Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Nail histopathology requires a sound knowledge of the anatomy of the nail apparatus and excellent clinical-pathological correlation. The first major challenge for the nail histopathologist is to obtain interpretable biopsies, i.e., specimens that are correctly sampled by the nail surgeon, but also that are correctly handled and processed in the pathology laboratory. This requires input from the physician, who is expected to provide high-quality clinical information. A drawing showing the lesion and the type of biopsy performed is helpful. A standardized form with a nail sketch should always be sent with the clinical information.
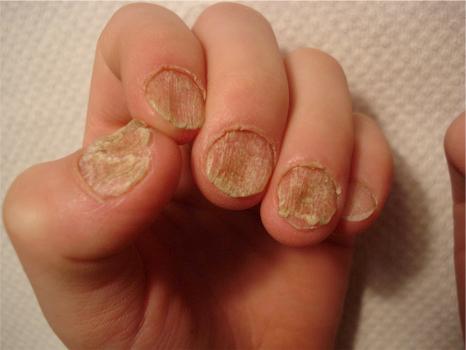
Almost any skin disease may affect the nail apparatus. The diseases that are most often biopsied, restricted to the nail apparatus, and those that show different histologic features when they are located in the nail apparatus, will be considered in this chapter.
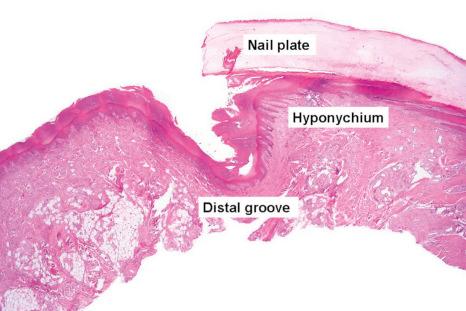
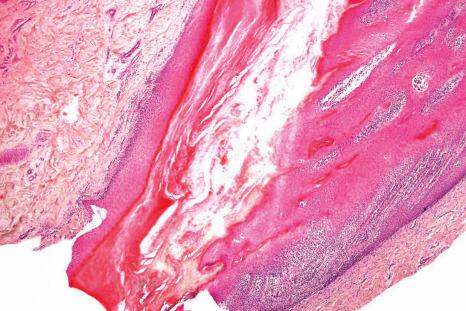
The nail plate, also abbreviated as ‘nail’, is a semihard keratin plate, slightly convex in the longitudinal and transverse axes. It is set in the soft tissues of the dorsal digital extremity from which it is separated by the periungual grooves (proximal, lateral, and distal). It stems from the nail matrix located in the proximal part of the nail apparatus. The nail plate and matrix are proximally covered by a skin fold called the proximal nail fold (PNF). The lunula, also known as ‘half-moon’, is a whitish crescent, distally convex, visible at the proximal part of some nails and more specifically those of the thumbs and the big toes. It corresponds to the distal part of the matrix. From the latter, the nail plate grows toward the distal region, sliding along the nail bed to which it adheres closely. The nail bed is the major area seen through the nail plate. The nail plate only separates from the nail bed at its distal part called the hyponychium ( Fig. 23.1 ).
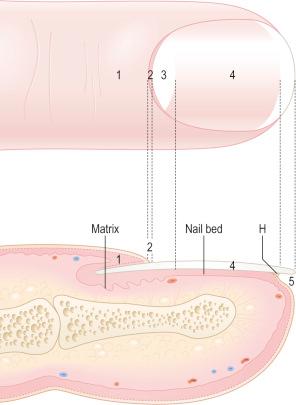
The upper surface of the nail plate is smooth while the under surface is corrugated with parallel longitudinal grooves that interdigitate with opposing ones on the nail bed surface, enhancing adhesion of the nail plate to the nail bed.
The nail grows continuously. In 1 month, fingernails grow about 3 mm and toenails about 1 mm.
The origin of the nail plate proper remains debatable. Most studies agree and show that at least 80% of the nail plate is produced by the matrix. It should be added that the main source of nail plate production is the proximal part of the matrix. This probably explains why distal matrix surgery or nail bed surgery has a low potential for scarring as compared to proximal matrix surgery. Some studies suggest that the nail bed produces 20% of the nail plate, whereas others suggest that the nail bed hardly participates in the making of the nail plate.
The nail plays an important role in everyday life. It protects the distal phalanx from trauma and provides counterpressure to the pulp, which is essential for tactile sensation involving the fingers. The nail allows scratching as a reaction to itch and can be used as a means for attack or defense. Finally, the esthetic importance of the nail should not be forgotten.
The main indications for nail biopsies are inflammatory nail disorders restricted to the nails, longitudinal melanonychia, and nail tumors. Different types of biopsies can be performed ( Fig. 23.2 ).
The longitudinal nail biopsy allows examination of all the nail apparatus components, but leaves a slightly narrowed nail ( Fig. 23.3 ).
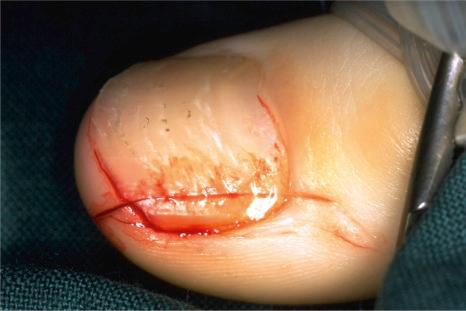
Matrix biopsies should preferably be taken after partial nail plate avulsion. A 3-mm punch biopsy is the most frequently used. Transverse crescentic biopsies are also sometimes obtained. The more recently described matrix shave biopsy is mainly used for longitudinal melanonychia.
In the nail bed, a 3- to 4-mm punch biopsy or a longitudinal elliptic biopsy are the most frequently employed.
Distal nail plate samples, which are easily obtained with nail clippers, can be used for the diagnosis of onychomycosis.
