Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As a result of the vast progress that has been made very recently in sequencing the entire human genome and in illuminating pathways that are involved in tumorigenesis, it has become clear that the malfunctioning of mutant proteins is the central cause of human cancers.
These proteins—growth factors, growth factor receptors, G-proteins such as ras-p21, the mitogen-activated protein kinases, and nuclear proteins such as Myc, Fos, Jun, and p53—are critical in signal transduction pathways in which proliferation signals from growth factors at the cell membrane are transduced to the nucleus and stimulate cell division.
The mutations in these proteins result in amino acid substitutions or deletions that cause them to be permanently activated. Mutations in regulatory domains of the genes encoding these proteins can result in protein overexpression, which can also result in continuous mitogenic signaling.
These proteins and antibodies to them can be used to detect the presence of cancers in patients at early stages—and even to predict their future occurrence.
Although mutated signal transduction proteins are involved in many different human cancers, it appears that there are patterns of expression of mutated proteins that typify specific cancers.
As a result of the vast progress that has been made recently in sequencing the entire human genome and in illuminating pathways that are involved in tumorigenesis, it has become clear that the malfunctioning of mutant proteins is the central cause of human cancers. This has given rise to a whole new field of proteomics—that is, detection of these aberrant proteins in the blood and other body fluids of patients. This chapter explains these new approaches and emphasizes that many of these aberrant proteins are actually involved in the transmission of proliferation signals on signal transduction pathways, prominent examples of which are discussed. This chapter explains signal transduction pathways in cell proliferation; illustrates how different mutant proteins on these pathways can cause abnormal, continuous proliferation signaling, leading to cell transformation; demonstrates how these proteins and antibodies to them can be used to detect the presence—and even to predict the future occurrence—of cancers in patients; and shows how new proteins that are strongly associated with different types of cancers have been discovered and can be used to detect these cancers at early stages.
Control of the process of cell division in eukaryotic cells, especially in the higher forms of life, is vital to the processes of cell proliferation and differentiation. The fine balance between these two processes is regulated by numerous proteins that interact in the cell to ensure that this balance is maintained. Virtually all these proteins, many of which are critical in regulating the cell cycle, are encoded by oncogenes. Mutations in oncogenes can result in the production of proteins that become either permanently activated in stimulating cell growth and proliferation (e.g., the ras gene–encoded p21 protein). There can also be silencing or attenuation of genes and their products that inhibit cell proliferation (e.g., the p53 protein), otherwise known as tumor suppressor genes. Both events give rise to malignant tumor cells.
Knowledge not only of the existence of these oncogenes and their encoded oncoproteins but also of the mechanisms by which they exert their effects has resulted in a new series of highly sensitive assays for both mutated oncogenes and their encoded mutant proteins. Assays using amplification methods such as real-time polymerase chain reaction (RT-PCR) for mutated oncogenes are discussed extensively in Part 8 and are further discussed in this chapter. However, the main focus of this chapter is how the detection of oncogenic proteins , or oncoproteins, that occur in the serum of patients with malignant tumors enables the diagnosis of malignancy to be made, often at an early stage of tumor development. Because the finding of elevated levels of any of these oncoproteins or mutated forms of these proteins in human serum indicates the likely presence of a malignant tumor, these proteins are also referred to in this chapter as tumor markers . Additionally, in view of the enormous strides that have been made in PCR and other molecular technology, allowing the detection of very few copies of aberrant DNA, we also discuss in this chapter recently developed new detection methods for oncogenic DNA present in the sera and other body fluids of patients with cancer, further allowing early tumor detection.
Oncogenesis, the process by which normal cells become malignant, involves multiple steps that can be broadly classified into tumor initiation and tumor promotion. Mitogenesis itself is a multistep process that commences at the cell membrane as a result of the activation of a growth factor receptor, which then activates other membrane and cytosolic proteins and second-messenger molecules that transduce the mitogenic “signal” to the nucleus. The pathway in which this orderly progression of activation of successive proteins, mostly kinases, occurs from the cell membrane to the nucleus is referred to as a signal transduction pathway.
A number of the steps on the signal transduction pathway that are involved in the transduction of the growth factor–initiated signal to the nucleus have become elucidated, but many of the steps that are involved are still not fully understood. One well-established pathway, for the ras oncogene–induced mitogenic signaling pathway, is summarized in Figure 77.1 . This figure shows that when a growth factor, such as epidermal growth factor (EGF), HER2/ neu , or insulin, binds to its receptor on the cell surface, it induces receptor activation. Most commonly, receptor activation is a result of receptor dimerization as illustrated in Figure 77.2 . The activated receptor in turn activates several intermediary proteins (Grb-2 and SOS, in Fig. 77.1 ) that activate the all-important G-protein (i.e., guanosine triphosphate [GTP]–binding protein), ras-p21. This membrane-bound 21-kDa protein, containing 189 amino acids, is activated when the SOS protein induces it to bind GTP in place of guanosine diphosphate (GDP). In its activated (GTP-bound) state, ras-p21 directly activates Raf ( ; ), which in turn induces a sequential protein kinase cascade beginning with the kinase MEK (formerly called MAP kinase kinase) and mitogen-activated protein (MAP) kinase encoded by the ERK gene, as shown in Figure 77.1 .
