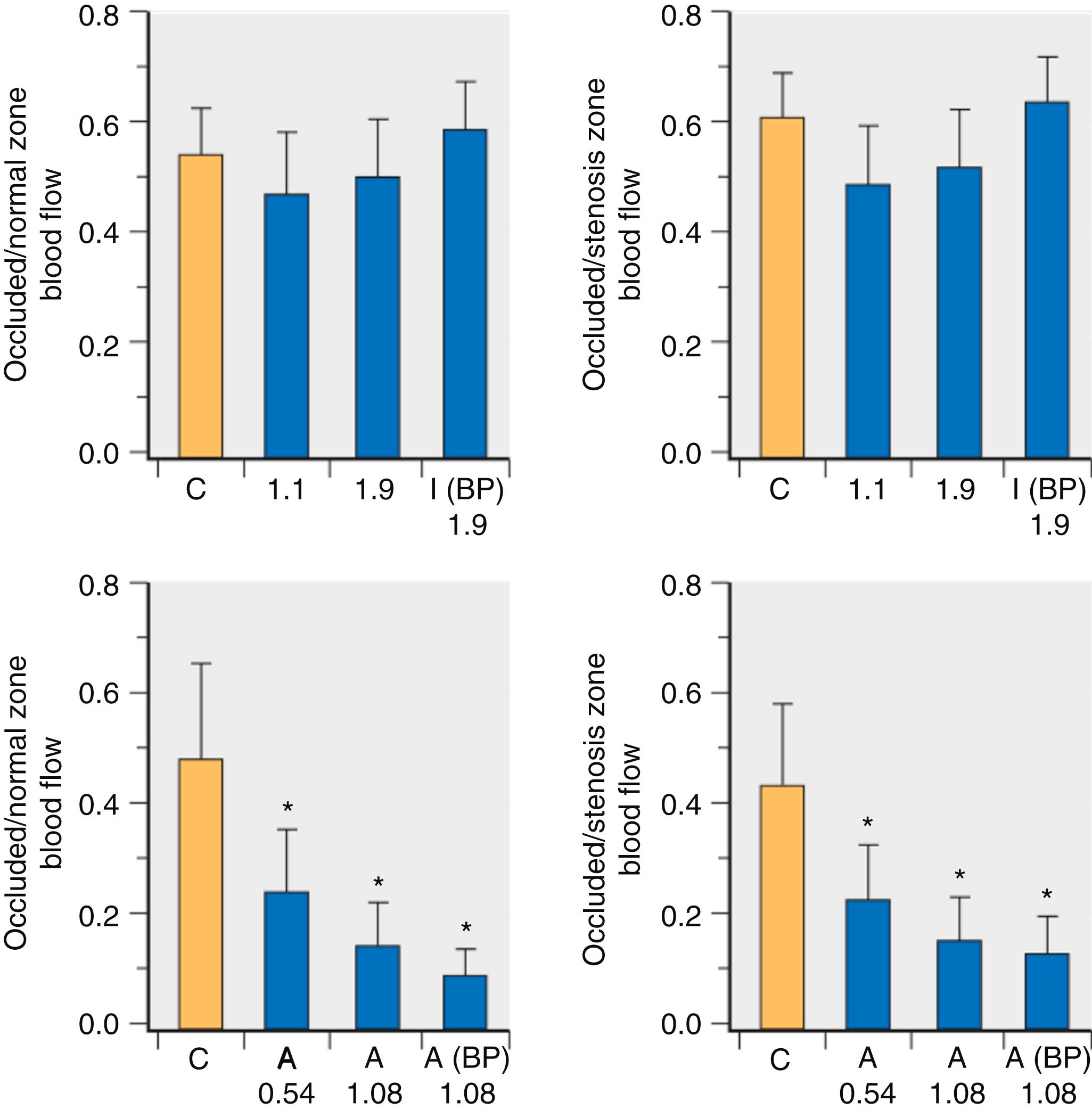
The potentially adverse effects of isoflurane on ischemic myocardium were avoided if coronary perfusion pressure was restored in experimental models of coronary artery disease. Subendocardial blood flow in the perfusion bed distal to a critical coronary stenosis was reduced during isoflurane-induced declines in arterial pressure, but treatment of hypotension restored subendocardial blood flow to values observed before the volatile anesthetic was administered. Nevertheless, the transmural distribution of coronary blood flow between the subendocardium and subepicardium (endo/epi ratio) decreased during isoflurane anesthesia despite control of arterial pressure because subepicardial blood flow increased to a greater extent than subendocardial flow. This relative increase in subepicardial perfusion accounted for the decline in the endo/epi ratio in the absence of an absolute reduction in subendocardial flow. Restoration of coronary perfusion pressure to baseline levels during isoflurane anesthesia also increased coronary collateral blood flow and normalized myocardial oxygen tension in the ischemic zone. Investigations in dogs with steal-prone anatomy (complete occlusion of a coronary artery with collateral flow from an adjacent vessel having a critical stenosis) demonstrated that isoflurane does not alter collateral-dependent or ischemic zone myocardial blood flow, modify endocardial to epicardial coronary blood flow distribution, or affect ST-segments when diastolic arterial pressure is held constant. Coronary collateral perfusion was unchanged during isoflurane or halothane anesthesia in dogs when mean arterial pressure was 50 mm Hg. Thus there was also no evidence of coronary steal in a canine model of coronary artery disease with isoflurane, desflurane, or sevoflurane independent of coronary stenosis severity or coronary collateral development. The effects of volatile anesthetics contrast to those obtained with adenosine, a potent coronary vasodilator that produces coronary steal when arterial pressure is maintained at control levels in models of multivessel coronary artery disease ( Fig. 7.17 ).
The isoflurane-coronary steal hypothesis was conclusively debunked in 1991 after three large-scale clinical trials were published confirming the safety of isoflurane in patients undergoing CABG surgery with or without steal-prone anatomy. These studies convincingly demonstrated that isoflurane does not cause coronary steal in patients at risk for intraoperative myocardial ischemia. In fact, the strongest predictor of intraoperative ischemia was preexisting ischemia and not anesthetic technique. The only hemodynamic event definitively related to intraoperative ischemia in patients undergoing coronary artery surgery was tachycardia. Perioperative use of β 1 -adrenoceptor antagonists to prevent myocardial ischemia strongly supports this contention. Interestingly, desflurane caused ischemia more often in patients undergoing coronary artery surgery compared with sufentanil because of increases in myocardial oxygen demand resulting from tachycardia and hypertension occurred during induction with the volatile anesthetic. Steal-prone anatomy was identified in 23% patients with hemodynamically significant coronary artery stenosis, but the incidence of ischemia was similar with this specific anatomic arrangement compared with other coronary artery disease configurations during desflurane anesthesia. The incidence of new electrocardiographic changes, postoperative myocardial infarction, and mortality were also similar in patients undergoing coronary artery surgery independent of anesthetic technique or the presence of steal-prone anatomy. Thus despite the findings that isoflurane, desflurane, and sevoflurane are mild coronary vasodilators, these medications do not cause coronary steal and myocardial ischemia when tachycardia and hypotension are avoided in patients with coronary artery disease.
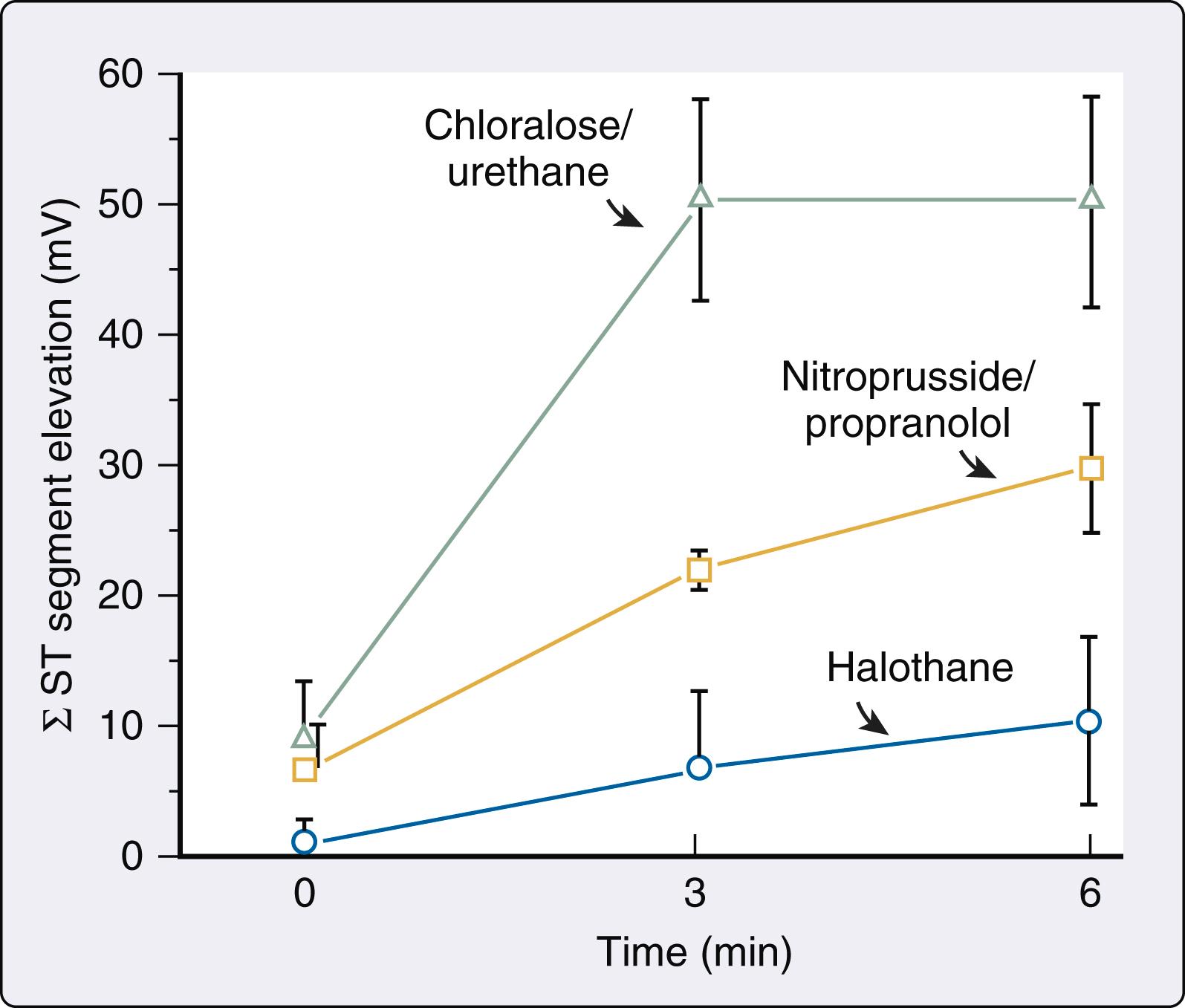
The controversies about isoflurane-induced coronary vasodilation and the implication that this anesthetic may cause coronary steal proved to be unfortunate distractions from other evidence indicating that volatile anesthetics exert protective (rather than detrimental) effects during myocardial ischemia and reperfusion injury ( Box 7.4 ). Seminal experiments published in 1976 were the first to demonstrate that volatile anesthetics are capable of exerting an antiischemic effect. Bland and Lowenstein used epicardial electrocardiographic mapping to show that halothane reduces the severity of myocardial ischemia estimated using the sum of ST-segment elevations that occurred during sequential coronary artery occlusion and reperfusion cycles in dogs. Because halothane also decreased heart rate, mean arterial pressure, and rate-pressure product, it was assumed that the antiischemic effect of the volatile anesthetic resulted from its ability to reduce myocardial oxygen demand. This ability of volatile anesthetics to favorably alter the primary hemodynamic determinants of myocardial oxygen demand, cause modest coronary vasodilation, and enhance coronary vasodilator reserve led to the suggestion that these medications may be beneficial to the ischemic heart. Subsequent contemporaneous work extended these initial findings. , Most notable among these early studies was an investigation by Gerson et al., who showed that halothane caused greater reductions in the sum of ST-segment elevations during a series of coronary occlusions and reperfusion than was observed during administration of propranolol and sodium nitroprusside, doses of which were chosen to mimic the halothane’s cardiovascular effects ( Fig. 7.18 ). Despite the study’s limitations, its results were important because they suggested for the first time that favorable changes in hemodynamics leading to reductions in myocardial oxygen demand were not the sole mechanism by which volatile anesthetics protect the heart against ischemic injury. Indeed, contemporaneous evidence appeared indicating that volatile anesthetics modulate autonomic nervous system activity. As previously discussed, volatile anesthetics inhibit baroreceptor reflex–mediated control of the circulation through depression of central nervous system integration of afferent baroreceptor input, attenuation of efferent autonomic nervous system activity, and partial blockade of ganglionic transmission and end-organ responsiveness. As a consequence of these actions, volatile anesthetics reduce sympathetic nervous system activity and blunt the autonomic nervous system’s response to hypotension. This attenuation of sympathetic nervous system activity by volatile anesthetics was associated with a decrease in the major determinants of myocardial oxygen demand, which is clearly beneficial when oxygen supply (coronary blood flow) is restricted.
