Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The immune system consists of cellular and humoral components that defend the body against invading microorganisms. Deficiency of individual factors of the immune system can leave an individual susceptible to different infections.
The lymphoid cells of the immune system are composed of T cells that act directly against foreign antigens and B cells that differentiate into immunoglobulin (Ig)-secreting plasma cells capable of synthesizing immunoglobulins that combine with foreign antigens. Antigen-presenting cells are crucial for cell-to-cell communication during the direction of an immune response.
The mechanisms by which lymphocytes differentiate into T cells and B cells involve rearrangement of genes for the T-cell antigen receptor and of genes for immunoglobulins, respectively.
Lymphocytes from patient specimens are characterized by detection of surface proteins, termed cluster of differentiation , or CD markers , which designate their subset and function.
Additional cellular elements are natural killer lymphocytes plus neutrophils, eosinophils, and the effectors of IgE-mediated hypersensitivity—basophils and mast cells.
In addition to immunoglobulins or antibodies, humoral elements include a cascading series of enzyme activation in the complement system that results in enzymatic destruction of foreign cells and recruitment of inflammatory cells by cytokine actions.
Histocompatibility antigens play a major role in antigen presentation to T cells and form the immunologic identity of an individual, which is important in rejection of organ transplants.
Immunologic injury can occur as the result of excessive response to specific antigenic exposures, as in an allergic response, or as a result of circulating immune complexes.
Autoimmune disease results when the mechanisms for maintaining immunologic tolerance to self-(auto)antigens fail, resulting in the formation of various autoantibodies plus activation of the cellular immune system against the patient’s own tissues.
Laboratory measurements of the immune system involve relatively simple techniques for counting lymphocytes and their subsets as well as for quantifying overall concentrations of immunoglobulins and complement proteins. More complex methods—such as detection of gene rearrangements, autoantibody characterization, and allergen reactivity with IgE—are useful for diagnosing various disorders of the immune system.
Therapeutic approaches to disorders of the immune system include stimulation or replacement of deficient components and reduction or removal of other factors that are abnormal or are present in excess.
The immune system is structured to recognize, respond to, and destroy a wide variety of invading organisms such as bacteria, viruses, fungi, and parasites that otherwise would be capable of promoting infection that is harmful to the body. Discovery of components of the immune system has very often followed investigation of serious infections and the specific reactions that the body uses to combat pathogenic organisms. In general, this immunologic function can be summarized as searching for foreign (or nonself) antigens that do not belong in the body and then destroying them. In this process, the immune system also maintains surveillance over the appearance of new or foreign antigens on tumor cells and attempts to destroy them while leaving unharmed the normal (or self) antigens on healthy cells. Disease states may arise as a result of various aspects of immunologic function going awry, as in hypersensitivity reactions, autoimmune disease, and various immunodeficiency disorders. When functioning properly, the immune system is responsible for rejection of allogeneic organ/tissue transplants and graft-versus-host disease; the quest for tolerance in transplantation continues. This overview highlights components of the immune system and their functions along with some of the significant clinical disorders of immunity.
The lymphoid cells of the body reside in the lymph nodes, the spleen, the mucosal surfaces, and the circulation. They derive from multipotential hematopoietic stem cells, with their production moving progressively from the yolk sac in the embryo to the liver in the fetus, and, finally, to the bone marrow in the infant through adult ages ( ). The various lymphoid cells are conveniently identified by the presence of unique protein markers on their surfaces that endow those cells with particular functions.
T lymphocytes undergo differentiation in the thymus. After originating in the bone marrow, prothymocytes pass from the cortex to the medulla of the thymus, during which time they undergo maturation. This involves a selection process such that self-reactive thymocytes are eliminated, while thymocytes that can recognize antigens through interactions with molecules of the major histocompatibility complex (MHC) are retained ( ; ). During maturation, they undergo genetic programming in which the gene for the T-cell antigen receptor (TCR) is rearranged to produce protein receptors that are invariant in their antigen specificity for the life span of that T lymphocyte as well as for all its descendant cells. T lymphocytes account for about 60% to 70% of all lymphocytes in the blood. They are also found in paracortical areas of lymph nodes and within periarteriolar lymphoid sheaths in the spleen.