In the laboratory, nail biopsies should be handled by dedicated technicians and read by a dermatopathologist with particular expertise in nails. It should be appreciated that nail dermatopathology is time consuming, even for experienced people.
Inking small nail biopsies must be exacting because China ink may be responsible for pigment artifacts which can make histologic examination more difficult.
If the nail plate is still attached, the keratin in the nail plate can be too hard for ready cutting with a microtome, so some softening method may be required. Our method is to use Mollifex Gurr (VWR Int Ltd). Paraffin blocks are dipped in the Mollifex Gurr before cutting. The length of time (2–12 hours) is dependent on the thickness of the nail plate. Omura places the formalin fixed specimen in a 3 molar solution of potassium hydroxide (KOH) for 1 hour, before paraffin embedding. Prior to cutting, the blocks are placed in KOH for 30 minutes and in a solution of detergent and ammonia for 20 minutes. Five percent trichloroacetic acid in 10% formalin, 5% trichloroacetic acid with a modification of the water-soluble Carbowax embedding method, ‘chitin softening solution’, and softening in cedar oil have also been suggested.
The sample needs to be correctly oriented. We usually try to perform sections parallel to the longitudinal axis of the nail. Lateral longitudinal biopsies are cut, starting from the central part of the nail and progressing toward the skin side. Three- to four-millimeter punch biopsies are embedded as such (without having been previously cut in two). A few sections are first made. If they are correctly oriented, other sections are performed. For nail biopsies, multiple sections are systematically performed.
Special stainings and immunohistochemistry are often necessary.

The lateral nail folds are comparable to normal skin. Hyperkeratosis related to chronic trauma may be observed.
The PNF has a dorsal surface, which is in continuity with, and similar to, the epidermis of the digit. The ventral surface of the PNF is a flat and rather thin squamous epithelium that keratinizes with a stratum granulosum and a stratum corneum. At the angle between its dorsal and ventral parts, the PNF produces a thick stratum corneum called the cuticle. The latter acts as a seal between the nail plate and the PNF, thus protecting the ungual cul-de-sac ( Figs 23.4 and 23.5 ).
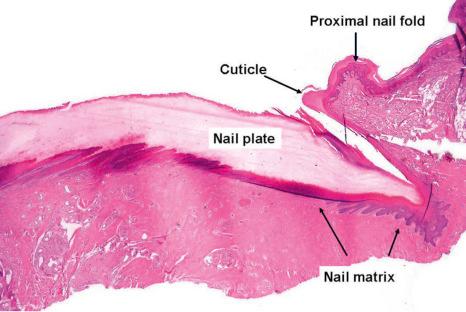
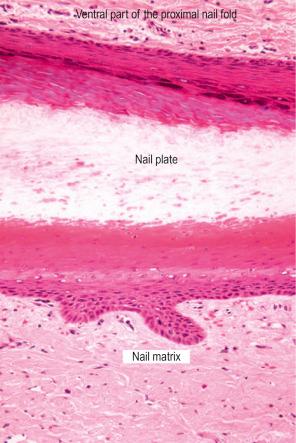
Both structures are located under the nail plate and are typically devoid of a stratum granulosum. The boundary between the nail matrix and nail bed is barely visible on histologic examination ( Fig. 23.6 ).
The nail matrix is a multilayered epithelium with three different compartments: basaloid, prekeratogenous, and keratogenous ( Fig. 23.7 ). The latter is characterized by an eosinophilic onychogenous band, devoid of keratohyaline granules ( Fig. 23.8 ). It gives rise to the nail plate: the proximal part of the matrix contributes to its dorsal aspect and the distal part of the matrix gives rise to its ventral aspect. The nail matrix epithelium is the sole site of hard keratin synthesis. In the midline of the nail unit, the matrix epithelium is thick with long, oblique rete ridges, distally oriented. Laterally, the matrix rete ridges are less marked. Distally, near the nail bed, the matrix epithelium is thinner and its onychogenous band markedly reduced.
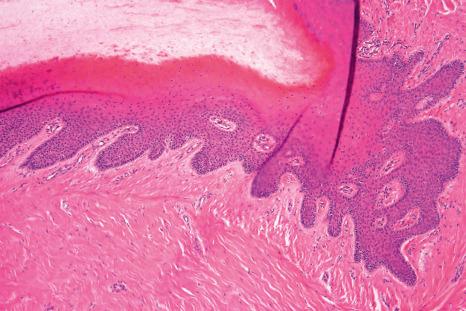
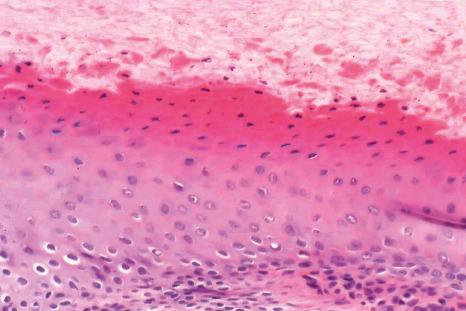
Over the bulk of its area, the nail bed epithelium is thin, reduced to a few cellular layers. In longitudinal sections, it appears flat while in transverse sections, pronounced rete ridges are observed, parallel to the longitudinal axis of the nail. The keratinization is abrupt with no granular cell layer. The nail isthmus designates a transitional zone between the most distal part of the nail bed and the hyponychium, preventing onycholysis. The stratum granulosum reappears only at the hyponychium, which represents the distal thickened part of the nail bed and is bordered by the distal groove and the digital pulp ( Fig. 23.9 ).
The basement membrane of the nail apparatus is almost identical to that of the skin.
The nail matrix and nail bed dermis do not contain pilosebaceous appendages. In the matrix, the dermis comprises two parts: a thin papillary dermis and a relatively thick reticular dermis. The nail bed comprises a single, relatively homogeneous compartment. Eccrine sweat glands are usually absent. However, sweat ducts have been observed by in vivo microscopic examination, near the distal end of the nail bed. Glomus bodies, which are specialized arteriovenous anastomoses involved in temperature regulation, can also be observed in the dermis.