Favorable alterations in the hemodynamic determinants of myocardial oxygen demand
Direct negative inotropic effect
Dilation of arteriolar resistance vessels (decrease in left ventricular afterload)
Increase in myocardial oxygen supply (modest coronary vasodilation; minor effect)
Decrease in sympathetic nervous system tone
Attenuation of intracellular Ca 2+ overload during ischemia
Inhibition of reactive oxygen species production during reperfusion
Attenuation of postischemic neutrophil adhesion in coronary vasculature
Acute and delayed preconditioning; postconditioning
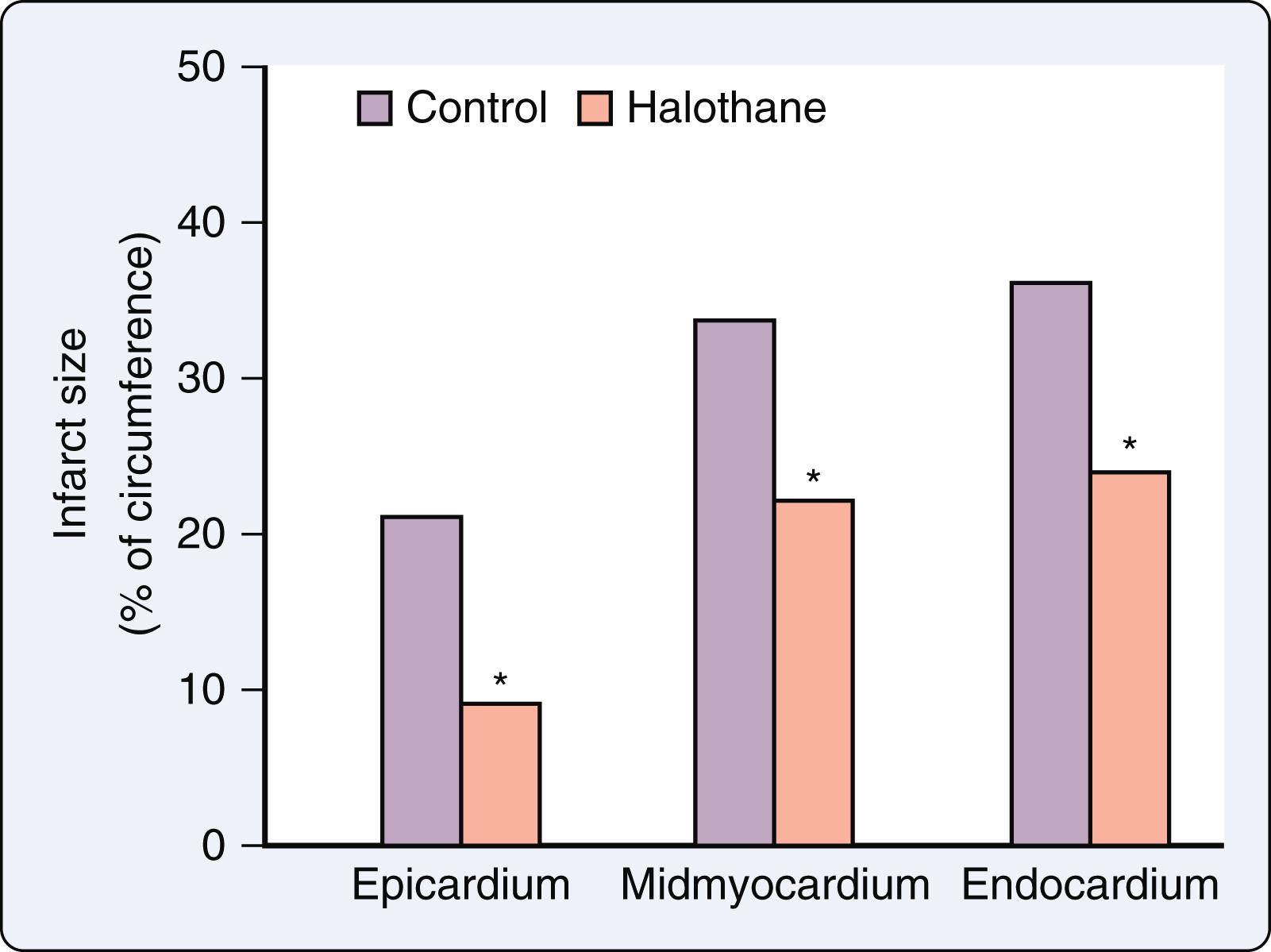
Seven years after Blank and Lowenstein published their findings, Davis et al. demonstrated that halothane reduces the size of an experimental myocardial infarction produced in dogs by suture ligation of a coronary artery ( Fig. 7.19 ). Isoflurane caused a similar effect. Two other studies showed that volatile anesthetics accelerate the functional recovery of postischemic, reperfused (“stunned”) myocardium. This form of reversible myocardial injury occurs as a consequence of a transient coronary artery occlusion that is of insufficient duration (typically less than 20 min) to cause tissue necrosis, but is considered to be a precursor to infarction in the spectrum of ischemic injury. Prolonged contractile dysfunction lasting hours to days is observed in stunned myocardium despite the complete restoration of coronary blood flow and a preserved responsiveness to positive inotropic drugs. Enflurane facilitated the recovery of LV pressure and preserved myocardial high-energy phosphate concentrations in isolated hearts subjected to global ischemia and reperfusion. Subsequently, halothane and isoflurane were also shown to enhance the functional recovery of postischemic-reperfused myocardium in a canine model ( Fig. 7.20 ).
Acute myocardial ischemia profoundly disrupts Ca 2+ homeostasis, leading to inadequate mitochondrial ATP synthesis, intracellular Ca 2+ overload, and almost immediate contractile dysfunction. Enhanced neutrophil-endothelium interaction during reperfusion facilitates transmigration of activated neutrophils into the myocardium, where they release additional large quantities of reactive oxygen species (ROS) along with proteolytic enzymes that further exacerbate this pathological process. As discussed earlier, volatile anesthetics are known to cause substantial changes in Ca 2+ regulation that decrease intracellular Ca 2+ availability in the cardiac myocyte. As a result, the finding that halothane directly reduces Ca 2+ accumulation after myocardial ischemia was proposed to be a third mechanism (see Box 7.4 ) by which volatile anesthetics protect against ischemic injury.
Additional data appeared in the wake of the isoflurane-coronary steal controversy indicating that volatile anesthetics possess relevant antiischemic effects. Halothane and isoflurane protected against hypoxia-reoxygenation injury, decreased the incidence of arrhythmias, improved contractile function, and reduced release of the metabolites adenosine, inosine, and lactate after global ischemia and reperfusion in isolated hearts. Halothane inhibited formation of platelet thrombi by a cyclic adenosine monophosphate (cAMP)-dependent mechanism and reduced cyclical variations in coronary blood flow associated with a critical coronary artery stenosis. Volatile anesthetics attenuated ROS-induced impairment of LV pressure development in isolated hearts, providing another potential mechanism by which volatile anesthetics may cause antiischemic effects. Volatile anesthetics also inhibited neutrophil adhesion in the coronary circulation after ischemia and reperfusion. Halothane exerted protective effects during cardioplegia-induced electrical and mechanical arrest in isolated hearts, providing further evidence that preferential alterations in myocardial oxygen supply-demand relations and sympathetic nervous system activity are not entirely responsible for the antiischemic actions of volatile anesthetics.
In 1986, Murry, Jennings, and Reimer described a phenomenon in which a brief ischemic insult confers protection against a subsequent ischemic episode of similar or greater magnitude. Four brief 5-minute periods of coronary artery occlusion interspersed with 5-minute episodes of reperfusion reduced myocardial infarct size resulting from a subsequent, prolonged 40-minute episode of ischemia and reperfusion. This phenomenon was termed “ischemic preconditioning” (IPC) and is now recognized as a landmark discovery in the history of cardiac physiology. The study demonstrated, for the first time, that the heart recognized and rapidly adapted to stress to render itself more resistant to additional injury. IPC has been described as a type of “vaccination” that promotes the myocardium’s “self-preservation” by altering its phenotype. Since IPC was first reported, its mechanisms, limitations, and potential clinical applicability have been exhaustively studied. Many other stimuli, including a wide variety of drugs, NO, ROS, endotoxins, inflammatory cytokines, heat stress, and intense exercise, also stimulate the development of this prosurvival phenotype and protect myocardium against ischemic injury without the need for a prior episode of brief ischemia. Most notable from the perspective of this chapter, volatile anesthetics are among the many drugs that have been shown to mimic the beneficial effects of IPC through essentially identical intracellular mechanisms.