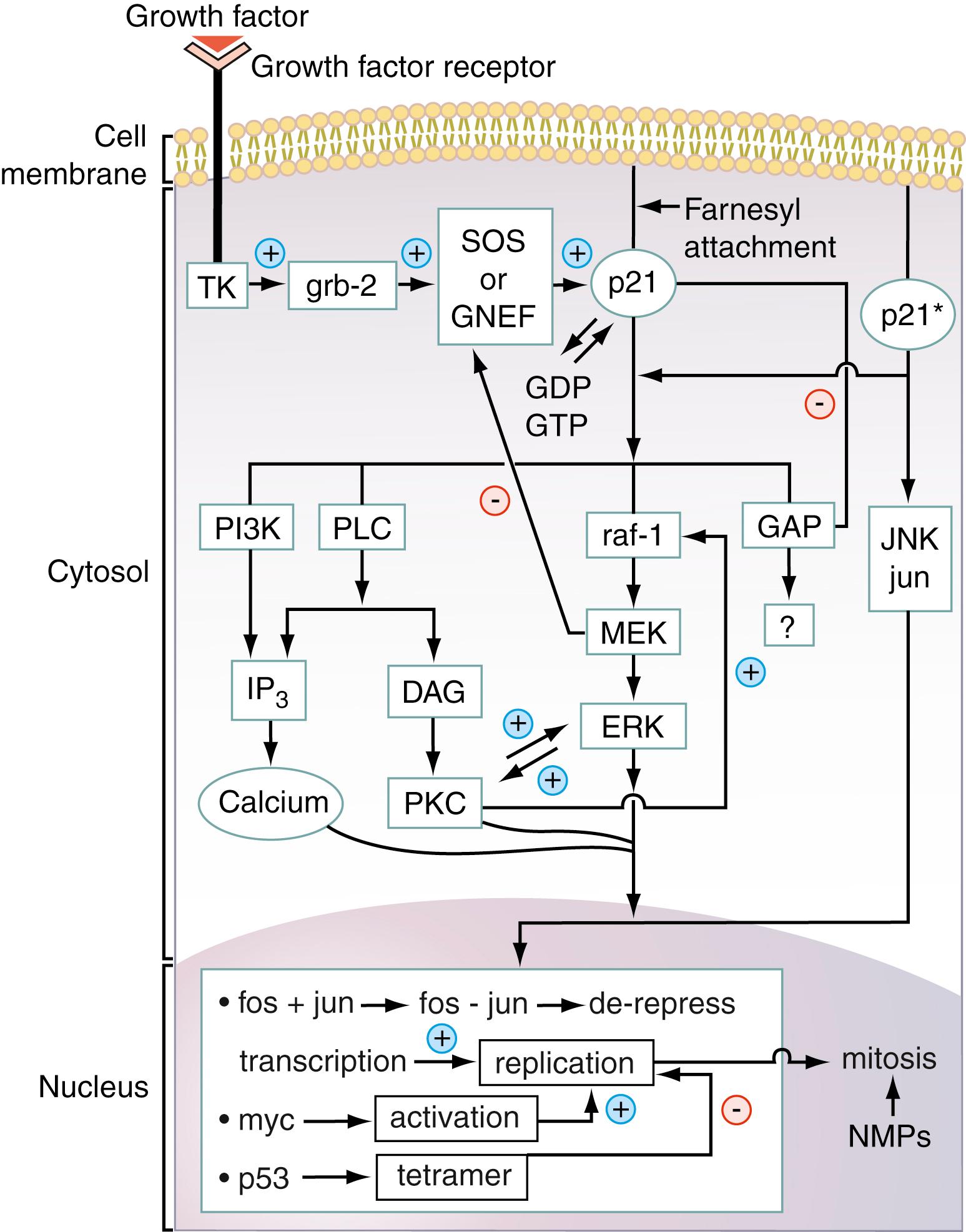
MAP kinase directly activates nuclear transcription factors, one of these being Fos. This important protein forms a heterodimeric complex with another nuclear transcription factor, Jun, which is directly activated by Jun kinase (JNK). The Fos–Jun complex, also called AP1, binds to specific regions of genomic DNA, inducing the transcription of mitogenic proteins such as the cyclins. Transcription of these proteins is blocked by the antioncogene p53 protein (see Fig. 77.1 ), which further induces the transcription of antimitotic proteins such as Bax and caspases that are critical to the process of apoptosis in which the cell dies in a programmed manner.
Figure 77.1 shows that there are several other important nuclear oncogene–encoded proteins such as Myc, a 75-kDa protein that is overexpressed in Burkitt lymphoma. This oncogene protein is not directly on the ras signal transduction pathway. Interestingly, there are cell lines that when transfected with either the ras oncogene or with the myc oncogene do not undergo cell transformation but when transfected with both oncogenes simultaneously do undergo cell transformation. These results indicate that ras and myc may be interdependent and are an excellent prototypical example of the multistage nature of oncogenesis.
Figure 77.1 also illustrates that in normal mitogenic signal transduction, antiproliferative proteins, also called antioncogene proteins—that is, products of tumor suppressor genes, such as p53—become activated and downregulate mitogenic events. If these proteins become mutated at critical positions in their polypeptide chains, they become inactivated, allowing mitogenic events to occur with less inhibition and regulation. Numerous other antioncogene proteins occur, including p16, mutations that have been implicated as a major causative factor of bladder cancer; Rb (retinoblastoma antioncogene protein); and APC (adenomatosis polyposis coli) protein, mutations of which have been implicated in causing familial adenomatous polyposis and colon cancer.
A central feature of the signal transduction events summarized in Figure 77.1 is that the activation cascades are ordered and are under tight regulatory control. Thus, for example, whereas SOS activates ras-p21, GTPase-activating protein induces hydrolysis of GTP bound to Ras-p21, causing its inactivation (see Fig. 77.1 ). Activated MEK downregulates SOS, diminishing GDP–GTP exchange by Ras-p21 ( ).
If one or more proteins on pathways such as the one shown in Figure 77.1 become mutated so they cannot be downregulated, continuous mitogenic signaling becomes possible, leading ultimately to neoplasia. The more such mutations occur, the more likely it is that the cell will undergo malignant transformation. Thus, progressive lesions in a mitogenic pathway or in more than one mitogenic pathway may correspond to the multiple steps in carcinogenesis. This model has been employed in the explanation for the pathogenesis of colorectal cancer ( ) and pancreatic cancer ( ).
These considerations have critically important practical consequences for both the diagnosis and treatment of patients with cancer. In colon cancer, overexpression of the epidermal growth factor receptor (EGFr) can occur, resulting in continuous mitogenic signaling. In addition, as a second occurrence, the ras gene encoding the ras-p21 protein can become mutated, resulting in expression of oncogenic ras-p21 and also resulting in continuous mitogenic signaling. Note in Figure 77.1 that ras occurs downstream of the growth factor receptor (EGFr in this case). If elevated levels of EGFr are found in the serum or tissue of a patient with colorectal cancer, an effective therapy is to treat the patient with anti-EGFr agents such as monoclonal antibodies (e.g., cetuximab and panitumumab). Unfortunately, treatment with EGFr inhibitors has no effect on oncogenic ras-p21 signaling. Therefore, it is necessary, prior to treatment, to ascertain whether oncogenic ras-p21 is present ( ). If it is not present, EGFr inhibitor administration is effective. If oncogenic ras-p21 is present, however, this treatment cannot be employed.
Table 77.1 summarizes the mechanisms by which each type of signal transduction element has been found to induce cell transformation, beginning with growth factors, progressing to growth factor receptors, then to G proteins and the kinase cascades, and finally to nuclear proteins. These mechanisms are discussed in more detail in each section of this chapter devoted to these different signal transduction proteins.
| Pathway Element | Mechanism of Action |
|---|---|
|
|
|
|
|
|
|
|
A number of these pathways seem to be activated in specific types of neoplasia. The ras-pathway itself has been found to be activated in so-called solid tissue (epithelial cell) tumors such as pancreatic cancers (>90%) and colon cancers (∼70%).
As discussed in Chapter 78, Chapter 79 , a number of other signal transduction pathways leading to mitogenesis have been discovered. Several of these interact with the ras pathway described above. Two prominent examples of these are the Akt-mTOR (target of rapamycin) pathway and the JAK-STAT (Janus kinase–signal transducer and activator of transcription) pathway.