No genuine hypodermis is present in the nail, but a cushion-like layer of adipocytes can be observed in the matrix.

The nail plate is made up of parallel layers of keratinized, flat, and completely differentiated cells called onychocytes. The latter are firmly adherent and not desquamated, in contrast to corneocytes. Remnants of nuclei are frequently observed in the proximal, ventral part of the nail plate.
Microscopic examination of the distal part of the nail, which can easily be sampled with nail clippers, is useful in clinical practice for the diagnosis of onychomycosis. In transverse sections, three zones (characterized by different staining affinities) can be identified at the distal part of the nail: the upper (or dorsal) nail plate, which makes up approximately one-third of the nail; the lower (or ventral) nail plate, which makes up two-thirds of the nail; and the subungual keratin.
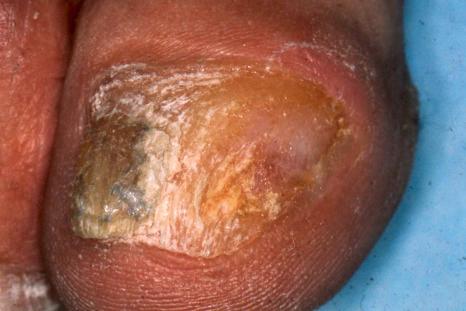
Onychomycosis is the most frequent cause of nail abnormality representing 18% to 50% of nail diseases. In the United Kingdom, North America, and Australia, nail mycoses affect 2% to 3% of the general population and up to 15% of older people. Six types of onychomycosis are now recognized: distal and lateral subungual onychomycosis (DLSO) ( Fig. 23.10 ), superficial onychomycosis (SO), proximal subungual onychomycosis (PSO), endonyx onychomycosis (EO), total dystrophic onychomycosis (TDO), and mixed pattern onychomycosis (MPO). DLSO is the most common form with invasion of fungal hyphae that begins at the hyponychium and spreads along the nail bed proximally, resulting in discoloration, thickening of the nail, subungual hyperkeratosis, and onycholysis. SO develops as an invasion of the dorsal surface of the nail plate, appearing as a white or, more rarely, black powdery and patchy discoloration. PSO is characterized by fungal invasion of the PNF followed by a deeper infection of the nail plate. It may be associated with paronychia. EO is characterized by direct invasion of distal nail plate and absence of nail bed invasion, resulting in a characteristic pattern with lamellar splitting and discoloration of the nail plate. In TDO, there is complete dystrophy of the nail plate. It can be secondary, resulting from complete progression of any of the different types previously mentioned, or primary, affecting immunocompromised patients, especially those suffering from chronic mucocutaneous candidiasis. MPO associates different types of onychomycosis, more frequently PSO and SO or DLSO and SO.
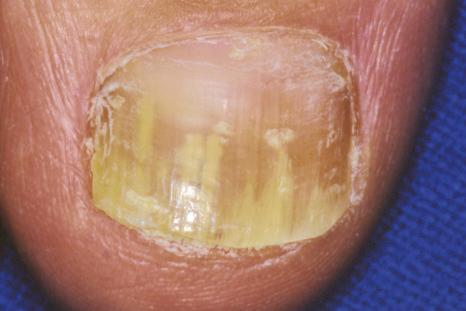
Onychomycoses may be caused by three groups of pathogens :
dermatophytes,
yeasts,
nondermatophyte molds.
Dermatophytes are by far the most commonly encountered, responsible for up to 80% of cases of onychomycosis in Central Europe. Trichophyton rubrum is isolated in 60% to 70% of cases followed by Trichophyton mentagrophytes var. interdigitale . Yeasts will grow in about 5% to 17% of cases, and in 7 out of 10 cases the responsible agent is Candida albicans in the fingernails and Candida parapsilosis in the toenails. Pathogenic molds ( Scytalydium , Aspergillus , Fusarium , Acremonium , and Onychocola canadensis ) are found in fewer than 5% of cases. The clinical classification of onychomycosis and their most frequent causative organisms are shown in Table 23.1 .
| Clinical type of onychomycosis | Main causative organisms |
|---|---|
| Distal and lateral subungual onychomycosis | Trichophyton rubrum Less frequently: Trichophyton mentagrophytes var. interdigitale Rarely: Epidermophyton floccosum |
| Superficial white onychomycosis | Trichophyton mentagrophytes var. interdigitale Rarely: Trichophyton rubrum Aspergillus terreus , Fusarium oxysporum , Acremonium spp. in tropical and subtropical environments Candida albicans in children |
| Proximal subungual onychomycosis | Trichophyton rubrum Exceptionally: Trichophyton megninii , Trichophyton schoenleinii , Epidermophyton floccosum |
| Proximal subungual onychomycosis with paronychia | Candida spp. Fusarium , Scopulariopsis brevicaulis , Aspergillus niger |
| Endonyx onychomycosis | Trichophyton soudanense Trichophyton violaceum |
Onychomycosis is usually diagnosed by direct examination and culture. Histologic examination of a nail sample stained with periodic acid-Schiff (PAS) is a useful complementary technique, especially when there is strong clinical suspicion, but negative results have resulted from fungal culture and KOH preparation. It enables one to:
confirm the diagnosis of onychomycosis,
specify the extent of nail plate invasion (subungual, superficial or total onychomycosis),
suggest the nature of the infecting agent (dermatophyte, yeast, mold),
store the histopathological slides for possible re-evaluation.
In the absence of onychomycosis, histologic examination of a nail plate sampling may disclose an alternative diagnosis especially psoriasis.
More and more studies indicate that staining with PAS is the most sensitive method for diagnosing onychomycosis. Others, however, consider that it is no more valuable than direct examination. This is probably dependent on the sampling technique used. In rare cases, histologic examination is the only investigation which confirms the clinical diagnosis of onychomycosis.