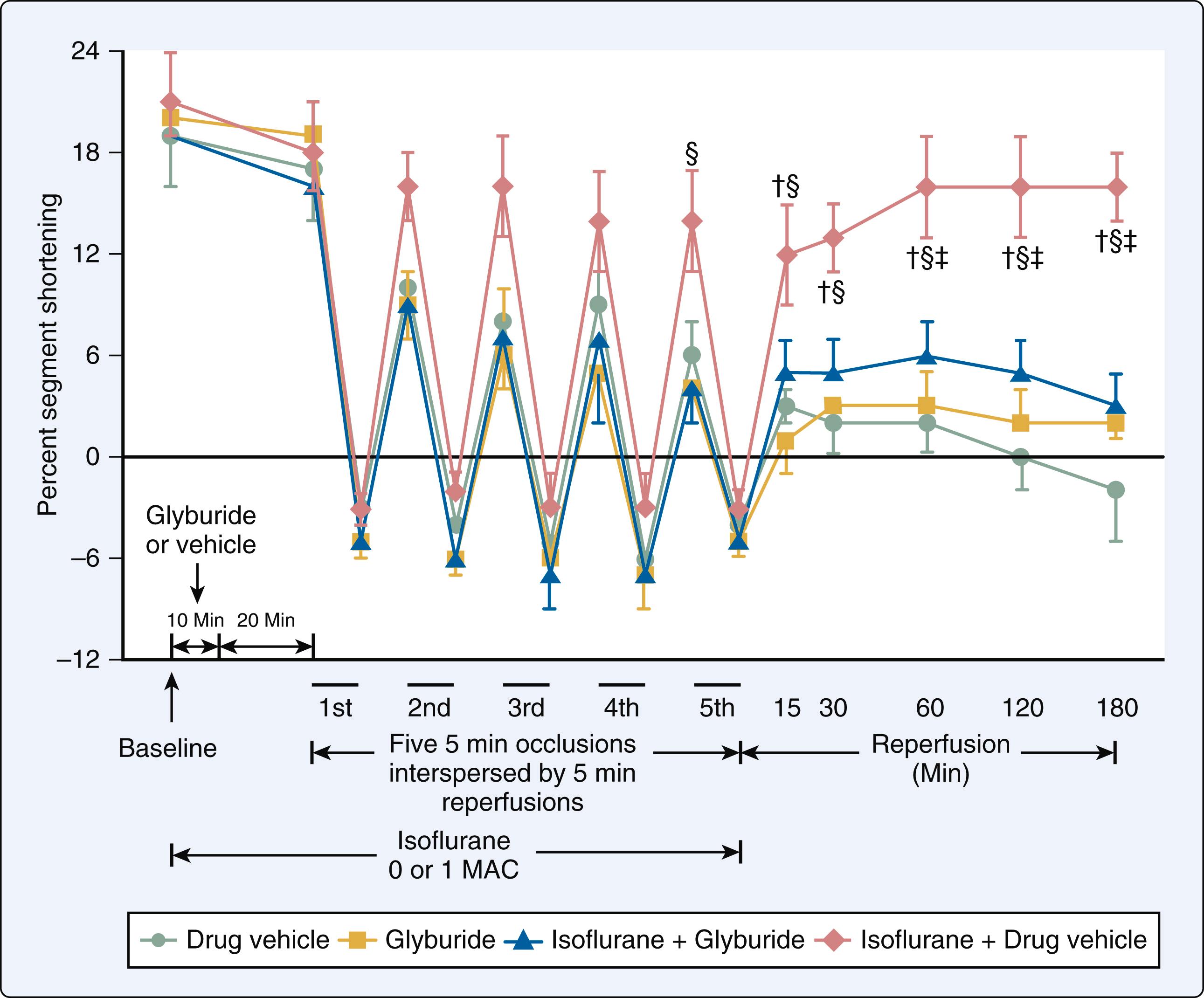
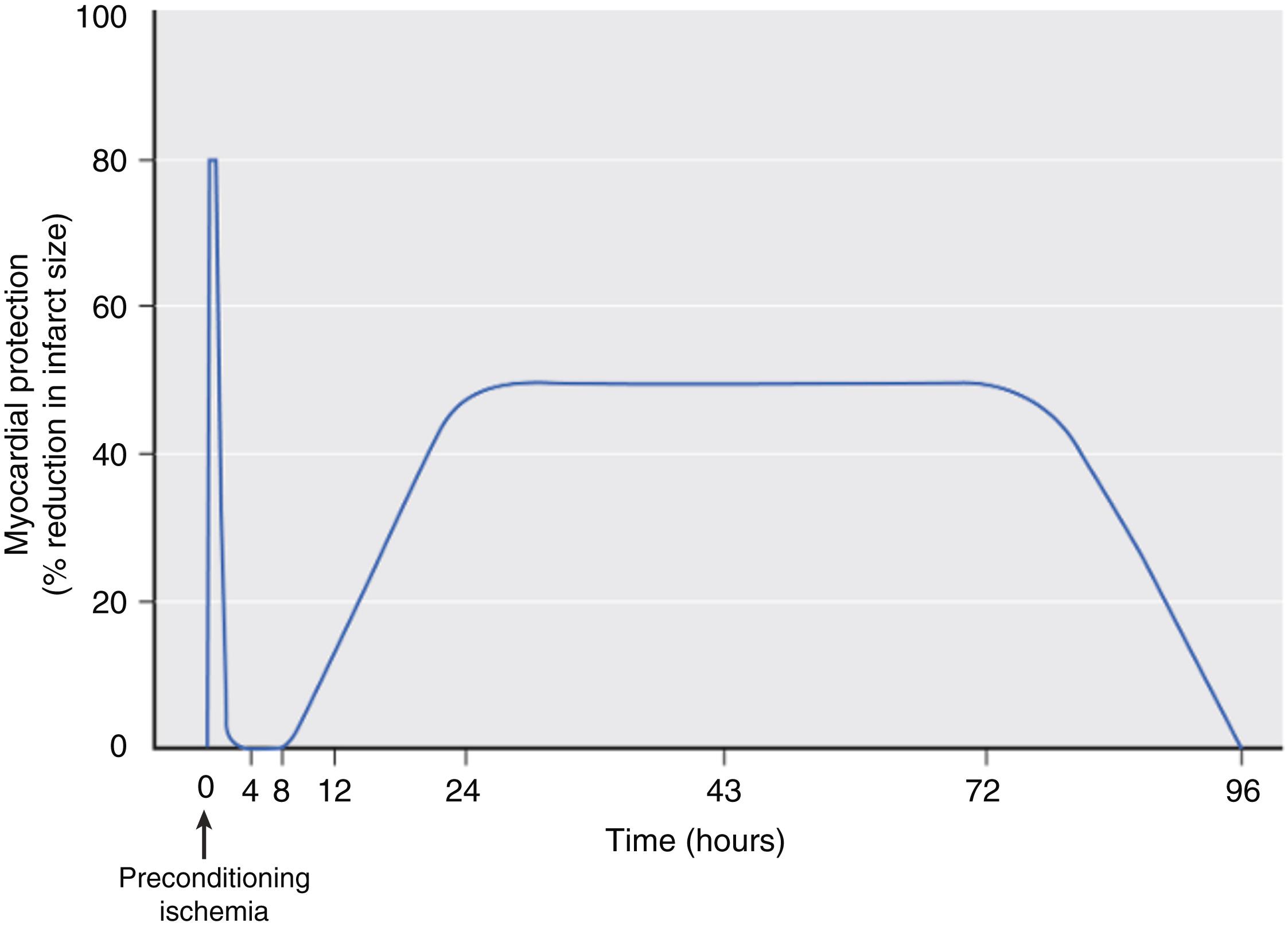
Three research groups first reported that administration of halothane or isoflurane before prolonged coronary artery occlusion and reperfusion reduced myocardial infarct size in isolated and intact hearts in 1997. This beneficial effect was coined “anesthetic preconditioning” (APC), occurred independent of changes in systemic hemodynamics and coronary collateral blood flow, and persisted despite discontinuation of the volatile anesthetic before coronary artery occlusion, establishing a “memory” period similar to that observed with IPC ( Fig. 7.21 ). These seminal investigations demonstrated that activation of adenosine receptors, protein kinase C, and K ATP channels mediate APC. A flurry of activity followed these initial descriptions of APC that comprehensively defined its responsible mechanisms in a wide variety of experimental preparations ranging from intracellular components to whole animal models. , , A second window of APC before prolonged myocardial ischemia (termed “delayed” or “late” preconditioning) and the ability of volatile agents to provide myocardial protection when administered immediately before and during early reperfusion after coronary artery occlusion (known as “postconditioning”) were also identified, and many of their corresponding mechanisms were characterized. , Acute APC provides an intense protection against infarction that occurs shortly after transient administration of a volatile anesthetic and persists for a brief duration (1–2 h), whereas delayed APC has a more protected period of myocardial protection (3–4 days) against ischemic injury that develops 6 to 12 hours after exposure to a volatile anesthetic ( Fig. 7.22 ). The mechanisms responsible for acute APC are quite different from those of its delayed form. Posttranslational modification of existing proteins mediates acute APC (explaining its rapid onset and relatively short duration of action), but synthesis of new proteins is required for the second window of APC (accounting for its delayed onset and more prolonged duration of protection). In contrast, acute APC and postconditioning by volatile anesthetics share almost identical mechanisms. It is essential for the reader to appreciate that the experimental data characterizing APC and its related forms are remarkably compelling. Indeed, the antiischemic effects of volatile anesthetics have been repeatedly validated in all mammalian species in which these phenomena were studied.
The intracellular processes that drive APC closely resemble those implicated in IPC and other types of pharmacological preconditioning ( Box 7.5 ). It is not our intention to provide a comprehensive discussion of this subject here as this topic is covered in detail in many reviews and textbook chapters. In general terms, alterations in ion channel function, G protein-coupled receptor activity, intracellular signaling kinases, regulation of apoptosis (programmed cell death), production of reactive oxygen and nitrogen species, gene expression, and mitochondrial physiology have been shown to play essential roles in volatile anesthetic–induced myocardial protection against ischemic injury. Several studies clearly documented that APC also occurs in human myocardium through mechanisms that are identical to those described in other mammals. Adenosine A 1 receptors and K ATP channels were shown to mediate isoflurane-induced protection against anoxic injury in human atrial myocardium. The mitochondrial K ATP channel plays a crucial role in APC by desflurane and sevoflurane, and isoflurane was shown to directly open human cardiac mitochondrial K ATP channels that had been isolated from LV myocardium and reconstituted in lipid bilayers. Sevoflurane- and desflurane-induced preconditioning was mediated by ROS in isolated human right atrial myocardium. A role for phosphatidylinositol-3-kinase signal transduction was shown during APC in human atrial trabeculae. Isoflurane also protected cardiac myocytes derived from human embryonic stem cells against oxidative stress–induced cell death through inhibition of the mitochondrial permeability transition pore. Thus the mechanisms responsible for APC in experimental animals are equally applicable in human myocardium.