A majority (>95%) of T lymphocytes have antigen receptors made of α- and β-subunits linked with disulfide bonds to form a molecular heterodimer that resides on the outer membrane of the cell in association with the CD3 molecular complex (CD3 is a pan–T-cell marker). The α- and β-subunit TCR proteins have variable, joining, and constant regions (α also contains a diversity region), with corresponding encoding regions in the TCR gene that undergo rearrangement, resulting in high specificity binding for a particular antigen. The CD3 proteins assist transduction of the signal to the interior of the cell when an antigen binds to the TCR on the lymphocyte surface ( ; ). A small percentage of T lymphocytes have a TCR composed of γ- and δ-subunits that similarly interact with CD3. These cells are generally found at mucosal surfaces of the gastrointestinal and respiratory tracts. T cell proliferations may be characterized as neoplastic (clonal) or benign (polyclonal) according to whether their DNA shows predominantly a single form of TCR gene rearrangement or a complete spectrum of such rearrangements, as normally occurs in a heterogeneous lymphocyte population.
Examination of T lymphocytes by flow cytometry focuses on a variety of surface markers. CD4 is found on about 60% of CD3 + cells; these are helper/inducer T cells that direct the functions of other cells of the immune system by secreting cytokines that stimulate various functions. Two distinct subpopulations of CD4 + helper cells have been identified: Th1 cells, which secrete interleukin (IL)-2 and interferon (IFN)-γ; and Th2 cells, which secrete IL-4 and IL-5. Th1 cells facilitate macrophage activation, delayed-type hypersensitivity, and the production of antibodies with opsonizing action. Th2 cells direct synthesis of other antibodies, such as IgE, and activate eosinophils. The marker CD8 is found on about 30% of T cells (thus, the normal ratio of CD4 + to CD8 + cells in the blood is typically 2:1). These CD8 + T cells exhibit cytotoxicity and suppressor activity in the immune response.
The mechanism of antigen recognition is different between CD4 + and CD8 + subsets of T lymphocytes. CD4 molecules bind to the MHC class II molecules on antigen-presenting cells, whereas CD8 molecules interact with MHC class I molecules. Accordingly, CD4 + T cells recognize antigens only in the context of MHC class II antigens, and CD8 + T cells recognize them only through MHC class I antigens. A subset of T helper cells termed, T regulatory (Treg) cells , are important for immune tolerance and prevention of autoimmune disorders (see Chapter 53 ). Treg cells have surface markers for CD4 and CD25 (IL-2 receptor); thus, they are driven by IL-2. The transcription factor FOXP3 plays a role in Treg function, and mutation in the foxp3 gene results in dysregulation of immune tolerance and progression to autoimmune diseases.
B lymphocytes make up roughly 10% to 20% of peripheral lymphocytes in the blood. They also are found in the bone marrow, lymph nodes, spleen, and other lymphoid tissues. In the spleen and lymph nodes, they aggregate into lymphoid follicles. Differentiation of B lymphocytes occurs in the bone marrow, where both positive selection and negative selection take place, as well as at peripheral locations. Antigenic stimulation of B cells leads to the formation of plasma cells that secrete immunoglobulins—the basis of specificity in humoral immunity. The B-cell antigen receptor complex uses surface IgM as the antigen-binding component. The antigen specificity of immunoglobulins derives from a rearrangement process in which both heavy- and light-chain genes are realigned. The heavy-chain gene has variable, diversity, joining, and constant regions, whereas the light-chain gene has variable, joining, and constant regions. Maturation of an antibody response entails switching from IgM to another heavy-chain type (usually IgG) as the result of further gene rearrangement, although the light-chain type remains fixed ( ).
B cells have on their surfaces receptors for complement—CD21, which is also the receptor for Epstein-Barr virus (EBV), thus making these cells susceptible to EBV infection, and for the Fc region of immunoglobulins they have CD19 and CD20, which are frequently used for immunologic identification of B cells.