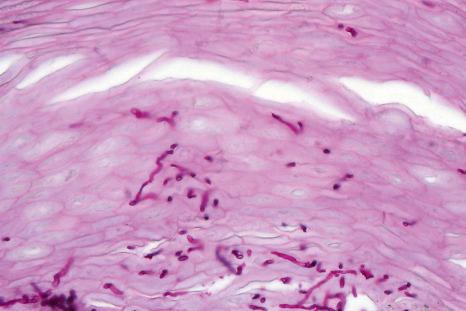
In subungual onychomycosis, the fungi are located in the subungual keratin from where they involve the ventral nail plate. In SO, they are usually restricted to the superficial part of the nail plate. However, they can also invade deeper. In total onychomycosis, the hyphae invade the entire nail plate and subungual keratin. Histologic examination does not allow precise identification of the infecting agent. This requires fungal culture or polymerase chain reaction (PCR). However, regular, straight, septate hyphae that tend to run parallel to the nail surface speak in favor of a dermatophytic infection while small round, yeast forms, some of them budding, pseudohyphae, and/or short filaments are observed in yeast onychomycosis ( Fig. 23.11 ). Spores without pseudohyphae can be contaminants and do not allow a diagnosis of onychomycosis. Truncated spores and irregular hyphae from which arise thin perforating filaments represent a mold infection ( Fig. 23.12 ).
Longitudinal nail biopsies are usually not performed in onychomycosis, except when another diagnosis such as psoriasis or lichen planus is clinically suspected. The histology is often psoriasiform with hyperplasia of the nail bed epithelium and exocytosis of neutrophils. Spongiosis is frequently present. The subungual keratin and nail plate may be thickened or thinned. They contain parakeratotic foci with neutrophil mounds. PAS staining allows the correct diagnosis to be made ( Figs 23.13–23.15 ).
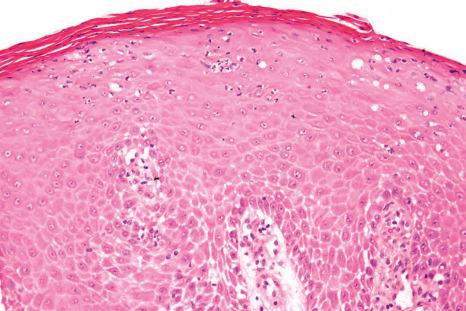
Numerous infections can affect the nail apparatus, particularly the periungual folds. Lesions are generally only biopsied when:
the clinical appearances are atypical (e.g., an extensive herpetic whitlow in a human immunodeficiency virus [HIV]-positive patient, or a paronychia-like cutaneous leishmaniasis),
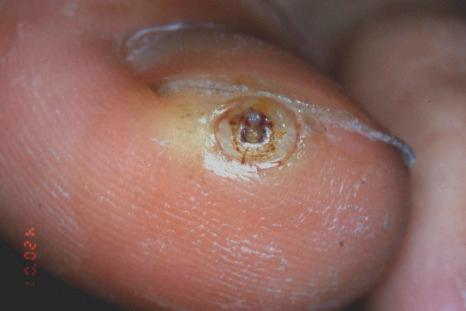
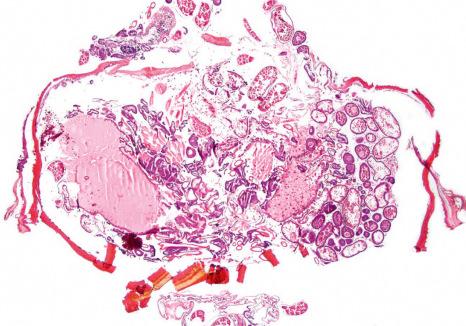
if excision constitutes a treatment modality (e.g., excision of subungual tungiasis ( Figs 23.16 and 23.17 ),
to exclude a malignant tumor.
The histologic appearances of these infections are similar to those observed in the skin.
Periungual warts are frequently seen in children and young adults. They are mainly located around the nail, leaving the nail plate generally unaffected. They present as firm, keratotic papules with sizes ranging from a few millimeters to several centimeters in diameter. Subungual warts generally involve the hyponychium, located in the distal nail bed, and cause subungual hyperkeratosis, or onycholysis. Presentation under the PNF is rare, although the clinical appearance is typical, with a paronychia-like inflammatory reaction and distal wart papillae emerging from under the cuticle ( Fig. 23.18 ).
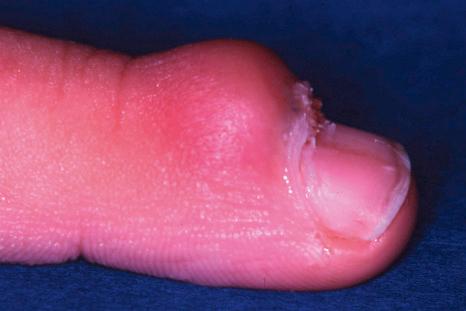
The diagnosis of a periungual wart is generally made from the clinical appearances. However, resistant periungual warts in adults require a biopsy, particularly when only one finger is involved, in order to exclude Bowen disease or an amelanotic melanoma.
Periungual warts are benign epithelial tumors caused by the human papillomavirus (HPV), usually HPV 2 and 4. The histologic appearances include acanthosis, papillomatosis, parakeratosis, and koilocytes in the most superficial layers. In cases of HPV 1-induced infection (myrmecia), the keratinocytes contain numerous inclusions resembling eosinophilic coarse-grained keratohyaline.
The nail has a limited repertoire of clinical manifestations. This is why nail biopsies are often necessary to reach an accurate diagnosis, especially when there is no associated skin or hair involvement.
The interpretation of nail biopsies is difficult because punch biopsies, rather than longitudinal nail biopsies, are often performed and because in almost all specimens a degree of parakeratosis, spongiosis, and rare neutrophils are present.
Nail involvement is frequent in psoriasis, occurring in 10% to 50% of patients. It is estimated that over a lifetime, between 80% and 90% of psoriatic patients will suffer nail disease. Fingernails are more frequently affected than toenails. Several nails are usually affected, but involvement of a single nail can sometimes be seen.