Ion channels (K ATP , K Ca )
G protein-coupled receptor agonists (adenosine, opioid)
Protein kinases (PKC, PTK, PI3K, ERK1/2, MAPK, eNOS, iNOS, mTOR/p70s6K, COX-2, HO-1, JNK, 5ʹ-AMPK, GSK-3β, 12-LO)
Prostaglandins (PGI2, PGE2), 12-HETE
Heat shock proteins (Hsp 27, 70, 90)
Glucose transporter-4
Reactive oxygen and nitrogen species
Transcription factors (NF-κB, HIF-1α, CREB)
Caveolin-3
Altered balance between pro- and anti-apoptotic proteins and their regulators
mPTP
Electron transport chain components (complexes I and III)
Modulation of interleukins, TNF-α, selectins
Enhances antioxidant defense (superoxide dismutase, catalase, peroxidase)
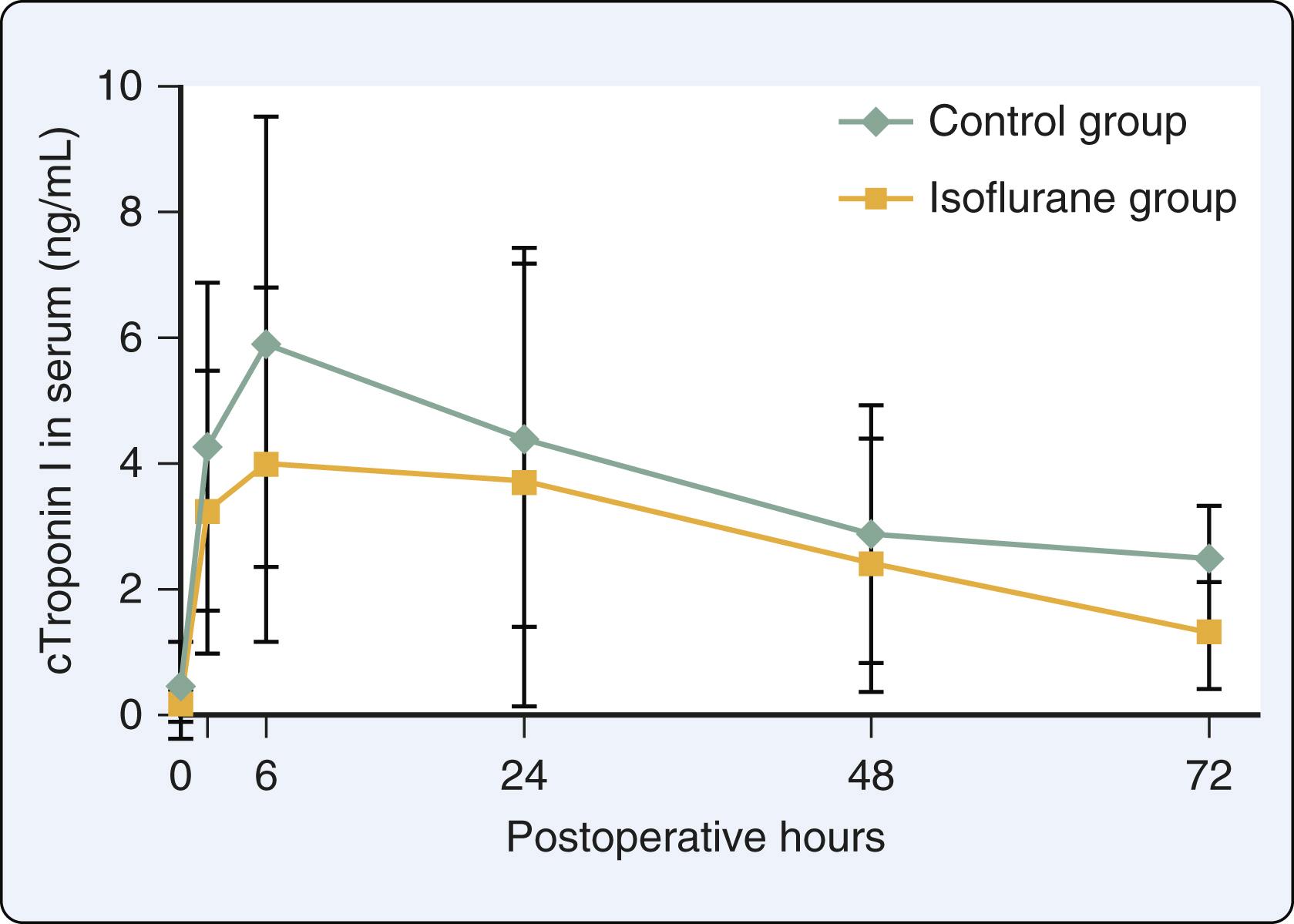
Less than 3 years after APC was first described, two groups of investigators published single-center proof-of-concept clinical trials demonstrating that APC may occur in patients at risk of perioperative myocardial ischemia undergoing cardiac surgery. Penta de Peppo et al. randomized 22 patients undergoing CABG using cardiopulmonary bypass and reported that brief (5 min) administration of enflurane before cardioplegic arrest improved the recovery of LV contractility. The same research group also studied 40 patients undergoing CABG randomized to receive brief exposure to isoflurane or placebo before aortic cross-clamping and administration of cardioplegia. Exposure to isoflurane reduced release of troponin I and creatine kinase-myocardial band (CK-MB) in patients with preexisting LV dysfunction, but not in those with normal LV function. Belhomme et al. examined the effects of a 5-minute administration of isoflurane (2.5 MAC) followed by discontinuation of the anesthetic for 10 minutes before application of the aortic cross-clamp and cardioplegic arrest in 20 patients undergoing CABG. Isoflurane preconditioning increased ecto-5ʹ-nucleotidase (a contributor to adenosine production and a marker of protein kinase C activation) activity in right atrial tissue biopsies and reduced postoperative release of troponin I ( Fig. 7.23 ) and CK-MB. These three studies provided the first clinical evidence that APC may reduce myocardial injury associated with CABG using cardiopulmonary bypass in patients who are susceptible to ischemia.
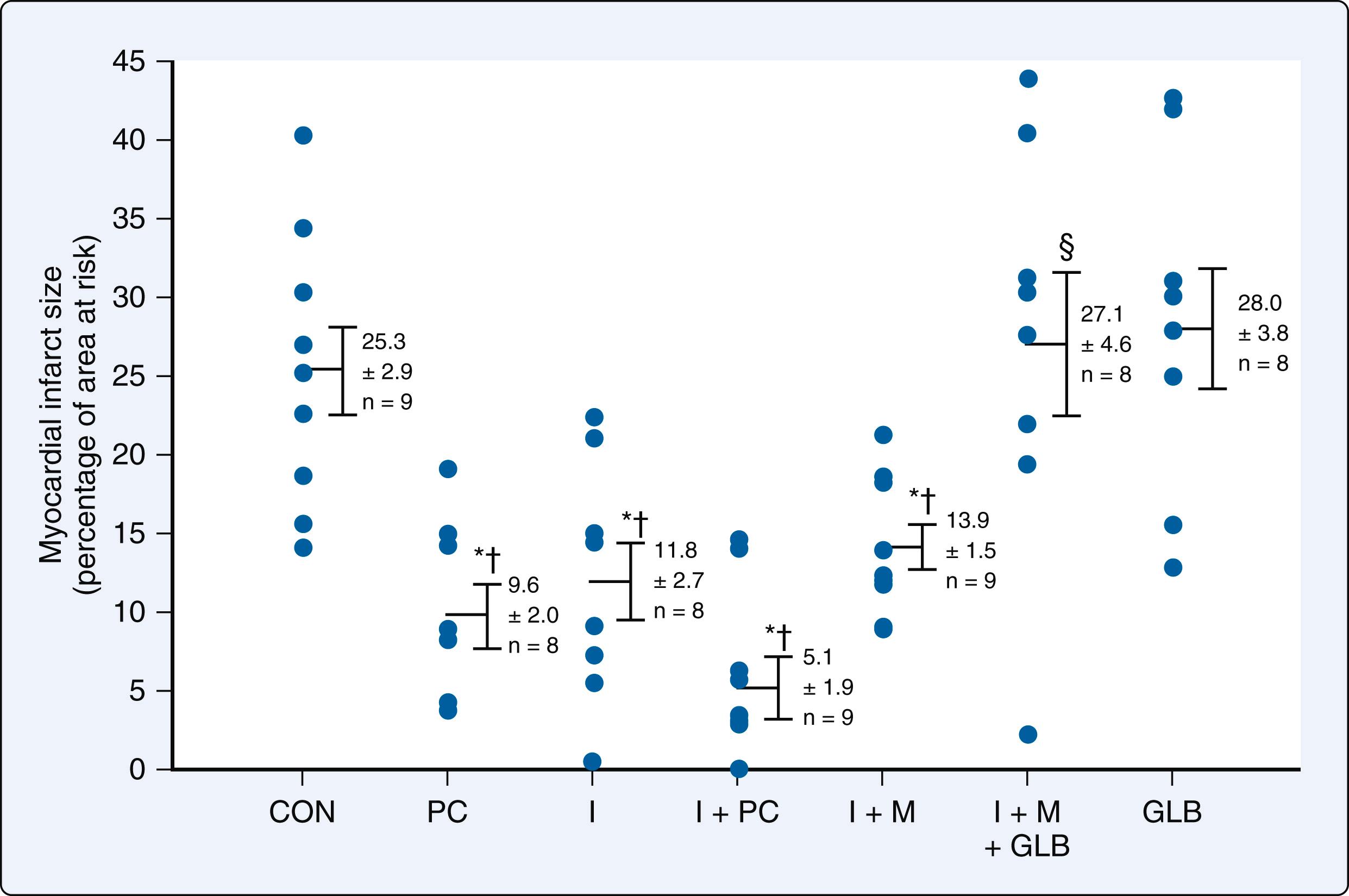
Many additional small-scale, single-center, trials using strict preconditioning protocols were subsequently published that supported these findings, demonstrating that pretreatment with volatile agents reduced biochemical markers of necrosis concomitant with relative preservation of LV function after cardiopulmonary bypass. Of those studies demonstrating positive results with APC, one small trial of 72 patients undergoing CABG also suggested that APC reduces mortality, but others did not, most notably, a large 3,400-patient subanalysis of the recent multicenter prospective Mortality in Cardiac Surgery Randomized Controlled Trial of Volatile Anesthetics (MYRIAD). Several confounding factors may be responsible for these disappointing results ( Box 7.6 ). It is highly likely that any beneficial effects of APC were attenuated, if not completely abolished, because cardiac surgical patients are usually elderly and suffer from other coexisting conditions such as diabetes mellitus, hyperglycemia, obesity, and cardiomegaly that are known to block the phenomenon. These patients are also commonly treated with medications to manage coexisting disease (eg, sulfonylurea agonists) and receive drugs (eg, opioids, vasoactive medications) during the perioperative period that modulate APC. In addition, the severity of ischemia produced in experimental models of prolonged ischemia- or hypoxia-reperfusion injury (the conditions under which the efficacy and limitations of APC were originally established) is much greater than is typically sustained during aortic cross-clamping when meticulous myocardial protection techniques are used. Indeed, the relatively small amount of myocardium at risk for ischemic injury is probably the single most important factor why many clinical investigations conducted to date failed to demonstrate that volatile anesthetics produce consistently beneficial effects or favorably affect long-term outcome in patients undergoing cardiac surgery.
Presence of advanced age and coexisting diseases that block APC
Use of medications that adversely modulate APC
Low ischemic burden compared with laboratory studies
Immediate treatment of intraoperative myocardial ischemia when it occurs
Myocardial protection techniques during cardiopulmonary bypass and aortic cross-clamping
Maintenance of coronary perfusion pressure and blood flow (OP-CAB)
Vigilant anesthesiologist
The intentional production of severe ischemia-reperfusion injury and the observation of its pathological consequences without any intervention to mitigate them are characteristic features of virtually all laboratory studies in which APC is examined. For example, a prolonged coronary artery occlusion followed by several hours of reperfusion is commonly used to produce a large myocardial infarction in intact hearts, the size of which often exceeds more than 50% of the LV area-at-risk. This experimental model provides a clear-cut, easily quantified endpoint (infarct size; typically measured with triphenyltetrazolium chloride staining) that may be used to discriminate whether administration of a volatile anesthetic reduces the magnitude of injury. In contrast, substantially less myocardium is typically at risk during elective cardiac surgery unless an unanticipated occlusion of a major coronary vessel occurs. Systemic and topical hypothermia, intermittent or continuous antegrade and retrograde cardioplegia, and LV decompression are typically used to minimize global ischemic damage during cardiopulmonary bypass in patients undergoing most types of cardiac surgery. Similarly, maintenance of coronary perfusion pressure with intravenous fluids or vasoactive medications during changes in cardiac position and use of intracoronary shunts to provide continuous coronary blood flow during construction of distal anastomoses are common techniques that also serve to minimize ischemia during off-pump coronary artery surgery. The role of the vigilant cardiac anesthesiologist also cannot be overemphasized. Active prevention, prompt recognition, and aggressive treatment of myocardial ischemia are central objectives when caring for a patient undergoing cardiac surgery who is at risk of this complication. More subtle clinical endpoints (eg, biochemical assays of myocardial necrosis or function, use of vasoactive medications, duration of intensive care unit or hospital stay) are also used required to measure generally less intense ischemic injury. As a result, the relative efficacy of APC observed clinically is almost certainly less apparent than that seen in the laboratory.
