Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Thyroid nodules are common and usually benign but may be malignant, and since there is currently no non-invasive method to determine which ones are malignant, standard of care involves thyroid fine-needle aspiration (FNA). Thyroid FNA is widely accepted for the evaluation of thyroid nodules, since it is rapid, safe, cost-effective, and accurate when definitive. When the cytomorphological diagnosis is benign, thyroid FNA has a negative predictive value that exceeds 95%, and when the cytomorphological diagnosis is malignant, the positive predictive value exceeds 99%. Nonetheless, approximately 20% of all thyroid aspirates yield an indeterminate or suspicious only result, with associated malignancy rates that range from 5% to 85%.
Cytomorphologically indeterminate nodules result from three inherent limitations of cytopathology. First, cytopathology deals with a sampling of a heterogeneous, non-uniformly distributed tissue and extracellular material. Second, the interpretation of the morphological findings in heterogeneous, non-uniformly distributed cells involves human sequential image processing so is prone to interobserver subjectivity. Third, cytopathological sampling cannot capture evidence of capsular invasion that is a sine qua non finding for the diagnosis of malignancy on histological sections. Thus, indeterminate findings are unavoidable. However, the manner in which they have been communicated historically has led to significant confusion and a lack of good evidence-based management guidelines merely because the nomenclature was too unstandardized.
With the number of thyroid aspirates increasing due to growing availability of imaging techniques and possibly underlying changes in the molecular epidemiology of thyroid cancer, there was a substantial need for standardized conventions on reporting thyroid cytopathology results, especially the indeterminate ones. Thus, in 2007, a multidisciplinary panel assembled by Dr Andrea Abati convened in Bethesda, MD, at the National Cancer Institute and participated in a State of the Science Conference that developed evidence-based consensus guidelines for the reporting of thyroid cytopathology. These were later formalized into The Bethesda System for Reporting Thyroid Cytopathology or TBSRTC.
Within TBSRTC are six tiers of malignancy risk that are associated with specific management recommendations. These are summarized in Table 23-1 . This section focuses on the morphological criteria and diagnostic considerations of each of the categories, while the subsequent sections address relevant developments in the application of TBS and the incorporation of molecular testing within the context of TBSRTC.
| Diagnostic Category | MP (%) | Clinical Management |
|---|---|---|
| Insufficient for diagnosis | 1–4 | Repeat FNA, preferably with ultrasound guidance |
| Benign | 0–3 | Clinical follow-up |
| AUS | 20–25 | Repeat FNA |
| Suspicious for follicular or Hürthle cell neoplasm | 15–30 | Surgical lobectomy |
| Suspicious for malignancy | 60–77 | Surgical lobectomy or near total thyroidectomy |
| Malignancy | >97 | Near total thyroidectomy ± 131 I therapy ± central lymph node dissection |
The non-diagnostic category consists of specimens that have too few follicular cells or are too severely compromised to be readable. The anecdotal quantitative minimum – six groups of follicular cells that contain at least 10 cells each – can be excepted when other diagnostic findings are present such as abundant colloid, a lymphocytic abundance that would indicate Hashimoto thyroiditis (HT), or rare atypical findings ( Fig. 23-1 ). While specimens containing copious numbers of cystic macrophages without follicular epithelium are technically non-diagnostic, the use of this category should be noted as such, as cystic papillary thyroid carcinoma (PTC) may yield aspirates that are indistinguishable from those containing cyst contents alone.
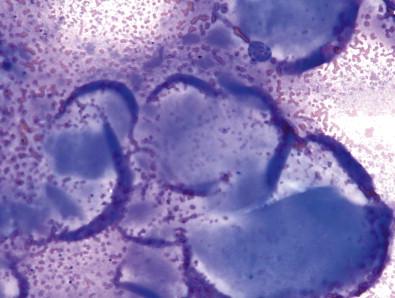
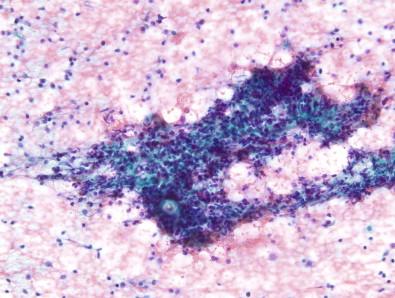
Three types of aspirates are categorized as benign: cellular aspirates with morphologically benign follicular epithelium and colloid; aspirates containing abundant colloid with scant benign follicular epithelium; and aspirates containing numerous polymorphic lymphocytes with any amount of benign follicular epithelium. Of these, the most common benign thyroid aspirate consists of benign follicular epithelium arranged in macrofollicles that are associated with colloid or exist in a background of watery colloid. In these specimens, the cellularity is so great that counting follicles is irrelevant. However, as shown in Fig. 23-1 , the abundance of colloid can be used to assure specimen adequacy even if the sample is paucicellular with scant benign follicular epithelium. The alternative variety of benign aspirate is that which contains findings suggestive of HT. While numerous lymphocytes and serologic evidence of HT can be enough to deem the specimen adequate and classify it as that of HT without follicular epithelium, the possibility of an intrathyroidal lymph node cannot be entirely excluded if colloid and follicular epithelium are totally absent. In these aspirates, the presence of some follicular epithelium is useful. As shown in Fig. 23-2 , the follicular epithelium often shows Hürthle cell changes; these are normal findings in HT that do not require extra commentary unless nuclear atypia is also present.