The clinical appearance depends upon the part of the nail involved. Matrix involvement results in pitting, leukonychia, nail plate thickening or thinning, onychorrhexis, and crumbling ( Fig. 23.19 ). Nail bed involvement gives rise to the ‘oil drop’ or ‘salmon patch’ signs, splinter hemorrhages, subungual hyperkeratosis, and onycholysis. PNF involvement can mimic chronic paronychia. Pitting, onycholysis, discoloration, and subungual hyperkeratosis are the most common symptoms.
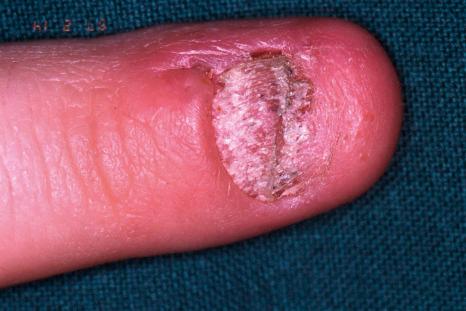
Pustular psoriasis of the nail, also known as Hallopeau acrodermatitis continua, is mainly observed in middle-aged females and has a chronic, relapsing course. It generally affects a single digit and is usually not associated with other manifestations of pustular psoriasis. Involvement of the nail bed with pustules, scale crusts, and onycholysis is seen.
Parakeratosis pustulosa typically affects a single fingernail (thumb or index) in girls younger than 7 years. It is a chronic condition not regarded as a specific disease but as a manifestation of atopic dermatitis, contact dermatitis, and psoriasis. It is characterized by erythematosquamous paronychia accompanied by intermittent vesicles and pustules, onycholysis, mild distal or lateral hyperkeratosis, and nail plate deformities. Although the disease generally resolves after a few years, some children develop psoriasis.
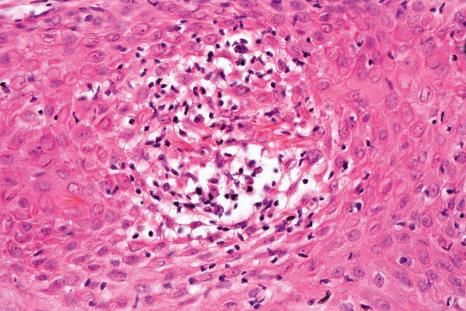
Precise descriptions of the histologic changes associated with the different clinical manifestations of nail psoriasis were first described by Zaias in 1969 and were reviewed in 2007. Histologic changes are rarely typical but often show similarity with those seen in the skin. However, nail psoriasis also displays some distinctive features. As with volar psoriasis, spongiosis is frequently present. The hyponychium loses its normally present granular cell layer, while, in contrast, the nail matrix and nail bed may develop a granular cell layer. The matrix hypergranulosis is analogous to epidermal parakeratosis. Mounds of parakeratosis with neutrophils and focal accumulation of proteinaceous serum-like material, in the nail plate or subungual keratin, are a good clue to the diagnosis. For Grover, hyperkeratosis with parakeratosis and a neutrophilic infiltrate (dermal or epidermal) were the most common findings ( Fig. 23.20 ). However, a definite diagnosis of psoriasis was possible in only 54% of the cases. The best results are obtained with longitudinal biopsies. Nail bed punch biopsies are useful in subungual hyperkeratosis, but rarely in onycholysis. Nail clippings showing severe parakeratosis with neutrophils, pits, or microscopic hemorrhage may suggest psoriasis, if onychomycosis has been ruled out.
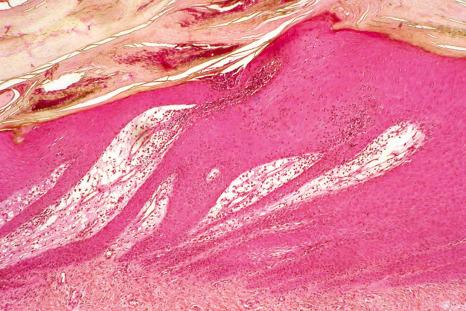
In pustular psoriasis, true spongiform pustules can be observed in the nail bed. They may coalesce to form intraepidermal or subcorneal macropustules ( Figs 23.21 and 23.22 ).
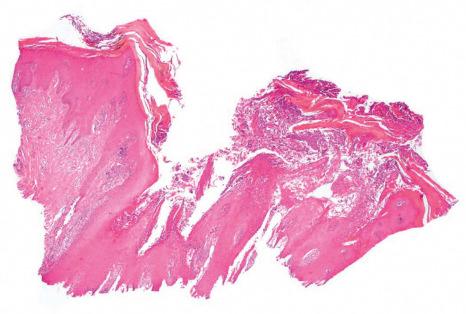
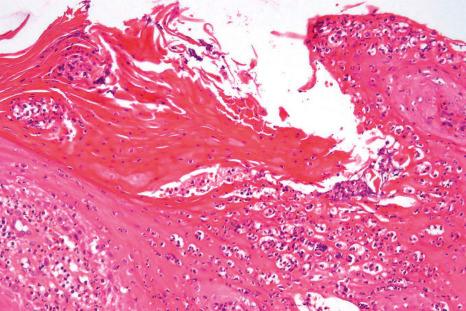
In parakeratosis pustulosa, histology may show spongiotic or psoriasiform changes.
A PAS stain should always be performed to exclude onychomycosis because the latter may clinically and histologically mimic nail psoriasis and because a mycotic superinfection or colonization is observed in 13% to 27% of cases of nail psoriasis.