Macrophages function as mononuclear phagocytes in inflammation. They also process ingested antigens and present them to immune effector cells in association with MHC molecules on their membranes ( Fig. 44.1 ). T cells are not activated by soluble antigens; thus, presentation of antigens in this manner is necessary for T-cell stimulation and induction of cell-mediated immunity ( ; ). Macrophages also secrete cytokines such as IL-1 for modulation of inflammatory processes. Macrophages can directly lyse tumor cells in their role of immunosurveillance and are effector cells for some types of cell-mediated immunity (e.g., delayed hypersensitivity).
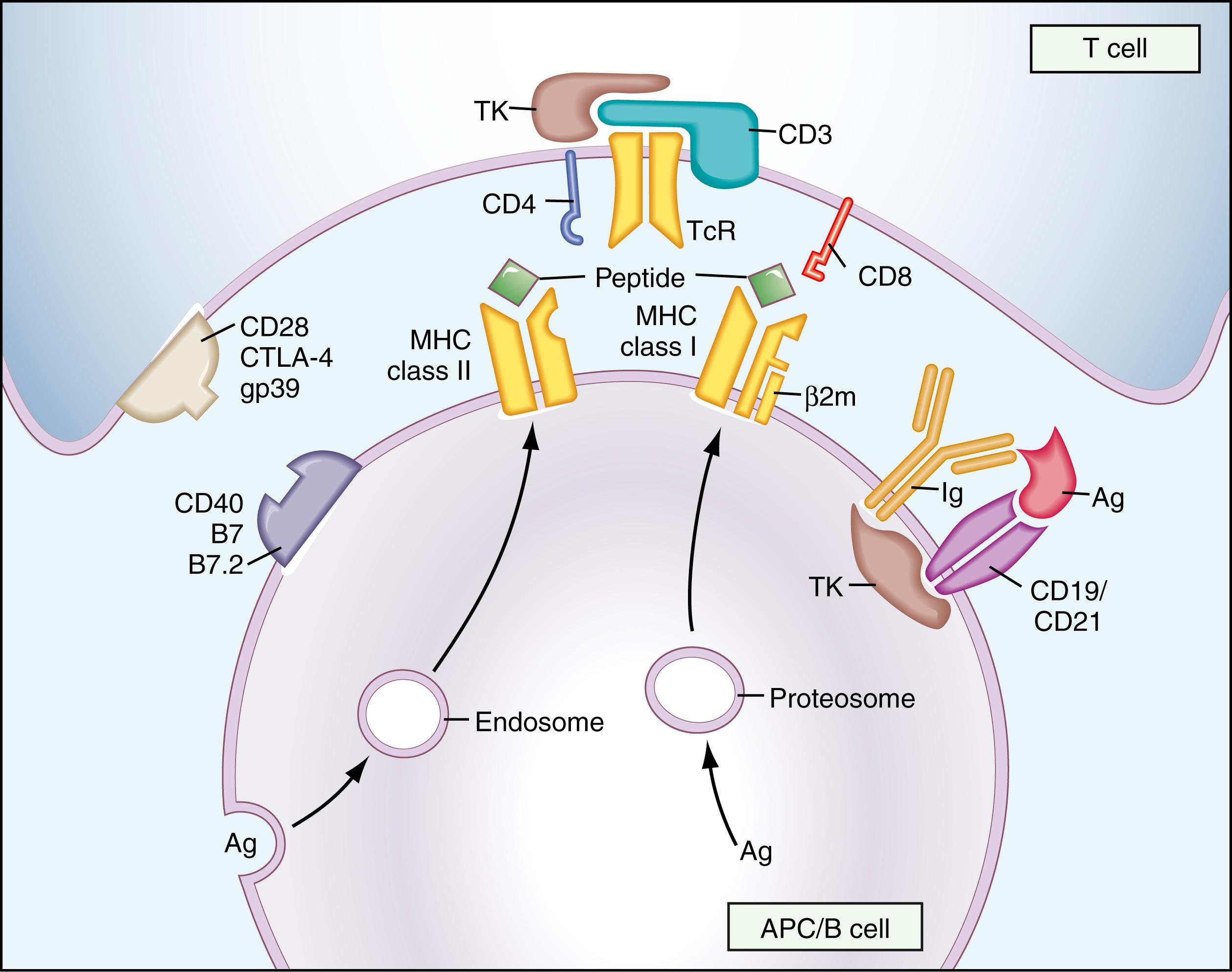
Dendritic cells (found in lymphoid tissues and in interstitial regions of other organs) and Langerhans cells (found in the epidermis) have extensive dendritic cytoplasmic processes that are rich in MHC class II molecules. Consequently, they are very efficient at presenting antigens (although they probably are not phagocytic) and are considered to be extremely important in that task within the entire immune system ( ).