The atypia of undetermined significance (AUS) or follicular lesion of undetermined significance (FLUS) category consists of nine distinct subtypes of compromised specimens that cannot be easily categorized into the other categories. These subtypes include:
Nuclear atypia in cyst-lining cells
Predominance of microfollicles in a sparsely cellular aspirate
Predominance of Hürthle cells in a sparely cellular aspirate
Focal features of PTC (focal nuclear atypia)
Predominance of Hürthle cells in a background suggesting HT
Morphological atypia in the setting of poor specimen preparation, including air-drying artifact and blood clot artifact
Follicular cell atypia (nuclear enlargement and/or prominent nucleoli) in patients with a history of radioactive iodine or repair due to cyst lesion involution
Atypical lymphoid infiltrate in which flow cytometric immunophenotyping could not be performed
Other atypical findings not classifiable in any of the other categories or subtypes of AUS.
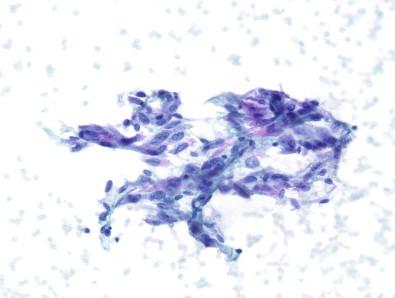
Of these, cyst-lining cells are a common mimicker of PTC, and this diagnostic confusion is exasperated when intranuclear inclusions are present. A cyst component in the aspirate – histiocytes with small nuclei and vacuolated cytoplasm – is helpful in alerting the cytopathologist to the possibility that the atypical cells may be cyst-lining cells. However, these are not often seen, and the cystic radiographic component may not be appreciable on ultrasound. Thus, some intrinsic features of cyst-lining cells can be useful. As seen in Figs 23-3 and 23-4 , the presence of a tapering cytoplasmic tail, binucleation, and abundant granular cytoplasm all are useful in delineating cyst-lining cells from follicular cells with features suspicious for PTC.
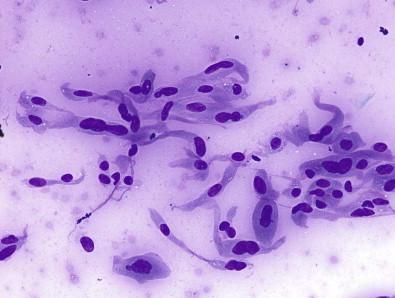
Microfollicles are a common finding in specimens that are interpreted as AUS. When microfollicles are seen, the index of suspicion is low if they are rare within in a cellular specimen that also contains abundant colloid; the index of suspicion increases when they are more prominent or are found within a scant specimen that contains little or no colloid. As seen in Fig. 23-5 , microfollicles are small and usually comprise monomorphic follicular epithelial cells that may show some overlap or may be arranged tightly around a fleck of hard colloid. However, microfollicles do not appear the same in every specimen; Fig. 23-6 shows a case in which the microfollicles have abundant oncocytic cytoplasm and display some nuclear pleomorphism. Oncocytic cytoplasm invariably leads to a search for evidence of HT; when it is present, the decision about how to best categorize the aspirate – benign HT or AUS with microfollicles – is not entirely clear.
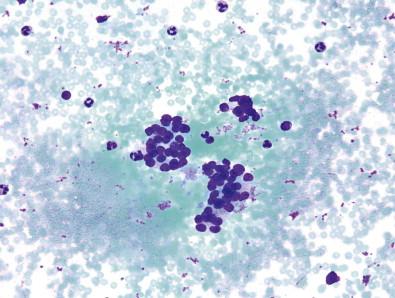
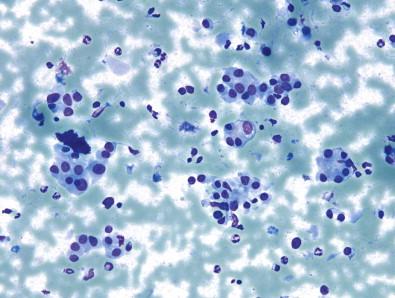
Of the remaining AUS subtypes, the subtype that includes focal nuclear atypia in an otherwise benign-appearing aspirate is the most notable yet difficult to describe or capture. As described elsewhere, focal nuclear enlargement, crowding, overlap, elongation, and grooves are the most common findings in this AUS subtype; inclusions usually are either classified as cyst-lining cells or prompt a higher risk cytological diagnosis. Additionally, the number of these findings that are present is usually low (less than 3), as more of these findings could enable the cytopathologist to arrive at a higher risk diagnosis such as “Suspicious for PTC” or even “PTC,” and these higher risk diagnoses will be described below.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here