Note that in Figure 77.1 , activated ras-p21associates with several “downstream” proteins, one of which is phosphoinositol-3-hydroxykinase (PI3K). This enzyme catalyzes the synthesis of inositol triphosphate (IP3) that is a critical second messenger molecule that is vital in mobilization calcium ions that are required for kinase activation. In addition, PI3K itself activates a vital kinase called Akt or protein kinase B, which in turn activates mTOR, a critical kinase. This protein is discussed in Chapter 24 as being vital in promoting activation of cyclin kinases that activate cyclins that in turn induce progression of T cells (and other cell types) from G1 to S phase in the cell cycle in blast transformation. Constitutive activation of Akt can therefore lead to uncontrolled cell proliferation and cancer. In the PI3K-Akt cascade, another protein becomes activated called phosphatase and tensin homologue protein (PTEN). This protein catalyzes the dephosphorylation of IP3 to inactivate this second messenger and inactivates phosphorylated Akt. Thus PTEN, like p53, is an anti-oncogene protein.
Certain growth factors, cytokines being prominent among them, interact with transmembrane receptors to induce receptor dimerization. In the dimerized state, each receptor binds to one kinase protein called Janus kinase or JAK that, in turn, induces phosphorylation of each receptor. After being phosphorylated, each receptor binds to kinases called signal transducer and activator of transcription proteins (STATs). JAK then phosphorylates each STAT such that each STAT molecule is doubly phosphorylated; in the doubly phosphorylated states, the two STAT molecules dimerize, dissociate from JAK, and enter the nucleus, where they act as transcription activators. Receptors that have been phosphorylated by JAK also bind to PI3K, which in turn activates Akt, which activates mTOR and activation of cyclins as discussed in the preceding paragraph.
Detection of mutated signal transduction proteins or high levels of the wild-type proteins in serum or body fluids is strongly suggestive of neoplasia. Therefore, many assays for different oncoproteins are now available commercially in kit form, including enzyme-linked immunosorbent assay (ELISA) assays for the growth factors, transforming growth factors (TGF)-α and -β and fibroblast growth factor (FGF); the growth factor receptor proteins EGFr and HER2/ neu , Ras-p21; and the nuclear proteins p53, Myc, and NMP22 from such companies as Abcam, Lifespan Biosciences, and Fischer Thermo Scientific. A number of studies also employ the technique of western blotting or immunoblotting, as described elsewhere in this book.
Numerous studies have documented alterations in oncogene, oncoprotein, or growth factor expression in terms of messenger RNA (mRNA) or protein in tumor tissue compared with normal tissue ( ; ; ). Several studies have examined oncoprotein or growth factor expression in biologic fluids such as urine or effusions and demonstrated the feasibility of using these peptides and proteins as markers for the presence of neoplasia ( ; ). Succeeding studies have confirmed these initial findings, resulting in the clinical use of these markers to detect the presence of neoplasia.
Recent studies have now revealed that oncogenes themselves can be detected in the body fluids of patients with different types of cancer. With the advent of microarray techniques for both gene expression and protein detection (see Chapter 70 ), it is now possible to assay for the presence of multiple oncogenes and oncoproteins in the body fluids, especially serum, of patients with cancer. A new, parallel approach for cancer screening has been developed, called proteomics , in which serum samples are subjected to mass spectroscopy and pattern analysis to detect the patterns of expression of proteins that are unique to the blood of patients with cancer. This type of analysis has recently resulted in the identification of not only oncoproteins but also other proteins that are expressed at abnormally elevated or abnormally low levels in specific types of cancer. In fact, these techniques are being applied to other body-derived fluids to find similar oncoproteins. For example, research on urine biomarkers for breast cancer remains investigational but promising ( ).
This chapter focuses on the identification of different oncoproteins and growth factors, as well as their respective genes, in the serum, plasma, or urine in patients with cancer or who are at risk for the development of cancer. It also focuses on expression of other proteins that occur at abnormally elevated or low levels in specific types of cancer. Table 77.2 summarizes some of the many (>100) known oncogenes and their functions in the cell. ELISA or western blotting kits are commercially available for all of these oncogene-encoded proteins.
| Oncogene | Protein Product b | Function |
|---|---|---|
|
EGF receptor | Binds to EGF; dimerizes; activates tyrosine kinases in signal transduction; works through ras |
|
GF receptor | Very similar to EGF receptor; works through ras |
|
β-chain of PDGF | Growth factor receptor; may work through ras |
|
Tyrosine kinase | Transduces signal through ras |
|
p21 proteins; H-, K-, and N-forms | G-proteins; bind to cell membrane and transduce signals through second messengers and Raf, GAP (?), JNK, PKC, and PLC |
|
Anti-ras oncogene; in ras family | Blocks ras action in cells. |
|
74-kDa protein | Phosphorylates MEK, which then phosphorylates MAPK |
|
MAPK family 43-kDa proteins |
Involved in cytoskeletal rearrangements and nuclear signaling |
|
62/64-kDa nuclear protein | Turns on transcription factors involved in replication |
|
Nuclear protein | Forms complex with fos |
|
Nuclear protein | Forms complex with jun; fos–jun complex activates transcription factors |
|
53-kDa nuclear antioncogene protein | Forms tetramers, then binds DNA segments to block transcription and replication |
|
236-kDa nuclear matrix protein | Involved in mitotic spindle formation |
a Only a few of the many (>100) oncogenes are listed in this table. The ones that are listed here encode proteins for which assays have been developed or are closely related to these proteins. No growth factors except platelet-derived growth factor ( PDGF) are included in this table.
b Assay kits for all of the oncogene-encoded proteins listed in this are commercially available.
Because various growth factors are believed to play a role in influencing cellular proliferation during tumorigenesis and because growth factors are actively secreted into the extracellular environment, they are attractive targets for detection in blood during cancer development. Several studies have demonstrated differences in blood levels of growth factors in patients with cancer and noncancer control participants.
TGF-α is a polypeptide with 50 amino acids that binds to the EGF receptor, which dimerizes upon binding to TGF-α or to EGF. TGF-β is a family of proteins labeled β 1 to β 5 . TGF-β 1 is a homodimer of two 12-kDa subunits linked together by disulfide bonds. When bound to its receptor, it induces activation of Smads (small body size gene in Caenorhabditis elegans worms) that are involved in DNA binding and transcriptional activation. Even though TGF-β has been found to be elaborated by many different types of human malignant tumors, elevated serum levels of this growth factor have been found predominantly in cancers of the liver ( ) and bladder (see later). Interestingly, TGF-β has been found to inhibit mitosis in specific cell lines in culture, such as mink bronchial epithelial cells. It is not clear how TGF-β is triggered to act as a tumor suppressor versus tumor promotor. The pathway for tumor promotion has been somewhat elucidated. TGF-β binds to the inhibitor of DNA binding 1 (ID1), a transcriptional regulator resulting in decreased levels of E-cadherin ( ), thereby diminishing the number of tight junctions between cells and causing loss of contact inhibition. This growth factor also has been implicated in the interconversions of epithelial cells into mesenchymal cells and vice versa. These interconversions (called epithelial-to-mesenchymal transformation (EMT) and mesenchymal-to-epithelial transition [MET], the reverse process) are thought to be steps involved in tumorigenesis ( ).