Nails are affected in 1% to 10% of patients with lichen planus and permanent damage of at least one nail occurs in up to 4% of patients. Approximately 25% of patients with nail lichen planus have clinical lesions at other sites before or after the onset of nail lesions. Fingernails are more frequently affected than toenails. Nail changes are not pathognomonic. However, a diagnosis should be considered when multiple nails are affected by lichenoid nail changes:
longitudinal ridges and splitting,
thinning of the nail plate, with or without dorsal pterygium (dorsal expansion of the PNF resulting from the fusion of the ventral aspect of the PNF with the underlying nail matrix and nail bed),
erythematous patches in the lunula and longitudinal melanonychia (less frequent).
All these features are caused by matrix involvement, the most frequent region affected. Involvement of the nail bed is also possible and results in subungual hyperkeratosis and onycholysis. Complete involvement of the nail matrix and the nail bed leads to a total loss of the nail plate with permanent atrophy of the nail area. Rarer presentations include erosive nail lichen planus, yellow nail syndrome-like features, onychopapilloma, and nail degloving. In children, nail lichen planus has three different presentations: typical nail lesions as above ( Fig. 23.23 ), trachyonychia, and idiopathic atrophy of nails. Nail biopsies are frequently performed due to the risk of permanent nail destruction and because treatment often necessitates systemic corticosteroid therapy.
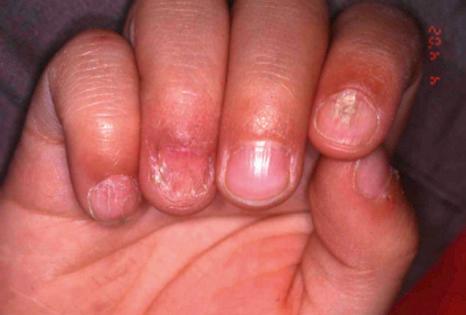
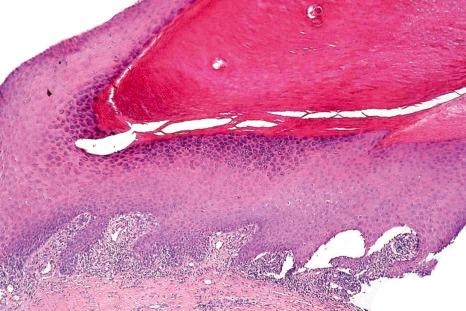
Histologic features of nail lichen planus were first described by Zaias. He observed that it can involve each of the nail unit constituents separately or together. The matrix is more frequently affected than the nail bed and PNF. Punch biopsies confirmed the diagnosis in 85.5% to 100% of the cases. Indeed, histologic changes are usually typical showing acanthosis, hypergranulosis, liquefactive degeneration of the basal cell layer with apoptotic cells, and a bandlike superficial lymphocytic inflammatory infiltrate in the superficial dermis ( Figs 23.24–23.26 ). In the matrix and nail bed, a compact horny layer replaces the normal nail plate above the zones of hypergranulosis. Spongiosis can be prominent. Numerous plasma cells in the infiltrate have been described.
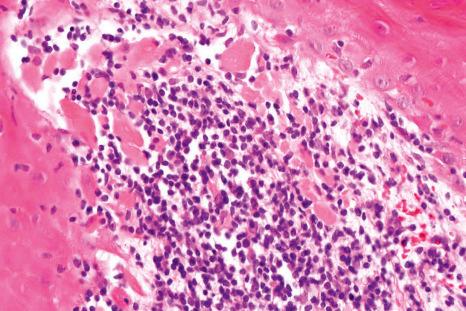
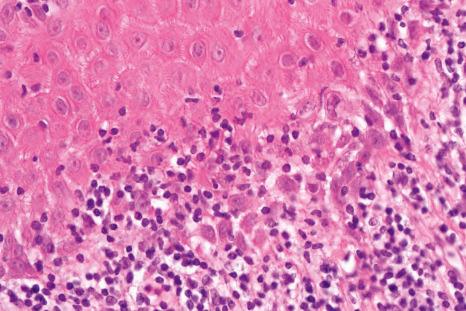
Matrix hypergranulosis is a major feature of nail lichen planus. However, it has also been observed in spongiotic trachyonychia, psoriasis, and pustular psoriasis. Nail lichen planus is indistinguishable from the nail changes seen in graft-versus-host disease and lichenoid drug reactions. In lupus erythematosus, the infiltrate is usually more perivascular, with thickening of the capillary wall. Lichenoid nail changes have been described in systemic amyloidosis and can be the first sign of the disease. Histology reveals typical amyloid deposits in the superficial dermis of the matrix.
Trachyonychia means rough nail. The nail is opaque and covered with thin scales ( Fig. 23.27 ). It shows excessive superficial longitudinal ridging. Thinning of the nail plate is responsible for brittleness, which may be associated with koilonychia (spoon nail deformation) and splitting at the free edge. The cuticles are often hyperplastic and ragged. Baran et al. described another form of trachyonychia, called the shiny type, characterized by multiple punctuate depressions that reflect light. Trachyonychia may affect one, several, or all 20 nails (twenty-nail dystrophy syndrome). Trachyonychia is mainly observed in three dermatological diseases: lichen planus, psoriasis, and alopecia areata. In alopecia areata, trachyonychia is observed in 12% of the cases in children and 3% in adults. The incidence of trachyonychia in psoriasis and lichen planus is unknown. Trachyonychia has also been described in association with atopic dermatitis, ichthyosis vulgaris, IgA deficiency, incontinentia pigmenti, pemphigus, and vitiligo. Idiopathic trachyonychia, i.e., without any concomitant skin or hair disease, has been reported mostly in children and males. Total resolution or marked improvement is observed within the first 6 years in 50% of cases. A nail biopsy is not routinely recommended.
Trachyonychia is best investigated with longitudinal biopsies. Trachyonychia associated with alopecia areata usually shows a mild to moderate lymphocytic infiltrate in the superficial dermis, exocytosis of lymphocytes, and mild to moderate spongiosis, without vacuolar degeneration of the basal cell layer ( Figs 23.28 and 23.29 ). The changes predominantly affect the ventral PNF, the proximal matrix, and the hyponychium. In so-called idiopathic trachyonychia, spongiotic alterations were observed in 83% of the cases, a psoriatic pattern in 13%, while 4% of cases were due to lichen planus. In another series, the proportion was different, with spongiotic dermatitis occurring in 45%, psoriasis in 26%, lichen planus in 18.5%, and non-specific changes in 10%. The results yielded in a third study cannot be compared, as 65.6% of cases were not idiopathic but associated with different known dermatoses.