Similar to the clinical findings with APC, more than 20 trials conducted to date also provided inconsistent results when volatile anesthetics were administered continuously for maintenance to patients undergoing cardiac surgery or solely during cardiopulmonary bypass. Many studies indicated that volatile agents exerted beneficial effects, whereas others did not. It is noteworthy that the common method of maintaining anesthesia with a volatile agent does not mimic APC because the anesthetic is not discontinued (“memory” period) at some point before the prolonged ischemic event (application of the aortic cross-clamp). The implicit assumption that continuous administration of a volatile anesthetic also produces myocardial protection against ischemic injury through identical mechanisms involved in APC is almost certainly invalid. Instead, it is much more likely that any beneficial effects observed under these circumstances are related to other factors, as previously discussed (see Box 7.4 ). A clinical trial by De Hert et al. provided a particularly useful example. These investigators demonstrated that only continuous administration of sevoflurane throughout and after cardiopulmonary bypass, and not APC or postconditioning with the volatile anesthetic per se, reduced postoperative troponin I concentrations compared with a propofol-opioid anesthetic in patients undergoing CABG using cardiopulmonary bypass ( Fig. 7.24 ). The conclusion of this study was clear: the actions of continuous administration of a volatile anesthetic on the hemodynamic determinants of myocardial oxygen demand, sympathetic nervous system activity, intracellular Ca 2+ homeostasis, ROS inhibition, and postischemic neutrophil adhesion in the coronary vasculature most likely played more important roles than APC in establishing its antiischemic effects (see Box 7.4 ).
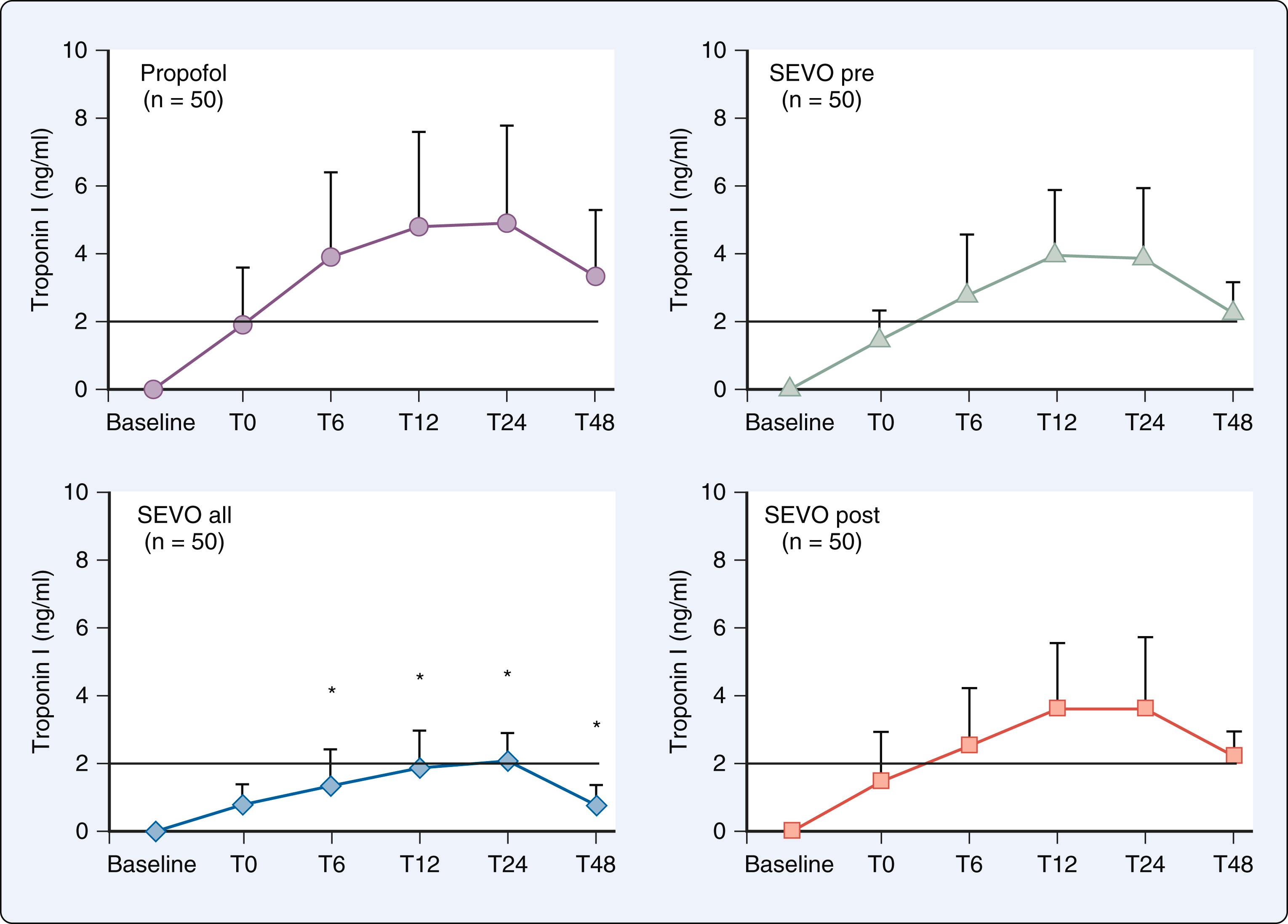
Several meta-analyses and longitudinal studies have been performed in attempt to clarify the implications of existing clinical trials, which varied widely in size, quality, and design (prospective versus retrospective, type of cardiac surgery, use of cardiopulmonary bypass). In general, the results supported the contention that use of a volatile anesthetic resulted in decreases in postoperative release of troponin I, preservation of LV systolic function, and reductions in positive inotropic drug requirements compared with total intravenous anesthesia (TIVA). However, many of these meta-analyses often included clinical trials without distinction between the method of administration of the volatile anesthetic (APC versus continuous) and consequently, the relative contribution of APC to these salutary effects cannot be ascertained. Some meta-analyses also suggested that use of volatile anesthetics is associated with reduced 1-year mortality compared with TIVA. However, this conclusion remains the subject of considerable debate, as other meta-analyses and the MYRIAD trial indicated that 1-year mortality after cardiac surgery was not influenced by anesthetic technique. The precise role of APC, if any, in the purported long-term beneficial effect of volatile anesthetics in this setting has not been established. The lack of consensus among meta-analyses about the impact of volatile anesthetics versus TIVA on long-term mortality after cardiac surgery is most likely multifactorial in origin. Study design, selection bias (choice of which trials to include, sampling of specific cohorts within individual trials, consistent exclusion of older “neutral” clinical trials), publication bias (tendency of journals to publish trials with positive compared with neutral results), and the adverse impact of inclusion of many small-scale, single-center trials are well-known constraints of meta-analysis. Such limitations almost certainly contributed to the contradictory conclusions about 1-year mortality and other outcome variables drawn by the meta-analyses when volatile anesthesia and TIVA were compared in patients undergoing cardiac surgery. ,
The MYRIAD trial provided more compelling insights about relevance of anesthetic technique in patients undergoing cardiac surgery than the often-contradictory meta-analyses. MYRIAD was a prospective, large-scale, multicenter randomized controlled trial testing the hypothesis that use of volatile anesthetics during coronary artery surgery was associated with a reduction in 1-year mortality compared with a propofol-opioid-based anesthetic technique. Investigators were encouraged (but not required) to use one of three strategies for administration of a volatile anesthetic, all of which were designed to mimic features of APC. The trial stopped for futility at its second interim analysis (after 5,400 patients had been enrolled; 10,000 patients were originally planned) by an independent data and safety monitoring board because no differences in any outcome variable were present between anesthetics groups nor could such differences be anticipated if more patients were studied. The study’s sample size also far exceeded that required by a priori power analysis to exclude β error, indicating the neutral results of MYRIAD were statistically sound within the context of its pragmatic design. MYRIAD demonstrated that 30-day and 1-year mortality was similar between anesthetic groups independent of whether coronary artery surgery was performed with or without cardiopulmonary bypass ( Table 7.2 ). Indeed, Kaplan-Meier survival estimates for all-cause mortality in patients receiving volatile anesthetics versus TIVA were nearly superimposable ( Fig. 7.25 ). Unlike the confusion wrought by the meta-analyses, the implications of MYRIAD were very clear: the choice of anesthesia is not a factor that influences outcome in patients undergoing cardiac surgery, as others have argued. Thus despite the overwhelming evidence that APC provides myocardial protection in experimental models of ischemia-reperfusion injury, these beneficial effects do not translate into clinically meaningful improvements in outcome in patients at highest risk for perioperative myocardial ischemia undergoing cardiac surgery.
| Outcome Variable | VA | TIVA | RR (95% CI) |
|---|---|---|---|
| N | 2709 | 2691 | — |
| All-cause 1-year mortality | 75 (2.8%) | 79 (3.0%) | 0.94 (0.69–1.29) |
| All-cause 30-day mortality | 38 (1.4%) | 34 (1.3%) | 1.11 (0.70–1.76) |
| Cardiac 1-year mortality | 33 (1.2%) | 43 (1.6%) | 0.76 (0.49–1.20) |
| Cardiac 30-day mortality | 20 (0.7%) | 24 (0.9%) | 0.83 (0.46–1.49) |
| Composite 30-day nonfatal myocardial infarction or mortality | 134 (5.0%) | 127 (4.7%) | 1.05 (0.83–1.34) |
| Readmission within 30 days of discharge | 56 (2.1%) | 61 (2.4%) | 0.91 (0.64–1.30) |
| Readmission within 1 year of discharge | 257 (10.1%) | 249 (9.8%) | 1.02 (0.87–1.21) |
Experiments in papillary muscle and isolated heart preparations consistently showed that nitrous oxide produces a direct negative inotropic effect, but inconsistent results about the influence of nitrous oxide on contractility were observed in experimental animals and healthy volunteers. These conflicting findings most likely occurred because of the effects of the anesthetic gas on systemic hemodynamics and autonomic nervous system reflexes. Studies using nitrous oxide alone are difficult to perform because the gas does not produce complete anesthesia at partial pressures less than 1 atmosphere (1 MAC = 1.04 atm). The effects of nitrous oxide on contractility may also be influenced by different baseline anesthetics. Preload recruitable stroke work derived from LV pressure-segment length relations was used to quantify changes in myocardial contractility produced by nitrous oxide in a canine model when the gas was administered during baseline isoflurane or sufentanil anesthesia and pharmacologic blockade of the autonomic nervous system. In this experimental preparation, 70% nitrous oxide decreased the slope of the preload recruitable stroke work relation by 28% and 41% during sufentanil and isoflurane anesthesia, respectively. This magnitude of reduction was similar to the decline in contractility observed during 1 MAC isoflurane ( Fig. 7.26 ). Notably, any nitrous oxide–induced myocardial depression is usually offset by simultaneous increases in sympathetic nervous system activity in the intact circulation. The negative inotropic effects of nitrous oxide may be more apparent in the presence of preexisting LV dysfunction. For example, nitrous oxide–induced depression of contractility was greater than the mild sympathomimetic effect of the anesthetic gas in patients with coronary artery or valvular heart disease and LV dysfunction because the reserve for additional sympathetic nervous system activation is limited.