TGF-β activity, which is determined using this technique, has been found to be markedly elevated in patients with hepatocellular carcinoma (HCC) but not in age-matched control participants ( ). More recently, TGF-β has been found to promote the formation of a tumor microenvironment together with other growth factors, such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and FGF, in hepatocellular carcinogenesis ( ).
In the sera of patients who have undergone surgical resection of these tumors, the activity of TGF-β is barely detectable, suggesting that the tumor was the source of the elevated levels of growth factor in serum. Interestingly, in many patients with HCC, TGF-β has been found to be elevated in urine ( ). In this regard, in a study on the development of HCC in patients with hepatitis C (see Chapter 22 ), TGF-β 1 was found to be elevated in the sera of patients with hepatitis C and hepatitis C–induced fibrosis and cirrhosis but was not elevated in the sera of patients in whom HCC subsequently developed, resulting from the antecedent disease. Thus TGF-β 1 may be an early marker for HCC, but lower levels may be required for progression of disease ( ). However, in other studies, TGF-β 1 was found to be elevated in small (<3 cm) HCC far more frequently than α-fetoprotein (AFP), the classic marker for HCC (see Chapter 75 ; ). Using a cutoff of 800 pg/mL for TGF-β 1 and 200 pg/mL for AFP, in which the specificities of both markers were 95% (i.e., few false-positive results), the sensitivity of TGF-β 1 was 68% versus only 24% for AFP. Thus TGF-β 1 may be a reliable marker for small HCC. The same conclusion has been reached in a study of HCC in which the sera of 23% of patients with this disease were positive for TGF-β 1 but not for AFP ( ). TGF-β 1 has also been found to be elevated in patients with metastatic prostate cancer ( ). Levels of TGF-β have also been associated with extraprostatic extension of the tumor. Serum levels TGF-β together with levels of interleukin-6 (IL-6) soluble receptor can be used to predict progression of the disease after surgery ( ).
Use of an ELISA with a monoclonal antibody directed against TGF-β 2 has revealed that TGF-β 2 is elevated in a high proportion of patients with invasive bladder cancer but not in patients with noninvasive bladder cancers or in cancer-free patients ( ; ; ). Recently, a new tumor marker for bladder cancer that appears to be more accurate in diagnosing both noninvasive and invasive bladder cancer is the nuclear matrix protein NMP22, detected in urine described later in this chapter.
Thus, serum assays for TGF-β are highly useful in diagnosing and following HCC. They are less useful in the diagnosis of carcinoma of the bladder, although for invasive bladder cancer, this growth factor has high sensitivity and specificity.
TGF-α has been found to be elevated in a large number of patients with epithelial cell tumors ( ; ), predominantly breast (almost 100%), lung, stomach ( ), colon, liver, and ovarian. It has been found to be elevated frequently in the sera of patients with gastric carcinoma ( ). In contrast, normal individuals have very low serum levels. With respect to liver, TGF-α has been found to be elevated in the sera of patients with HCC but not in patients with cirrhosis. The elevated levels were reduced in patients who had been treated for HCC ( ).
Recent studies confirm that TGF-α is an effective predictor of the occurrence of malignancies at an early stage. For example, in 36 patients who had a history of asbestosis and subsequently developed a cancer, mainly adenocarcinoma or squamous cell carcinoma of the lung, more than one third were found to be seropositive for TGF-α. Blood samples from all of these patients were collected and then banked at the time of the diagnosis of asbestosis and before the diagnosis of malignancy. Significantly, all but one of these seropositive patients were found to have elevated TGF-α levels in their banked blood ( ).
TGF-α thus appears to be an excellent marker for the presence of malignancy. Unlike the case for TGF-β, these elevations are not tumor specific. Nonetheless, it is clear that TGF-α is extremely useful in screening patients for the presence of malignant solid tissue tumors.
PDGF, a protein of molecular mass 28 kDa, exists as a dimer of A and B chains, as the A–A, A–B, or B–B dimer forms. Either chain can be glycosylated, increasing the molecular mass to 30 kDa. This growth factor, originally isolated from platelets, binds to a transmembrane growth factor receptor. The B chain is encoded by the sis oncogene. It has been found to be a potent mitogen in lymphoid, myeloid, and fibroblastic cell lines. Overexpression of PDGF has been found in both lung and pleural tumors. It has also been examined in the blood of patients with cancer. Overall, this growth factor has been found to be significantly elevated in more than 15% of patients with carcinomas, sarcomas, and lymphomas but not at all in normal individuals. In patients with breast cancer, there is an excellent correlation between the stage of the cancer and the serum level of the growth factor ( ). Higher levels predict shorter survivals. In addition, PDGF-B (Sis) in breast tissue appears to correlate with increased cellular proliferation because its expression increases with both nonmalignant and malignant states and decreases after menopause. Elevated serum levels of PDGF-B have also been found to occur in esophageal carcinoma that has infiltrated into lymph nodes ( ). In malignancy, PDGF-B does not correlate with p53 expression, estrogen and progesterone status, or tumor grade ( ). Levels of PDGF-A and PDGF-B were found to be elevated highest in patients with ovarian cancer compared with normal, benign, and borderline patients ( ). The role of PDGF-B as a marker in colorectal cancer is less clear ( ). Serum PDGF-A levels in patients with cholangiocarcinoma were 1.4-fold higher than in normal control participants ( P = .014) ( ).
PDGF-receptor A (PDGFRA) mutations have also been implicated in the pathogenesis of gastrointestinal stromal tumors. Most recent genetic analysis reveals that exon 18 is the commonest site of these mutations, although exon 12 can be involved as well ( ). In addition, overexpression of PDGFRA has been found to occur in more than 20% of patients with triple-negative breast cancer, correlates with severity of disease (i.e., is a marker for disease progression), and correlates with prognosis, with higher levels indicating worse prognoses ( ).
As discussed later, both oncoproteins and antibodies to oncoproteins can be elevated in the sera of patients with cancer. Anti-PDGF antibody (PDGF-Ab) in serum has been found to be potentially diagnostic for pleural mesothelioma in patients with histories of exposure to asbestos. The serum levels of PDGF-Ab correlate positively with severity of the disease and show a significant negative correlation with survival—that is, higher levels correlate with shorter survival times ( ).