Natural killer (NK) cells constitute 10% to 15% of lymphocytes in the peripheral blood. They are neither T cells nor B cells and were formerly called null cells . NK cells have the function of lysing other cells without prior sensitization. They can attack tumor cells, cells infected with viruses, and others as well. Consequently, they form the initial defense against aberrant cells. NK cells are characterized by the surface markers CD16 and CD56, which are commonly used for their identification. CD16 is the Fc receptor for IgG; hence, NK cells are able to lyse selectively those cells that are coated with antibodies (antibody-dependent cell-mediated cytotoxicity, which is important in some hypersensitivity reactions). NK cells also secrete cytokines such as IFN-γ. It is interesting to note that NK cells are recognizable on examination of standard stained blood smears as large granular lymphocytes ( ).
These cells are not genetically programmed to recognize specific antigens or to interact with lymphoid cells in the induction of an immune response. Instead, they are effectors of immune reactions that are triggered by various factors.
Neutrophils are drawn to regions of inflammation by chemoattractants such as IL-8. They then release from their granules toxic substances and enzymes that digest cellular structures indiscriminately. Neutrophils also ingest cellular debris, as do eosinophils, and remove it from tissue sites.
Basophils (and their counterparts in tissues, the mast cells) have on their surfaces high-affinity Fc receptors that bind circulating IgE. Uptake of IgE onto the membranes of basophils apparently is not antigen dependent; instead, it is driven by mass action between the amount of total IgE and the available basophils. When the antigen (or allergen) comes into contact with basophil surface-bound IgE that recognizes it, those basophils become activated and release substances such as histamine, which mediate some hypersensitivity reactions. Thus, basophil specificity is directed by the particular IgE that is bound to its surface from the blood; theoretically, a basophil could have multiple different IgE molecules on its surface and, thus, could react with many different allergens.
Antibody molecules can be bound to B-cell surfaces for stimulation of further immunoglobulin secretion. They also circulate in the blood and appear at mucosal surfaces of the respiratory and gastrointestinal tracts, where they are likely to come into contact with potentially pathogenic microorganisms. The major immunoglobulins in the blood are IgM, IgG, and IgA. These antibodies presumably arise following exposure to a multitude of foreign antigens and may remain for years with continued secretion to replace molecules that are lost through normal clearance of proteins from the circulation. At mucosal surfaces, the primary type of antibody is dimeric IgA, which is secreted at that site ( ) (see Chapter 47 ). The other immunoglobulins are IgD (which resides on B-cell surfaces, where it may be involved in immune signal transduction but does not achieve substantial concentrations as free molecules in the blood) and IgE (which functions by binding to surfaces of basophils and stimulates the release of vasoactive substances from those cells in the presence of specific allergens).
Immunoglobulins in the blood function by attaching to antigens on the surfaces of foreign cells, bacteria, viruses, and the like, and by facilitating their destruction through nonspecific effectors such as complement and NK cell activation. Disease monitoring by immunoglobulin testing includes qualitative analysis for clonal versus polyclonal detection (e.g., for diagnosis of multiple myeloma) and quantitative analyses both for overall concentrations of IgM, IgG, and IgA (to detect possible selective or combined deficiencies or overproduction of immunoglobulin classes) and for titers of specific antibodies against individual antigens (e.g., isohemagglutinins, Pneumococcus, Haemophilus , tetanus, and diphtheria).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here