The possibility that idiopathic spongiotic trachyonychia is actually a variant of alopecia areata limited to nails has been suggested. However, the exact significance of spongiosis is not fully understood. Spongiosis is frequently observed with more specific changes in many nail disorders such as psoriasis and lichen planus. Typical spongiotic changes may also be associated with contact dermatitis, atopic dermatitis, and dyshidrosis. In all of these, the spongiosis usually extends to involve the periungual tissues. This is not seen in alopecia areata.
Nail lichen striatus is rare but probably under-reported. It has mainly been described in children and young adults and usually affects a single digit. It is characterized by a median or asymmetrical, longitudinal, lichenoid nail plate dystrophy ( Fig. 23.30 ). Upper limbs are more frequently affected than the lower limbs. When the typical cutaneous involvement characterized by small, flesh-colored papules in a linear distribution is present, diagnosis is easy. However, the nail dystrophy may appear before the skin involvement or can be isolated. Spontaneous regression after a median duration of 22.6 months is the rule.

A longitudinal nail biopsy from two cases of lichen striatus restricted to a nail showed a moderately dense bandlike lymphohistiocytic inflammatory cell infiltrate affecting the PNF, the nail bed, and the dermis of the nail matrix. It was associated with exocytosis, slight spongiosis, focal hypergranulosis, and dyskeratotic cells in the nail matrix epithelium and with slight focal spongiosis and exocytosis in the ventral PNF, nail bed, and hyponychium. Dyskeratotic cells surrounded by lymphocytes were also present in the nail bed.
Nails are affected in 92% of patients with Darier-White disease, and involvement may also be seen in the absence of any other evidence of the disease. The number of abnormal nails ranges from two or three, to all nails in a minority of patients. The fingernails are more severely affected. Characteristic features are longitudinal red and white streaks associated with distal wedge-shaped subungual keratosis.
Each component of the nail apparatus may be affected, but the most dramatic changes are seen in the nail bed. White longitudinal streaks and subungual keratosis are characterized by epithelial hyperplasia with marked parakeratosis and numerous multinucleated (from 2 to more than 20 nuclei) epithelial cells ( Fig. 23.31 ). The longitudinal red streaks are due to mild epithelial hyperplasia and vasodilatation. The histologic findings in the nail bed of Darier disease differ from those of the skin: presence of multinucleate epithelial giant cells and near-absence of inflammatory infiltrate. The matrix may be completely spared. However, if the distal nail matrix is affected, typical changes of Darier disease may be observed. The proximal matrix is rarely affected.
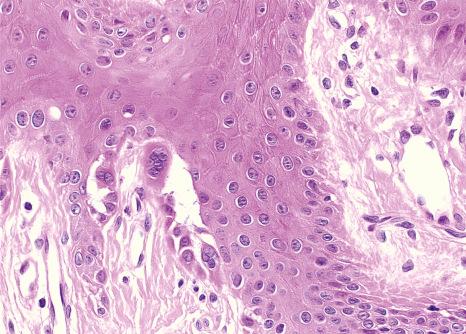
Multinucleate giant cells in the nail bed epithelium were thought to be specific to Darier disease. In fact, they may also be observed in several unrelated nail conditions such as onychopapilloma (see erythronychia) and Bowen disease. A case of focal subungual warty dyskeratoma and three cases of acantholytic dyskeratotic acanthomas of the nail have been described. Clinically, they presented as median longitudinal erythronychia with distal onycholysis. Histologically, they were characterized by suprabasal clefts, acantholytic cells, grains and corps ronds.
Nail changes in pemphigus vulgaris may be more frequent than previously thought. Nail changes were present in 30 of 64 (47%) affected patients. Sixteen patients had onychomycosis and 14 had nail changes due to pemphigus, confirmed by nail biopsy. No correlation was found between duration or severity of the skin disease and nail involvement. Nail involvement may either be an isolated primary manifestation or, more frequently, it may accompany the initial mucocutaneous presentation. It may also occur just before or concurrent with a flare-up of a pre-existing disease. Nail symptoms are varied with chronic paronychia and proximal separation of the nail plate from the nail matrix and/or the nail bed, with subsequent nail shedding (onychomadesis) being the most common. Several nails are usually affected, mainly fingernails. Rare cases of chronic nail involvement restricted to one toenail may lead to permanent loss of nail. Nail biopsies are only performed when chronic paronychia or onychomadesis precede the mucocutaneous lesions or when only one nail is affected. In this instance, a subungual tumor ( Fig. 23.32 ) or herpetic whitlow must be excluded.
Suprabasal clefting and acantholysis, typical of pemphigus vulgaris, are observed in nail matrix, nail bed, and PNFs, as well as are intercellular deposits of IgG and C3 on direct immunofluorescence.
There are fundamental differences between the nail and skin melanocytic populations.
The number of melanocytes is much lower in the nail than in the skin. Nail matrix melanocytes are about 200/mm 2 compared with around 1150/mm 2 in the epidermis. They also differ by their usual quiescence. In the proximal matrix, most melanocytes are dormant and do not produce any pigment, while in the distal matrix 50% are dormant and 50% are active. In the nail bed, melanocytes are even rarer (approximately 50/mm 2 ) and they are dormant.
Melanocytes in a suprabasal position are physiological in the matrix. In the proximal matrix, melanocytes are located within the lower two to four germinative cell layers, while in the distal matrix, they are located in the first and second layers.