Basic fibroblast growth factor (bFGF) is a protein containing 155 amino acids. It is a growth factor for mesenchymal cells but has also been found to have relatively high concentrations in the central nervous system (CNS). Interestingly, bFGF has been found to be present in high concentrations in the sera of patients with epithelial cell tumors. Prominent among these tumors is renal cell carcinoma. More than 50% of patients with this disease have markedly elevated serum levels of bFGF ( ; ), as determined either by ELISA or by enhanced chemiluminescent assays. This growth factor is also elevated in the sera of more than 50% of patients with CNS tumors, 90% of patients with lung cancers ( ), and more than 60% of patients with lymphomas ( ). It is not elevated, however, in the sera of large populations of normal (control) individuals.
In patients with colorectal cancer with tumors 30 mm or smaller, serum bFGF levels were 7.65 ± 1.11 pg/mL, whereas those with tumors greater than 30 mm had bFGF levels of 8.53 ± 3.22 pg/mL. Although these are small differences, it has been found that serum bFGF levels could be used to predict tumor size in colorectal cancer ( ).
Elevated serum levels of bFGF have been found to be a good prognostic factor in patients with non–small cell carcinomas of the lung (NSCLCs; ). More recently, the extent of overexpression of bFGF appears to correlate with prognosis for both small cell carcinoma of the lung and NSCLC, with higher levels indicating worse prognosis ( ). Thus, TGF-α and TGF-β, PDGF, and bFGF all appear to be elevated in the sera of a significant number of patients with epithelial cell tumors but are not completely tumor specific. TGF-α has some specificity for breast cancer and TGF-β for HCC. bFGF is elevated in a variety of malignancies, including nonepithelial cell tumors, such as CNS tumors and lymphomas. PDGF shows little specificity for tumor type, but high levels in serum indicate the presence of malignancy.
Given the development of protein–antibody and gene arrays, it is possible to assay the sera and other body fluids of cohorts of patients who are at risk for developing cancer because of such factors as occupational exposure for multiple oncogene proteins concurrently. Discovery of the elevation of or the presence of oncogenic mutations in one or more of these proteins in a patient’s serum or other body fluid identifies the patient as either having a cancer or developing one in the near future. Recently, serial levels of both TGF-β and PDGF were determined by ELISA (see Chapter 45 ) on a cohort of workers with long-term exposure to asbestos. PDGF, but not TGF-β, was found to predict both the development of NSCLC and (1) the radiographically determined severity of the disease and (2) the levels of PDGF correlating with severity of cancer and with fibrosis of the lung, which may be a predisposing factor ( ). These studies parallel the results of other studies, such as the study with TGF-α described earlier, in which the sera of patients with occupational or environmental risks have been found to be positive for one or more oncogene proteins prior to appearance of a cancer.
Earlier studies have suggested that EGF is elevated in the sera of some patients with stomach cancer ( ) and cancer of the tongue ( ) but unchanged or decreased in other cancers ( ). However, recently it has been found that EGF is significantly elevated in bronchial alveolar lavage fluid (BALF) in patients with lung cancer ( ). Levels of EGF, Ca-125 (serum marker for ovarian cancer; see Chapter 76 ) and Ca 15-3 (breast cancer mucin; see Chapter 76 ) were studied in cohorts of patients with lung cancer and a control group with no lung cancer. Only EGF was found to be significantly elevated in the patients with lung cancer. The sensitivity was found to be 63.6% and the specificity 65.7%, indicating that use of EGF in BALF may be a promising approach to aid in diagnosis of lung cancer.
Hepatocyte growth factor (HGF) is a growth factor that is cleaved posttranslationally into α and β chains that are connected by a disulfide bond and binds to the c-MET receptor that is present on many different types of cells. Binding of HGF to c-MET results in activation of processes that result in cell proliferation, motility, migration, and invasion ( ). Elevated serum levels of HGF have been reported in HCC, but this growth factor appears to be unique in that elevated levels also occur in nonmalignant liver diseases ( ), diminishing its utility as a tumor marker for this disease. On the other hand, HGF has been found to be an important marker for evaluating prognosis and response to treatment of melanoma ( ).
This growth factor induces growth of endothelial cells and contributes to the growth of blood vessels in tumors. In addition, it has been found to have numerous functions outside of the vascular system including bone formation, hematopoiesis and cell migration of neurons in the central nervous system. This growth factor has been implicated in inducing tumor cell resistance to chemoradiation and is considered an anti-apoptotic factor enabling tumor cell growth. Significantly, VEGF has been found to be present in the bronchoalveolar lavage fluid of patients with NSCLC ( ). Following up on this finding, a recent Asian study was performed in which sera from 180 patients with NSCLC, 136 patients with benign pulmonary nodules, and 119 healthy control participants were subjected to ELISA assays for VEGF ( ). Patients with NSCLC had significantly higher serum concentrations of this growth factor than those from patients with benign pulmonary nodules from healthy control participants ( P < .0001). The areas under the receiver operating characteristic (ROC) curves for VEGF as a diagnostic marker for NSCLC was 0.839 with a sensitivity of 0.75 for NSCLC and a specificity of 0.933 compared with benign pulmonary nodules and a ROC area of 0.824 with a sensitivity of 0.75 and specificity of 0.956 for NSCLC compared with healthy control participants. These values were significantly higher than for other markers for NSCLC such as CA125 (normally used for ovarian cancer; see Chapter 75 ), CEA (see Chapter 75 ) and cytokeratin 19 fragments (Cyfra 21-1) used in immunohistochemical (IHC) evaluation of lung tissue. On the other hand, use of the VEGF ELISA assay together with ELISA assays for these other three biomarkers raised the sensitivities to significantly higher values (0.84 and 0.85, respectively), although the specificities were slightly reduced to 0.9 and 0.92. Also of interest, VEGF was found to detect NSCLC at early stages with a significantly higher efficacy than any of the other markers and was found to be elevated before surgical removal of the tumors and was significantly reduced postoperatively, indicating its use for monitoring tumor status. Thus, VEGF appears to be a strong candidate for screening patients with this disease.
Growth factor receptors are often structured such that they contain three major domains: an extracellular domain that binds to the growth factor, a transmembrane domain often containing hydrophobic amino acid sequences that interact with lipid in the membrane lipid bilayer, and an intracytosolic domain that often contains a tyrosine kinase. Normally, growth factors bind to their growth factor receptors, generally inducing the receptors to dimerize. For receptors that contain intracytosolic tyrosine kinases, this dimerization activates these kinases, starting in motion a sequence of protein activations that occur on a signal transduction pathway. Figure 77.1 illustrates this phenomenon on the ras pathway. Activation of the receptor tyrosine kinase (TK in Fig. 77.1 ) results in phosphorylation of tyrosine residues of the adapter protein grb-2 that then binds both to the receptor tyrosine kinase and to the guanine nucleotide exchange protein, SOS, that in turn binds to ras-p21, causing it to exchange GDP for GTP, resulting in its activation.