![Figure 7.26, Effects of nitrous oxide on (N 2 O) on indices of myocardial contractility in the presence of isoflurane (I; data are presented as percent of control [I only]). ∗Significantly ( P < .05) different from I only; †Significantly ( P < .05) different from I + 30% N 2 O. CO , Cardiac output; PRSW , preload recruitable stroke work; %SS , percent segment shortening. Figure 7.26, Effects of nitrous oxide on (N 2 O) on indices of myocardial contractility in the presence of isoflurane (I; data are presented as percent of control [I only]). ∗Significantly ( P < .05) different from I only; †Significantly ( P < .05) different from I + 30% N 2 O. CO , Cardiac output; PRSW , preload recruitable stroke work; %SS , percent segment shortening.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/CardiovascularPharmacologyofAnesthetics/25_3s20B9780323829243000077.jpg)
The actions of nitrous oxide on LV diastolic function have been incompletely studied. Modest increases in maximal lengthening velocity and maximal rate of decline of force were observed in ferret papillary muscle concomitant with decreases in contractile state. No changes in the rate of isometric or isotonic relaxation were observed, indicating that nitrous oxide does not substantially modify myocardial relaxation. However, nitrous oxide did modestly increase indices of LV chamber stiffness and reduce early LV filling in a canine model, findings that were consistent with observations of nitrous oxide–induced LV diastolic dysfunction after cardiopulmonary bypass in patients undergoing CABG surgery. Nitrous oxide caused dose-related reductions in the intracellular Ca 2+ transient in vitro, suggesting that myocardial depression was related to decreases in Ca 2+ availability. Nitrous oxide did not affect myofibrillar sensitivity to Ca 2+ or Ca 2+ uptake and release from the SR. In addition, the diastolic Ca 2+ transient was unaffected by nitrous oxide, suggesting that this anesthetic gas does not alter Ca 2+ kinetics responsible for myocardial relaxation.
Reversible AV dissociation was reported in humans anesthetized with nitrous oxide and volatile or opioid-based anesthetics. Addition of nitrous oxide to halothane also lowered the threshold at which arrhythmias occur. This observation may result from a combination of sympathetic nervous system stimulation by nitrous oxide and myocardial sensitization to epinephrine by halothane. The incidence of arrhythmias was less during a nitrous oxide–opioid-based anesthetic compared with halothane anesthesia.
Nitrous oxide caused pupillary dilation, diaphoresis, and increases in systemic vascular resistance, central blood volume, and forearm vascular resistance in volunteers during volatile anesthesia, effects that were consistent with activation of the sympathetic nervous system. A subsequent study demonstrated that nitrous oxide increases sympathetic nerve traffic measured using sympathetic microneurography in human volunteers, especially during the first 15 to 30 minutes of exposure. Baroreceptor reflex–mediated control of heart rate was impaired during administration of nitrous oxide, but regulation of sympathetic outflow to peripheral blood vessels was preserved, suggesting that neural regulation of arterial pressure was intact.
The hemodynamic consequences of nitrous oxide are relatively minor, but assessment of the effects of anesthetic gas in humans is often complicated by concurrent administration of volatile anesthetics, opioids, or other medications in the presence of cardiovascular disease. Typical clinical concentrations of nitrous oxide (between 40% and 70%) caused modest increases in heart rate in healthy volunteers ( Box 7.7 ). Small increases in heart rate also occurred when hyperbaric concentrations of nitrous oxide were administered or when the gas was added to a volatile anesthetic. In contrast, nitrous oxide caused modest reductions in heart rate in patients with coronary artery disease anesthetized with isoflurane and when combined with high-dose morphine or fentanyl (a commonly used anesthetic technique before fast-track cardiac anesthesia came into vogue). Nitrous oxide (60%) produced modest hypertension in humans. Small increases in arterial pressure also occurred under hyperbaric conditions or when the gas was added to a volatile anesthetic in volunteers. These findings were consistent with a mild sympathomimetic effect. Other studies indicated that partial substitution of nitrous oxide for a volatile anesthetic either did not affect or modestly increased arterial pressure during isoflurane or desflurane anesthesia when a constant MAC was maintained. In contrast, reductions in arterial pressure were observed in patients with coronary artery disease receiving nitrous oxide with or without opioids.
Modest tachycardia
Modest increase in arterial pressure
Modest increase in stroke volume and cardiac output
Modest increase in systemic vascular resistance
Decrease in venous capacitance
Modest increase in central venous and pulmonary arterial pressures
Modest increase in pulmonary vascular resistance
Inhibition of lung uptake of norepinephrine
Small increases in cardiac output and stroke volume were noted during administration of 60% nitrous oxide in oxygen to healthy volunteers, but cardiac output remained unchanged in the presence of hyperbaric nitrous oxide. Cardiac output was greater in volunteers anesthetized with nitrous oxide and halothane than observed during halothane alone. The addition of nitrous oxide to isoflurane or desflurane anesthesia also modestly increased cardiac output. These findings are consistent with sympathetic nervous system stimulation by nitrous oxide. In contrast, nitrous oxide reduced cardiac output in patients with heart disease receiving opioids. Hyperbaric nitrous oxide (1.5 MAC) caused a modest increase in systemic vascular resistance. The addition of nitrous oxide to a volatile anesthetic also caused an increase in systemic vascular resistance. Pretreatment with a ganglionic blocker attenuated the relative increase in systemic vascular resistance observed during administration of halothane and nitrous oxide compared with halothane alone. This observation inferred that stimulation of the sympathetic nervous system by nitrous oxide was responsible for the modest increase in arteriolar resistance. Indeed, nitrous oxide–induced increases in systemic vascular resistance were reported in volunteers and patients with cardiac disease anesthetized with opioids.
Nitrous oxide increased venous tone and decreased venous capacitance in conscious volunteers. The anesthetic gas also modestly increased pulmonary artery pressures and pulmonary vascular resistance in patients with coronary artery disease anesthetized with morphine and diazepam or a volatile anesthetic. The combined effects of enhanced venous return, elevated pulmonary vascular resistance, and depressed contractility most likely contributed to the modest increases in central venous pressure observed with nitrous oxide in humans. Hyperbaric nitrous oxide also enhanced central venous pressure in association with increases in pulmonary vascular resistance. Nitrous oxide inhibited norepinephrine uptake by the lung, and subsequent increases in plasma norepinephrine levels detected in the pulmonary vasculature may play a role in the increase in pulmonary vascular resistance. Nitrous oxide–induced increases in pulmonary vascular resistance may be more pronounced in adults with pulmonary hypertension and children with increased pulmonary blood flow. Interestingly, elevations in pulmonary artery pressures and vascular resistance were described during administration of nitrous oxide to neonatal lambs. Such an increase in pulmonary vascular resistance has the potential to reverse a left-to-right shunt and cause arterial desaturation in patients with some forms of congenital heart disease.
Nitrous oxide does not produce direct effects on the coronary vasculature in vitro. The gas altered coronary blood flow in parallel with changes in myocardial oxygen consumption in dogs, consistent with intact metabolic control and the lack of a direct coronary vasomotor effect. However, nitrous oxide decreased myocardial segment shortening, increased postsystolic shortening, and redistributed transmural coronary blood flow preferentially to the subepicardium (decreased endo/epicardial ratio) in experimental models of coronary artery disease. Nitrous oxide decreased the recovery of contractile function of canine stunned myocardium, and, in contrast to the findings with isoflurane, the gas did not reduce myocardial infarct size in rats. These data provided compelling evidence that administration of nitrous oxide before or during transient or prolonged coronary artery occlusion and reperfusion is not associated with myocardial protection. A sympathetically induced increase in myocardial oxygen consumption mediated by nitric oxide may represent a potential mechanism for the absence of a beneficial effect during reversible or irreversible ischemic injury during administration of nitrous oxide. In the presence of a volatile anesthetic, nitrous oxide decreased myocardial oxygen consumption and extraction, which may exacerbate myocardial ischemia during reductions in arterial pressure in patients with coronary artery disease. However, the addition of nitrous oxide to volatile or opioid anesthetics did not increase the incidence of new regional wall motion abnormalities as assessed using transesophageal echocardiography in this patient population.
The alkylphenol propofol was introduced into clinical practice in 1986. , Propofol is currently the most commonly used sedative-hypnotic for anesthetic induction, including for patients undergoing cardiac surgery. The effects of propofol on myocardial contractility were the subject of considerable debate when the drug’s pharmacology was first being characterized. Propofol caused modest negative inotropic actions in papillary muscle and ventricular septum preparations, but the depression of myocardial contractility appeared to be less than that of sodium thiopental when plasma concentrations of the medications required for an equivalent anesthetic effect were compared. This conclusion was challenged by contemporaneous findings indicating that the negative inotropic actions of propofol and thiopental were similar in isolated hearts. In contrast, other studies suggested that propofol produces little if any direct impact on myocardial contractility. Most notable among these studies was an elegant investigation in which the actions of intracoronary infusions of propofol on regional contractile function (percent segment shortening measured using sonomicrometry) were examined in an open-chest canine model. Only calculated plasma concentrations (15–50 μg/mL) of propofol that substantially exceeded the range required for anesthesia in humans caused decreases in percent segment shortening, whereas lower plasma concentrations of the intravenous anesthetic that mimicked therapeutic levels (1.5–5 μg/mL) did not ( Fig. 7.27 ). Clinical concentrations of propofol also did not affect contractility in isolated human atrial muscle obtained from patients undergoing CABG ( Fig. 7.28 ).