The melanocyte density can be routinely measured as the number of intraepithelial melanocytes over a stretch of 1 mm epithelial-dermal junction of the nail matrix and/or nail bed. In normal nails, the density ranges from 4 to 9 melanocytes (mean: 7.7) per 1 mm of nail matrix epithelium. Melanocytes are small; some have dendritic process. Immunostainings with HMB-45 and Mart-1 (Melan A) are useful to better visualize the epithelial nail melanocytes. Immunostaining with S100 protein should not be used, at least alone, as many nail epithelial melanocytes do not express this antigen .
Most melanocytic lesions of the nail apparatus present as a longitudinal or a total melanonychia. A longitudinal melanonychia is a longitudinal pigmented band extending from the matrix up to the distal part of the nail plate, caused by the presence of melanin in the nail plate ( Fig. 23.33 ). In total melanonychia, the whole nail plate is pigmented. The melanosomes originate from matrix melanocytes and are transferred via their dendrites to differentiating matrix cells and will be incorporated in the nail plate. Longitudinal melanonychia may be the first sign of a nail apparatus melanoma, especially when it involves a single digit. This is why biopsies are generally performed. Lateral longitudinal excision (for lateral lesions) and matrix biopsies can be performed.
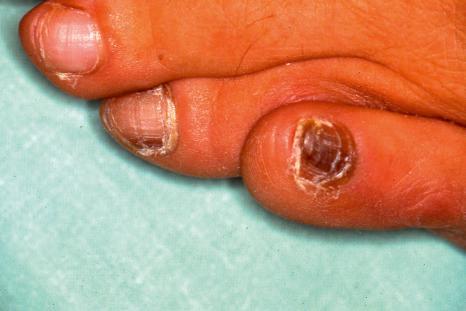
If the nail plate is available for histologic examination, brown melanin granules can be observed in the onychocytes. They appear black with the Fontana-Masson staining. In most examples, the pigment is located in the ventral nail plate, arising from the distal matrix. The pigment location in the nail plate is used to guide the matrix biopsy and to anticipate postsurgical sequelae, as a biopsy performed on the distal matrix carries a much lower risk of postsurgical dystrophy than a proximal matrix biopsy. Nowadays, the location of the pigment can also be determined by dermoscopic examination of the free edge of the nail plate. Moreover, it is recommended to perform the matrix biopsy after proximal nail avulsion and direct visualization of the pigmented area in the matrix.
Histologic examination of the nail matrix allows the identification of two broad groups of longitudinal melanonychia: melanocytic activation and melanocytic proliferation, frequently designated as melanocytic hyperplasia. Their relative incidence is completely different in adults and in children: 73% of single-digit lesions in adults are due to melanocytic activation, while 75% of single-digit lesions in children are due to benign melanocytic hyperplasia, mainly nevi.
In melanocytic activation, melanotic pigmentation of the matrix epithelium is seen, without any increase in the density of melanocytes.
Melanocytic hyperplasia is defined as an increased number of matrix melanocytes. Benign melanocytic hyperplasia can be subdivided into lentigo when benign melanocytes remain arranged in individual units or nevus when at least one nest is present. The proliferation of melanocytes may also be malignant, corresponding to in situ or invasive melanoma.
Although a number of clinical and dermatoscopic features can help in the distinction between benign melanonychia and melanoma, accurate diagnosis requires histologic examination.
A longitudinal pigmented band extending from the matrix but stopping at the distal part of the nail bed, and leaving the free edge of the nail plate unpigmented, has been described. It is due to a pigmented cornified acanthoma of the nail bed. This can be considered as the equivalent of a pigmented seborrheic keratosis. Melanin should not be confused with other chromogens that are not stained with the Fontana-Masson reaction. In the absence of melanin, subungual hematoma is the main differential diagnosis. It is characterized by degrading red blood cells. Perls reaction is always negative in the nail plate, as iron requires macrophage processing to be transformed into hemosiderin.
Longitudinal melanonychia due to melanocytic activation or stimulation is also called hypermelanosis, functional melanonychia, or melanotic macule. The etiologies are multiple and can be classified as physiological, local and regional, dermatological, systemic, and iatrogenic. Laugier-Hunziker syndrome as well as Peutz-Jeghers and Touraine syndrome can be added to this list. Melanocytic activation is more frequent in patients with darker phototypes and increases with age. The pigmented bands frequently involve several nails, but melanocytic activation is also responsible for 73% of single-digit longitudinal melanonychia in adults. Dermatoscopic examination reveals a grayish background usually associated with thin regular gray lines.
By definition, there is no increase in the density of melanocytes. Only some melanocytes with pigmented dendrites and pigmented keratinocytes are observed ( Figs 23.34 and 23.35 ). If the pigment is barely visible, a Fontana-Masson stain should be performed. A few melanophages are frequently present in the superficial dermis.
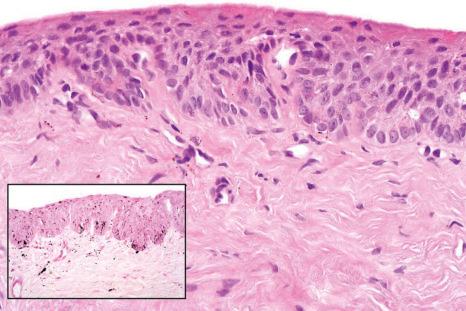
The pathologist can only diagnose melanocytic activation, but cannot determine its precise cause except in pigmented onychomycosis or pigmented Bowen disease.
Longitudinal melanonychia due to fungal infections may show melanin pigmentation of the matrix and nail plate together with nonpigmented fungus in the nail plate. It may also reveal brown-colored hyphae as in onychomycosis caused by the dematiaceous family.
In cases due to pigmented Bowen disease, typical features of Bowen disease are observed (see below) together with melanin pigmentation of the epithelium.
Melanocytic activation is sometimes difficult to differentiate from a lentigo with a slight increase in the melanocyte density. Immunostainings with HMB-45 and Mart-1 may be helpful. This, however, is of little therapeutic consequence, as both entities are benign.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here