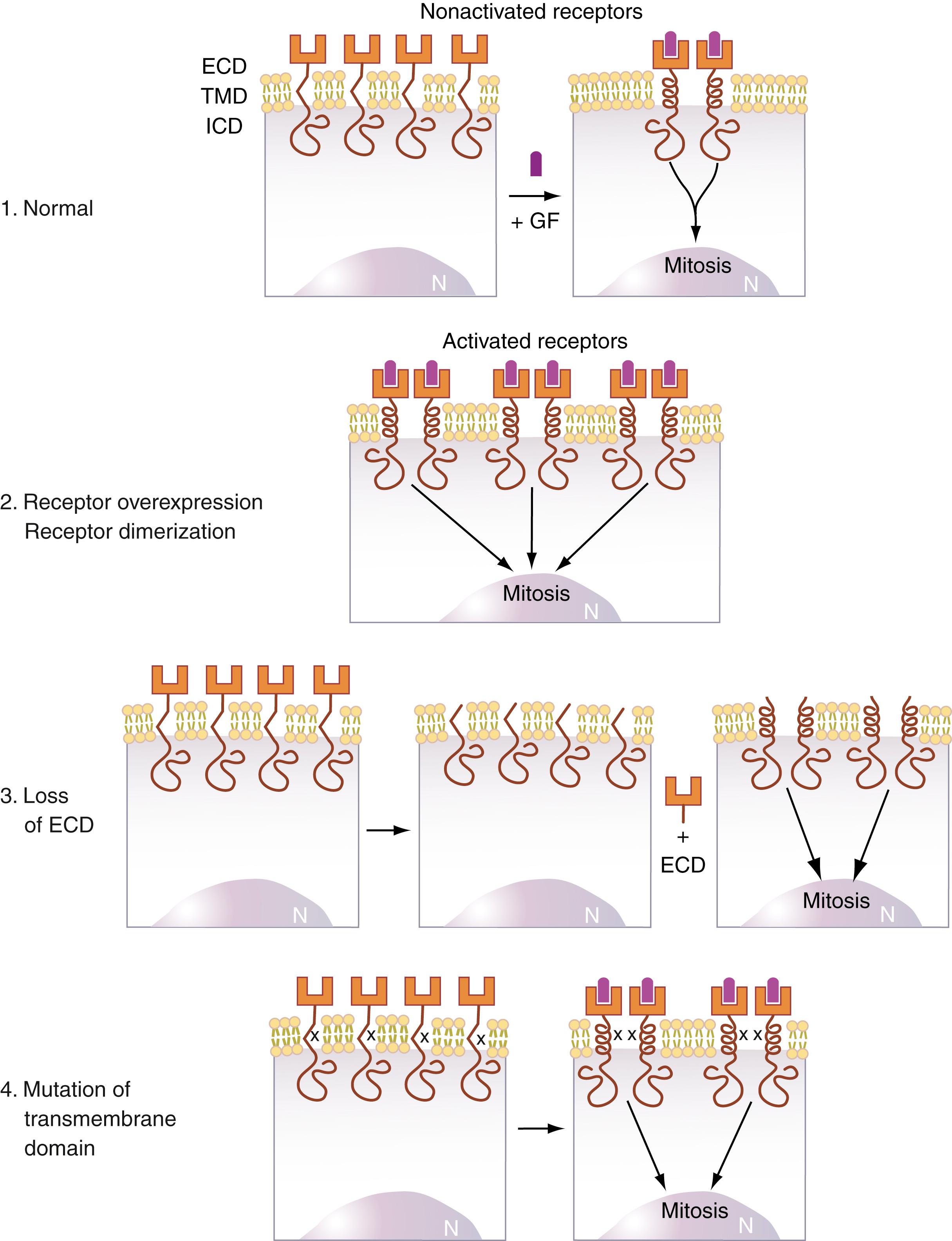
At the growth factor receptor level, there are several mechanisms by which uncontrolled mitogenesis may be initiated. These are summarized in Figure 77.2 . For both the EGF receptor and the neu (HER)–encoded p185 growth factor receptor protein, strongly associated with breast cancer ( ; ; ), receptor dimerization results in activation of tyrosine kinases involved in the phosphorylation of proteins that initiate the transduction of the mitogenic signal to the nucleus. Three pathologic mechanisms can cause abnormally prolonged receptor dimerization that results in continuous mitogenic signaling. These are loss of the extracellular binding domain (ECD), mutations in the transmembrane domain that promote dimerization ( ; ), and overexpression of the receptor.
Transmembrane growth factor receptors encoded by the erbB family of oncogenes (i.e., erbB, which encodes the EGF receptor, also called EGFr, and HER2/ neu [erbB-2]) are particularly attractive targets for detection in blood during cancer development because it has been found that in human cancers induced by these receptors, the mechanism appears to be proteolysis of the extracellular receptor-binding domain (see Fig. 77.2 , third illustration). The liberated extracellular domains, termed ECD , then enter the circulation and can be readily detected in serum using conventional immunoassay techniques ( , ; ; ).
Overexpression of or oncogenic amino acid substitutions in the amino acid sequence of EGFr and in the closely related HER2/ neu receptor are the primary causes of a large number of human epithelial cell tumors. These include colon, breast, lung, and ovarian cancers ( ). This observation has been extended to CNS tumors, in particular, glioblastoma multiforme ( ). As discussed earlier, serum EGFr is not only important for diagnostic purposes but also suggests a therapeutic strategy ( )—that is, administration of EGFr inhibitors, the efficacy of which can be monitored by assaying for serum EGFr.
A particularly important use of serum EGFr is in following patients with known histories of exposure to environmental agents known to cause specific tumors. Elevations of circulating levels of the ECD of this growth factor receptor have been studied in patients with asbestosis ( ), which is known to predispose to malignancies. It has been found that patients with ECD serum levels of 636 fmol/mL or higher either have an asbestos-associated malignancy (carcinoma of the lung or mesothelioma) or subsequently develop such a malignancy. Large numbers of normal individuals have been found to have much lower serum ECD levels. Thus, EGFr appears to be an excellent marker for asbestos-induced tumors. These results are also of interest because they suggest that the primary effect of asbestos as a carcinogen is to cause mutations in the EGFr gene ( ).
As discussed, many patients with colon cancers are treated with EGFr inhibitors. An important indication of efficacy of treatment is to measure circulating levels of EGFr. It was recently found that baseline levels of serum EGFr were strong predictors of outcome, higher values predicting better responses to treatment with EGFr inhibitors than lower values ( ).