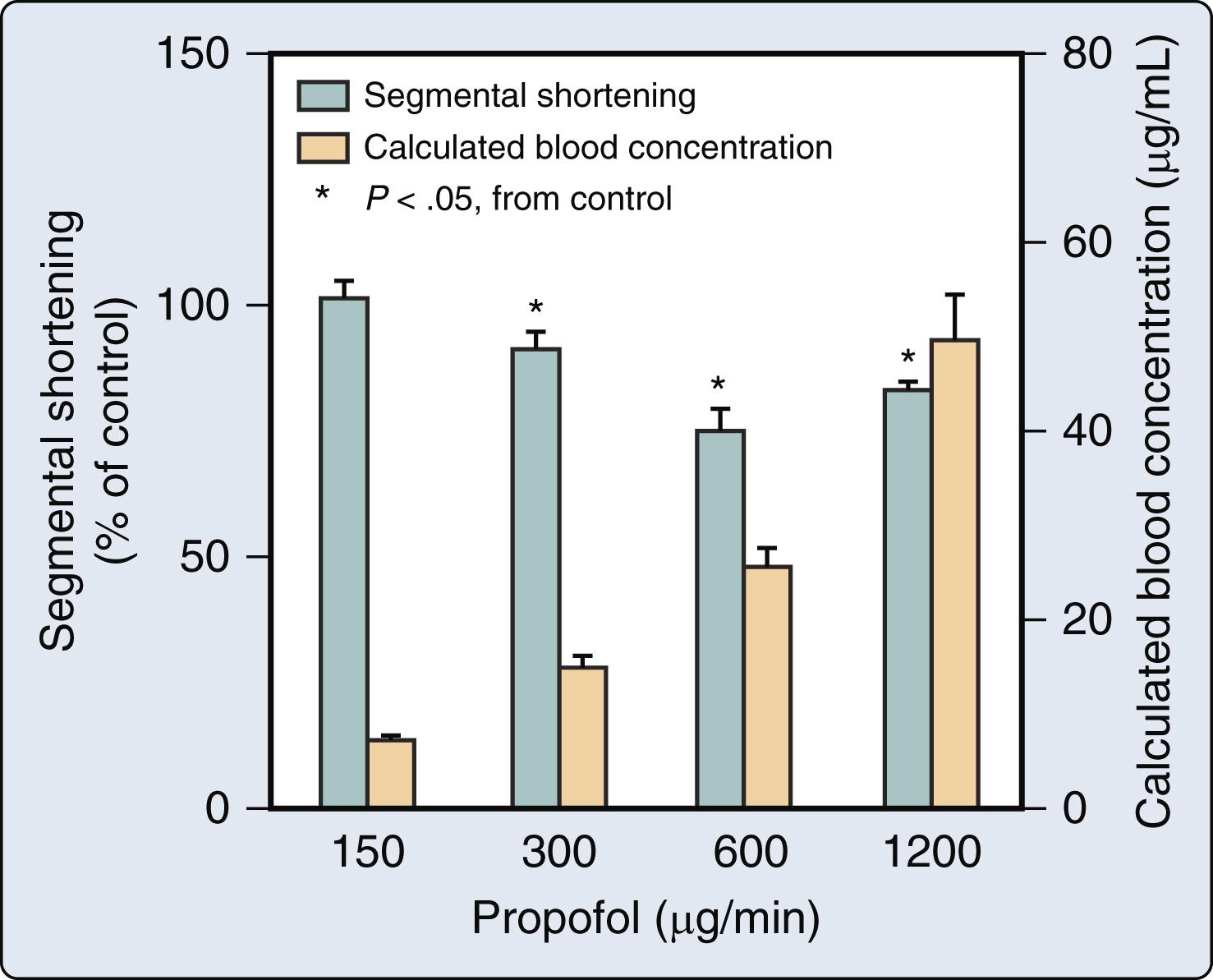
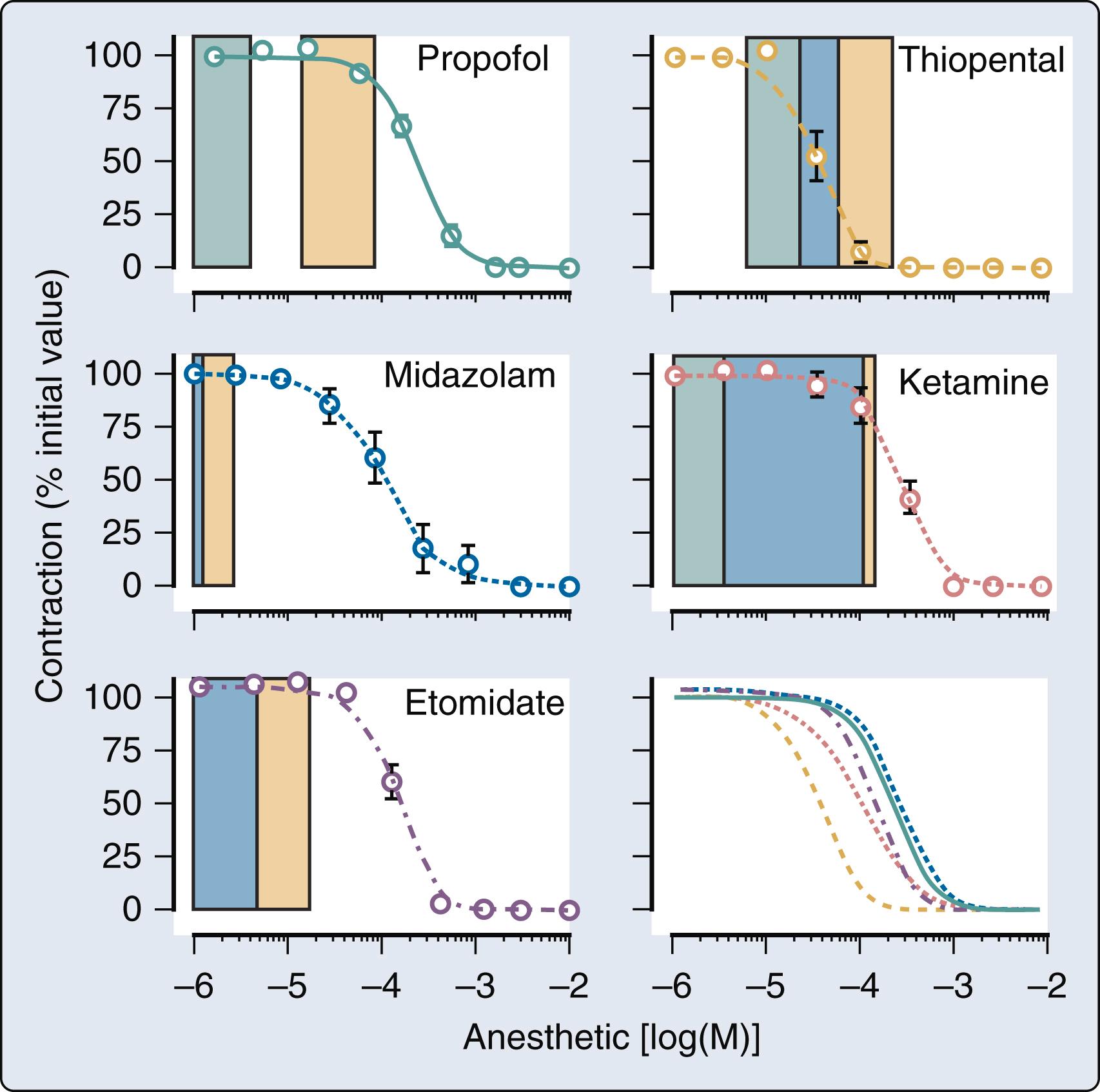
Contradictory findings about the relative impact of propofol on contractility were also reported in patients. LV ejection fraction was unchanged during administration of propofol to patients with coronary artery disease. Propofol also did not affect cardiac output or stroke volume when the drug was administered for anesthetic induction to patients undergoing CABG or noncardiac surgery. It is highly likely that reductions in LV afterload contributed to the preservation of these ejection phase indices of contractility. In contrast, infusions of propofol were shown to cause dose-related reductions in heart rate- and load-independent indices of myocardial contractility derived from LV end-systolic pressure-segment length or -volume relations (E es , preload recruitable stroke work) in canine models. , These findings suggested that propofol exerts direct negative inotropic effects, but this depression of contractility was most prominent when infusion rates of the intravenous anesthetic resulted in plasma concentrations that were much greater than the therapeutic range in humans. Analogous results were obtained during anesthetic induction with propofol using estimates of end-systolic pressure-volume relations in healthy volunteers or patients undergoing elective surgery. When viewed in their totality, the data indicated that rapid administration of larger induction doses of propofol and the resulting high plasma concentrations of the drug probably cause transient depression of myocardial contractility that contributes to hypotension. In contrast, infusions of propofol used for conscious sedation or maintenance of anesthesia generate plasma concentrations that are insufficient to produce clinically meaningful negative inotropic effects. Partial inhibition of transsarcolemmal Ca 2+ current through interaction with the dihydropyridine L-type Ca 2+ channel and attenuation of the Ca 2+ channel’s function likely mediate the effects of propofol on myocardial contractility.
The influence of propofol on myocardial contractility in models of and patients with preexisting LV dysfunction has been incompletely studied. The intravenous anesthetic did not cause more pronounced myocardial depression in patients with mildly compromised LV function undergoing CABG compared with those who had normal LV function. The negative inotropic effects of propofol were greater in isolated porcine cardiomyopathic ventricular myocytes than those produced by the intravenous anesthetic in normal myocardium. Similar results were obtained in human LV trabecular muscle obtained from explanted failing hearts. In contrast, the magnitude of propofol-induced myocardial depression quantified using regional preload recruitable stroke work was nearly equivalent in normal versus cardiomyopathic canine hearts ( Fig. 7.29 ). These findings suggested that propofol does not exert more pronounced negative inotropic effects in HFrEF. As observed in the normal heart, propofol-induced decreases in contractility in the failing heart were generally observed at plasma concentrations that exceeded the anesthetic range in humans. This concentration dependence of propofol’s negative inotropic actions was supported by the observation that propofol (1, 3, and 10 μg/mL) did not affect contractility in an experimental model of congenital hypertrophic cardiomyopathy.
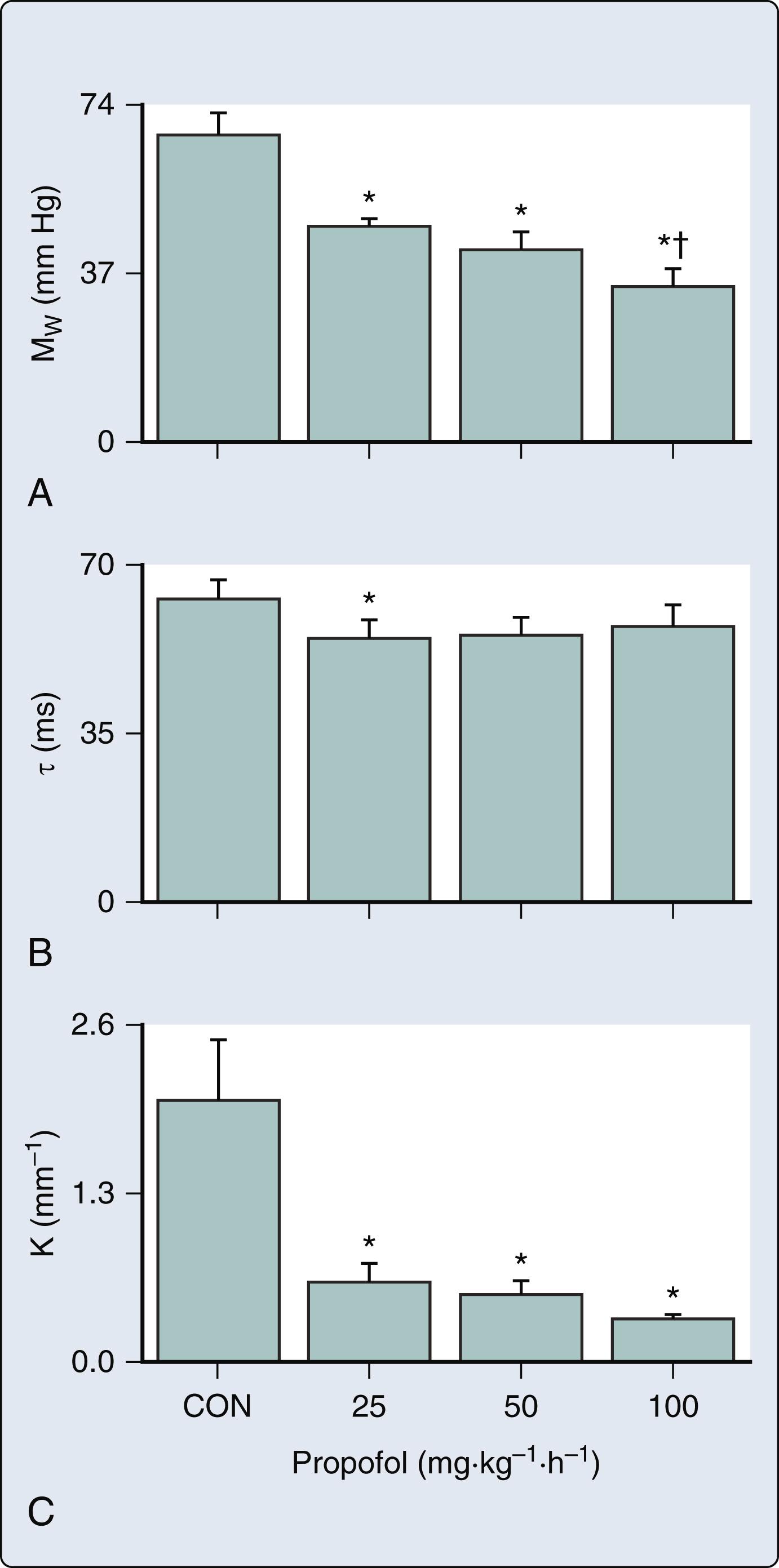
Propofol has little if any effect on LV diastolic function in the normal heart. Propofol did not affect the time constant of LV isovolumic relaxation or regional chamber stiffness measured invasively in a chronically instrumented canine model ( Fig. 7.30 ). Similar findings were reported in humans during propofol anesthesia. The intravenous anesthetic caused a modest decline in the time constant of isovolumic relaxation, reduced early LV filling, and decreased regional chamber stiffness in a canine model of HFrEF ( Fig. 7.29 ). These findings were comparable to those observed in patients with diastolic dysfunction undergoing CABG. The propofol-induced improvements in indices of diastolic function were most likely the result of favorable reductions in LV loading conditions rather than any intrinsic lusitropic effect. Propofol had a modest beneficial effect on diastolic function in isolated hearts exposed to ischemia-reperfusion injury. Conscious sedation with propofol did not affect transmitral and tissue Doppler indices of LV diastolic function measured using transthoracic echocardiography in patients with preexisting diastolic dysfunction. Propofol also did not alter mitral annular velocity (tissue Doppler imaging) in a similar group of patients.