Of considerable interest is the recent finding that serum levels of EGFr can detect the presence of prostate cancer ( ). Use of EGFr in detection of prostate cancer was motivated by the finding that overexpression of EGFr or critical amino acid substitutions in the amino acid sequence of prostate EGFr are strongly associated with prostate cancer. It was found that, using a cutoff value of 67.9 ng/mL for serum EGFr, the sensitivity and specificity of serum EGFr were 93.3% and 98%, respectively. In this study, it was further found that the best serodiagnostic marker for spread of prostate cancer beyond the prostate (e.g., into the seminal vesicle) was urokinase-type plasminogen activator receptor. These results suggest that both biomarkers may offer a new approach to diagnosis and monitoring of prostate cancer.
This protein is discussed in Chapter 75 as a marker for breast cancer and a number of other cancers. Here we consider its potential as a marker for different stages of this cancer and for other human epithelial neoplasms. Current American Society of Clinical Oncology (ASCO) guidelines recommend evaluating the HER2/ neu status of all primary tumors, primarily to guide therapy if trastuzumab is a suitable agent ( ). Because of the documented strong association between breast cancer and mutations in the HER2/ neu gene, many studies have examined the p185 erbB2 ECD in the blood of patients with cancer, particularly breast cancer. In prior studies on neu oncogene–induced overexpression of the p185 protein ( ), it was found that the level of expression of the neu oncogene in breast cancer biopsy tissue correlated with the extent of the tumor and was the best prognostic indicator of survival rates, exceeding even the extent of lymph node involvement as a prognostic indicator.
Results on quantitation of p185 ECD in the serum of patients with breast cancer parallel those of the prior genetic results. Between 25% and 50% of patients with stage III or IV breast cancer have been found to have markedly elevated levels of p185 ECD in their sera (40- to 190-fold higher than in the sera of normal control individuals) ( ; ; ). For patients from whom tumor biopsy material is available, there is an excellent correlation between serum ECD levels and tissue level of expression ( , ; ). There is also an excellent correlation between levels of serum ECD and recurrent disease ( ).
ECD levels in serum have also been found to be an excellent prognostic indicator for breast cancer ( ). In a study of patients with stage I to III breast cancer, it was found that presurgical serum ECD level was an independent prognosticator of disease-free survival in early breast cancer ( ). This is an important finding because it identifies patients with early breast cancer with complete apparent surgical removal who are at significant risk for recurrence. In a large study, ECD levels were elevated in the sera of 44% of HER2-positive and, perhaps more important, in 15.8% of patients with HER2-negative breast cancer who developed recurrence, suggesting that HER2 status may change with disease progression ( ).
Because serum levels of p185 ECD correlate with tumor load and stage, detection of incipient breast cancer using serum levels of p185 ECD is less effective for these patients. Overall, the rate of detection of stages I and II breast cancers using serum levels of ECD, based on conventional ELISA techniques, ranges from 10% to 15%. However, use of a sensitive ELISA assay for p185 ECD in patients with breast cancer resulted in discovery of carcinoma in situ in 43% of patients with this disease ( , ). This latter result indicates that the use of more sensitive assays for p185 ECD identifies a significant increase in the number of patients with carcinoma in situ (i.e., at an early stage of development).
Treatment for patients with breast cancer that is positive for HER2/neu is with one or more agents that block the HER2/neu receptor or inhibit its tyrosine kinase activity ( ). The anti-HER2/neu receptor blockers are two monoclonal antibodies trastuzumab, also known as Herceptin, that blocks growth factor–induced activation of the receptor and pertuzumab that blocks HER2/neu receptor dimerization. The tyrosine kinase inhibitor lapatinib blocks autophosphorylation by ATP of the HER/neu receptor (and of EGFr). Depending on the stage of the tumor, one or more of these agents are used often in combination with more general anti–breast cancer agents such as Taxol. Although serum levels of ECD have not been found to predict responsiveness of HER2/neu–positive tumors to these chemotherapy regimens, newer assay techniques are being investigated that can accomplish this goal ( ).
p185 ECD is also elevated in a high percentage of patients with pulmonary cancers. HER2 expression has been detected in about a quarter of specimens of NSCLCs ( ). More tellingly, a recent meta-analysis of 40 studies comprising more than 6000 patients revealed that HER2 overexpression was associated with a poor prognosis in patients with lung cancer ( ). This trend has been observed in another large study of HER2 mutations in patients in the Lung Cancer Mutation Consortium with adenocarcinoma of the lung ( ). Of 920 patients in this study, 24 (3%) were found to have HER2 mutations. Survival times for patients with HER2 mutations were lower than for patients with adenocarcinomas that were negative for mutations in HER2. Treatment with specific anti-HER2 agents as discussed earlier resulted in significantly prolonged median survival times.
Elevated serum levels of EGFr in early tumor detection have been employed to screen patients with known predisposition, such as pneumoconioses, for developing these cancers. In 70% of patients with pneumoconioses, elevated serum p185 ECD levels have been found prior to the onset of frank malignancy. In almost 100% of patients with this predisposing factor who have cancer of the lung, serum p185 ECD is markedly elevated ( ). Clearly, this protein is a highly sensitive marker for pulmonary cancers. In contrast, no elevations of p185 ECD have been found in the sera of large numbers of normal individuals ( ; ).
It was noted previously that TGF-β is a good marker for HCC. There are now strong indications that p185 ECD is also a sensitive marker for this disease. Serum p185 ECD has been found to be elevated in almost 100% of East Asian people who have known risk factors for developing this disease ( ; , ). However, tissue expression of c-erbB-2 in biopsies derived from patients with HCC and adenoma showed neither overexpression nor amplification by fluorescence in situ hybridization or IHC ( ). Furthermore, no such elevations have been observed in normal individuals of similar age and race or in those with exposure to risk factors for this disease but who did not subsequently develop cancer.
By looking at a subset of HCC patients, however, HER2/ neu IHC may be more instructive. It has been observed that HER2/ neu upregulation in response to hepatitis B infection, specifically the hepatitis B x antigen (HBxAg), is an important mediator of hepatocyte proliferation and ultimately of malignant transformation to HCC. Patients with hepatitis B with liver specimens that stain strongly for HER2/ neu develop HCC and have poorer survival. HER2/ neu may be a sentinel of malignant transformation in this subset of patients ( ).
Further studies indicate that there is amplification of HER2 mRNA in 14/17 HCC samples and in several human HCC cell lines. In these cells, HER2 appeared to induce upregulation of β catenin and inhibition of SMAD3 ( ). Peculiarly, however, HER2 was found to be downregulated in high-grade HCCs.
HER2 has been observed to be overexpressed in a significant number (4.4%–53.4%; mean, 17.9%) of gastroesophageal tumors (Abrahao-Machado & Scapulatempo-Neto, 2017). As found with other tumors in other tissues, survival times are lower for patients with HER2 overexpression compared with those found in patients with the same tumors without HER2 overexpression. Treatment of these patients with anti-HER2–directed agents appears to prolong life.
Elevated serum erbB-2 ECD levels have now been found to occur in patients with colorectal, pancreatic, prostatic, hepatic, and ovarian cancers ( ) at lower frequencies of detection (15%–20%) and were also found to be significantly higher in patients with nasopharyngeal carcinoma ( ). Excellent correlations between serum levels and tissue levels have been found in many of these cases. Serum p185erbB-2 ECD levels correlate well with the stage of oral cavity squamous cell carcinoma, as well as the response to therapy ( ). These levels do not correlate with tumor size, nodal status, or metastases. There is a direct correlation between serum levels of p185 ECD and tumor size for premalignant adenomas of the colon ( ). Because colonic neoplasia usually progresses through well-defined steps from adenoma to carcinoma with the malignant potential of adenomas increasing with size, serum erbB-2 ECD levels may be useful in monitoring this progression. Gene amplification and protein overexpression of c-erbB-2 were also found in adenocarcinoma derived from Barrett esophagus ( ). In addition, pretreatment serum ECD has been found to be a reliable prognosticator of recurrence of metastatic prostate cancer and significantly more reliable than tissue p185 ( ).
E-cadherin, a cell-adhesion transmembrane protein, has been found to be responsible for METs and EMTs, activities that stabilize tumors in their local environment. One of the major proteins involved in this interconversion is β-catenin that is transported across the nuclear membrane and stabilizes transcription of mRNA that encodes EMT-promoting factors. However, E-cadherin binds to β-catenin, inhibiting its ability to enter the nucleus. Downregulation of E-cadherin results in decreased binding of β-catenin, allowing increased entry of β-catenin to the nucleus, where it activates EMT. Less certain is the mechanism of METs, likely caused by upregulation of E-cadherin and binding of β-catenin, reducing EMT ( ).Thus E-cadherin is a negative regulator of EMT and cell transformation.
However, E-cadherin is cleaved intracellularly to so-called soluble E-cadherin or sE-cad that is released from cells. This form of E-cadherin has been found to break cell–cell junctions in murine mammary tumor cells, suggesting that sE-cad reduces contact inhibition of cell growth that can lead to uncontrolled proliferation. sE-cad has been detected in the serum of patients with cancer.
Elevated levels of sE-cad have been detected in NSCCL and bladder, colorectal, ovarian, prostate, skin, esophageal, gastric, and hepatocellular cancers ( ; ). The appearance of these cell adhesion molecules in blood circulation can indicate more than the mere presence of neoplasm and can be seen as a marker of metastases or poor prognosis. Indeed, elevated levels of sE-cad have been shown to be valid prognostic markers for gastric cancer and correlated well with late-stage colorectal cancer ( ; ). Most recently, it was found useful as a marker of preoperative chemotherapy response for breast cancer ( ). In this study, levels of sE-cad were significantly reduced in patients with pathological complete response compared to those without pathological complete response, and the decreases in the sE-cad levels were significantly different between these two groups. This may have great potential in identifying patients with breast cancer. The caveat to using this marker is that it is also found to be elevated in diseases causing inflammatory states, such as diabetes and HIV ( ; ).
Matrix metalloproteases (MMPs) constitute a class of many enzymes responsible for cleavage of the ECD of E-cadherin ( ). Their levels in serum have also been shown to be upregulated in the sera of patients with pancreatic ductal adenocarcinoma (PDAC), specifically MMP-1, -3, -7, -9, -10, and -12, compared with normal control patients’ sera. MMP-7 and -12 had specificities and sensitivities greater than 90%. MMP-2, however, was significantly downregulated in the sera of patients with PDAC ( ). Other useful tests are urinary MMP-2 (uMMP-2) and urinary tissue inhibitor of metalloprotease (TIMP-1). These markers were elevated to significantly higher levels in the urine of patients with pancreatic neuroendocrine tumors and PDACs than in the urine of normal control participants ( ).
As for growth factor receptors, signaling proteins that occur downstream of the growth factor receptors become oncogenic primarily from overexpression and amino acid substitutions at critical positions in their polypeptide chains. Less commonly, for some proteins, such as Raf (see Fig. 77.1 and Table 77.2 ), loss of regulatory domains may also lead to oncogenesis. Amino acid substitutions in signal transducing proteins cause these proteins to undergo conformational changes that result in their becoming permanently activated to stimulate cell division ( , , , , ).
This mechanism has been well documented for the ras oncogene–encoded p21 protein, for which substitutions of most amino acids for glycine 12 or glutamine 61 result in an oncogenic protein. Many p21 proteins with such substitutions have been cloned and directly microinjected into normal cells in culture such as NIH 3T3 cells ( ). The cells undergo malignant transformation that lasts until the added mutant protein is metabolized and cleared from the cells. Oncogenic mutant forms of the ras gene and of its encoded p21 protein have been found in approximately one of three common human epithelial cell tumors, in more than 90% of human pancreatic tumors, and 75% of human colon cancers ( ; ).
One of the consequences of amino acid substitutions in p21 and presumably in other signal transducing proteins is the abnormal activation of alternate, unregulated signal transduction pathways. Oncogenic ras-p21 protein (p21 in Fig. 77.1 ) has been found to interact directly with Jun, the nuclear transcription factor, and its activating kinase, JNK. This binding process results in the direct activation of these nuclear transcription–inducing proteins, thereby bypassing the normal cellular controls. This bypass or short-circuit pathway is shown on the right side of Figure 77.1 for p21 ( ; ; ; ). In addition, oncogenic ras-p21 protein requires activation of protein kinase C as a downstream event on its signal transduction pathway ( ), as illustrated in Figure 77.1 .
As noted previously, the ras oncogene–encoded p21 proteins are 21-kDa membrane-associated G-proteins that have been implicated in the growth signal transduction process from the cell membrane to cytoplasmic kinases. Qualitative (i.e., point mutations) and quantitative (i.e., overexpression) changes in p21 have been identified as contributing to human carcinogenesis ( ). By undefined mechanisms, p21 proteins gain access to the extracellular environment. Thus increased amounts of p21 or point-mutated forms of p21 can be detected by immunoblotting with monoclonal antibodies in the supernatant of cells in culture known to overexpress p21 or to express mutant p21, respectively ( ; ; ).
Similarly, mice with tumors that overexpress p21 or express mutant p21 are found by immunoblotting to have increased amounts of p21 or mutant forms of p21 in their serum, respectively ( ). These results suggest that the detection of increased p21 or mutant p21 in blood is possible in humans.
Because of its central role in mitogenic signal transduction, overexpressed or mutated p21 protein might be expected to be present in a wide variety of human tumors. Indeed, elevated serum p21 has been identified in the serum of up to 68% of patients with many different cancers, including breast, prostate, colon, lung, and liver cancer. On the other hand, only a small percentage of normal individuals have been found to have detectable serum levels ( ). Newer assays based on RT-PCR have detected the ras oncogene in the stool of a high percentage of patients with colonic cancer ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here