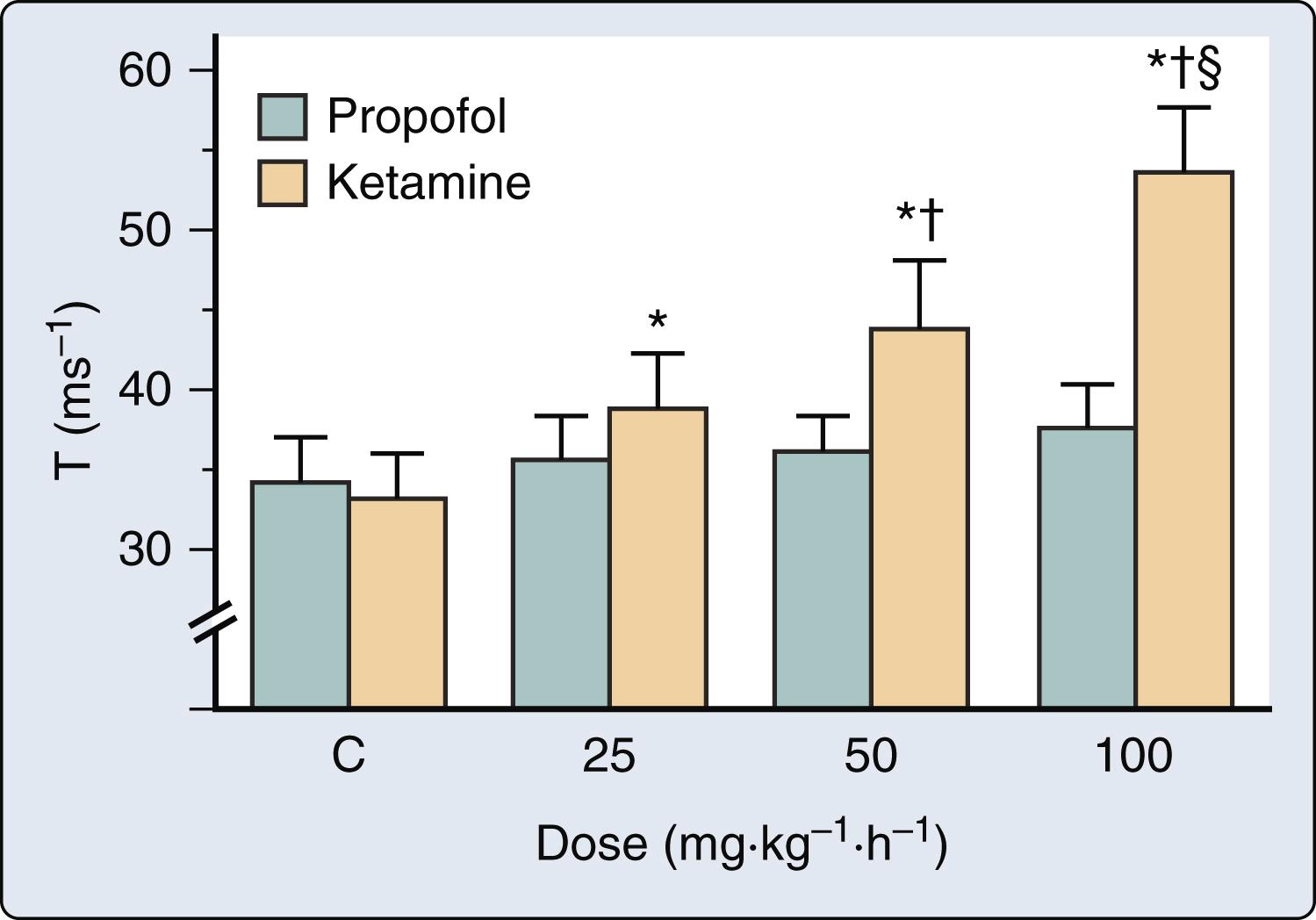
Arterial vasodilation is a major contributing factor to the decreases in arterial pressure observed during propofol anesthesia. Many clinical studies clearly documented that induction or maintenance of anesthesia with propofol is associated with declines in systemic vascular resistance and hypotension. An especially noteworthy study conducted in patients with total artificial hearts modified to maintain constant cardiac output convincing demonstrated that propofol is a potent arterial vasodilator. Anesthetic induction with propofol (2.5 mg/kg) caused a large decrease in systemic vascular resistance index that was largely responsible for the hypotension associated with the drug ( Fig. 7.31 ). Similarly, peripheral vascular resistance (the ratio of pressure to pump flow) and mean arterial pressure fell sharply after propofol (2 mg/kg) was administered during cardiopulmonary bypass when flow was maintained at a constant rate. As discussed previously in this chapter and elsewhere (see Chapter 5 ), systemic vascular resistance is not a quantitative index of LV afterload. The effects of propofol on aortic input impedance (Z in [ω]) interpreted using a three-element Windkessel model of the circulation were characterized. Propofol caused dose-dependent decreases in total arterial resistance (R) and increases in total arterial compliance (C) but did not alter arterial wave reflection. These findings indicated that the intravenous anesthetic reduces LV afterload not only by producing arteriolar vasodilation but also by favorably affecting the elastic characteristics of the aorta and proximal great vessels. Thus the impact of propofol on LV afterload is more pronounced than that of volatile anesthetics and more closely resembles those of sodium nitroprusside. Despite the reductions in LV afterload produced by propofol, intravenous infusions of the sedative-hypnotic adversely affected LV-arterial coupling (ratio of E es to E a ) and mechanical efficiency (ratio of SW to PVA) in an open-chest canine model because simultaneous negative inotropic effects were observed. These actions of propofol on LV afterload and LV-arterial coupling are likely to be most apparent when the drug is used for anesthetic induction because higher plasma concentrations are achieved than those observed during maintenance of anesthesia. Propofol caused reductions in R and C in a canine model of HFrEF that were of similar magnitude to those observed in the normal cardiovascular system. These data suggested that a decline in LV afterload is also an important cause of propofol-induced hypotension in patients with preexisting LV dysfunction.
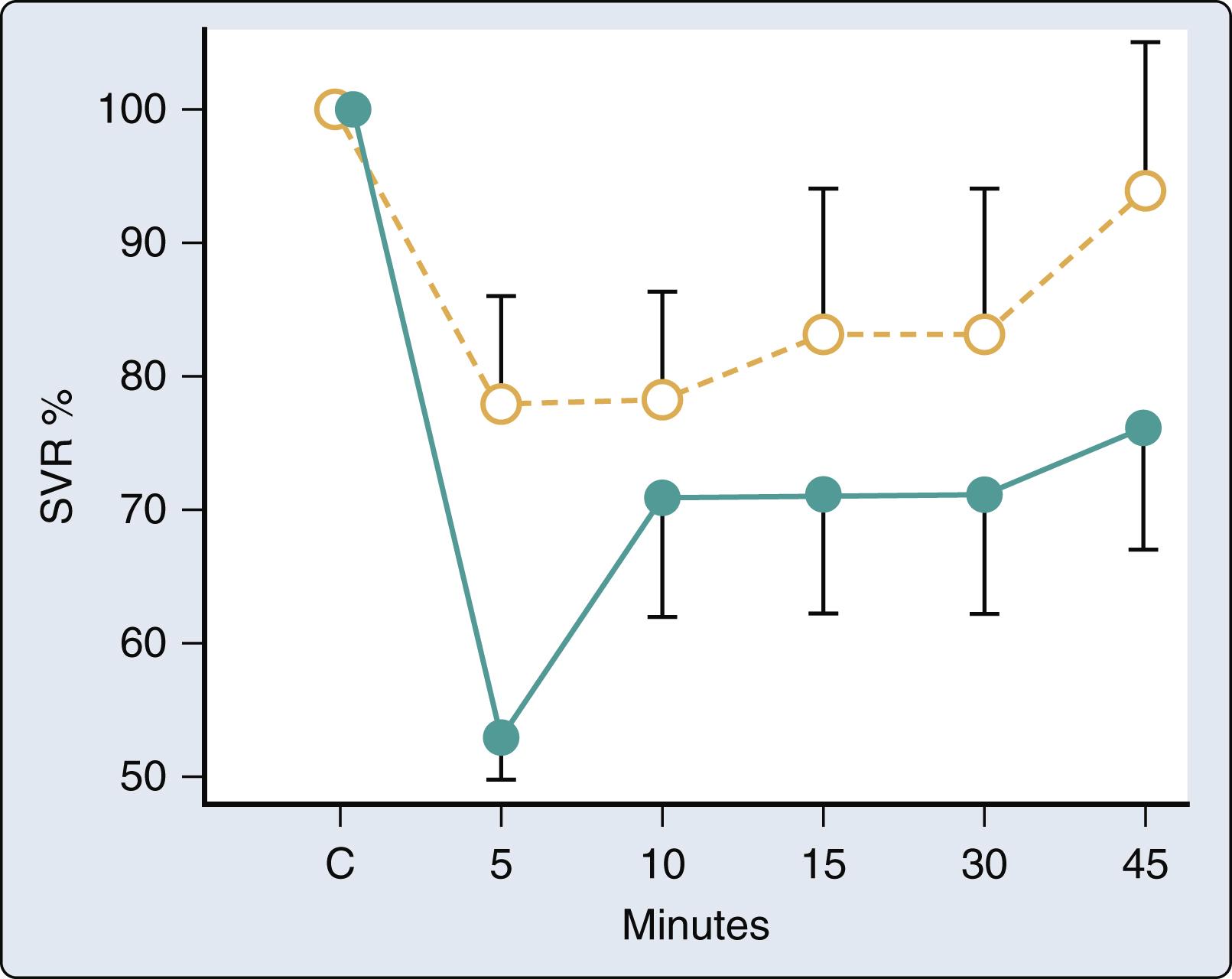
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here