Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The plasma concentration of calcium, phosphate, and magnesium is dependent on the net balance of bone mineral deposition and resorption, intestinal absorption, and renal excretion. The principal hormones regulating these processes are parathyroid hormone (PTH), calcitonin, 1,25-dihydroxyvitamin D, and fibroblast growth factor-23 (FGF-23).
The most common causes of hypercalcemia are primary hyperparathyroidism (elevated PTH) and malignant neoplasms (decreased PTH and usually elevated PTH-related peptide). They account for 80% to 90% of all patients with hypercalcemia.
The most common causes of hypocalcemia are chronic renal failure, hypomagnesemia, hypoparathyroidism, pseudohypoparathyroidism, vitamin D deficiency, and acute pancreatitis.
Biointact PTH measures biologically active PTH; it is useful in patients with impaired renal function when various metabolites accumulate and interfere with traditional PTH assays.
In patients undergoing surgery for primary hyperparathyroidism, intraoperative PTH measurements are useful in identifying whether the abnormal tissue is completely removed.
Osteoporosis is the most common metabolic disease of bone and is characterized by decreased organic bone matrix. Serial bone resorption marker measurements can predict early response to therapy.
Osteomalacia is failure to mineralize newly formed organic matrix (osteoid) in the mature skeleton.
Phosphatonins comprise a newly described cascade of hormones, enzymes, and proteins related to phosphate metabolism.
The skeleton is a metabolically active organ that undergoes continuous remodeling throughout life. This remodeling is necessary both to maintain the structural integrity of the skeleton and to fulfill its metabolic functions as a storehouse of calcium and phosphorus. Skeletal remodeling can be triggered by changes in mechanical forces or microdamage and by hormonal response to changes in circulating calcium and phosphorus levels. The skeleton also serves as the second line of defense against acidosis, and it is able to liberate buffers in the form of inorganic phosphates ( ).
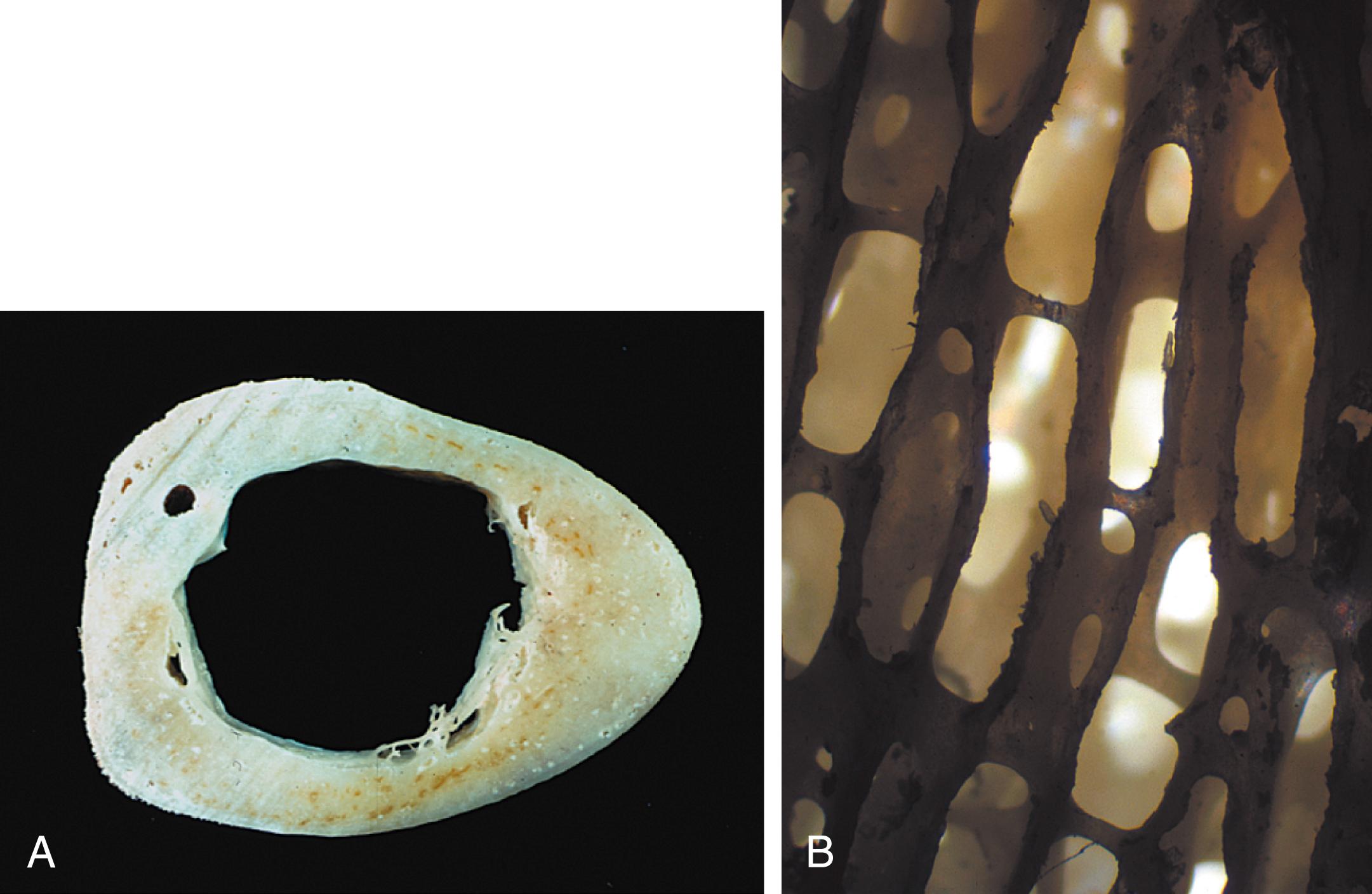
Bone can be classified into two types: cortical or compact bone, and cancellous or trabecular bone ( Fig. 16.1 ). Cortical bone plays an important role in the supportive, protective, and mechanical functions of the skeleton; it comprises the shafts of long bones and the outer envelope of all bones. It constitutes approximately 80% of skeletal mass, and it is 90% bone and 10% space (vascular canals, osteocyte lacunae, and canaliculi) by volume. Cancellous bone, which constitutes the remaining 20% of skeletal mass, is 25% bone and 75% space by volume. It is present at the ends of long and short tubular bones, within carpal and tarsal bones, and in the medullary cavities of vertebral bodies and flat bones. It is arranged in highly perforated vertical plates interconnected by horizontal struts and has a honeycombed appearance. It serves as a repository for hematopoietic cells and provides a large surface area for short-term mineral exchange ( ). Even though cancellous bone represents only 20% of the total skeletal mass, it provides such a large surface area—one that equals that of cortical bone because of its honeycombed structure—that it accounts for 50% of active bone turnover at any given time ( ). Both compact and cancellous bones are composed primarily of inorganic minerals (calcium and phosphorus) and an organic matrix. Approximately 90% to 95% of this organic matrix is type I collagen. The remaining 5% to 10% consists of noncollagenous proteins, including osteocalcin, osteopontin, osteonectin, thrombospondin, sialoproteins, and other less well characterized proteins. Osteoclasts actively resorb bone by producing hydrogen ions to mobilize the minerals and proteolytic enzymes to hydrolyze the organic matrix. Osteoblasts synthesize the organic matrix and control the mineralization of the newly synthesized matrix ( ).
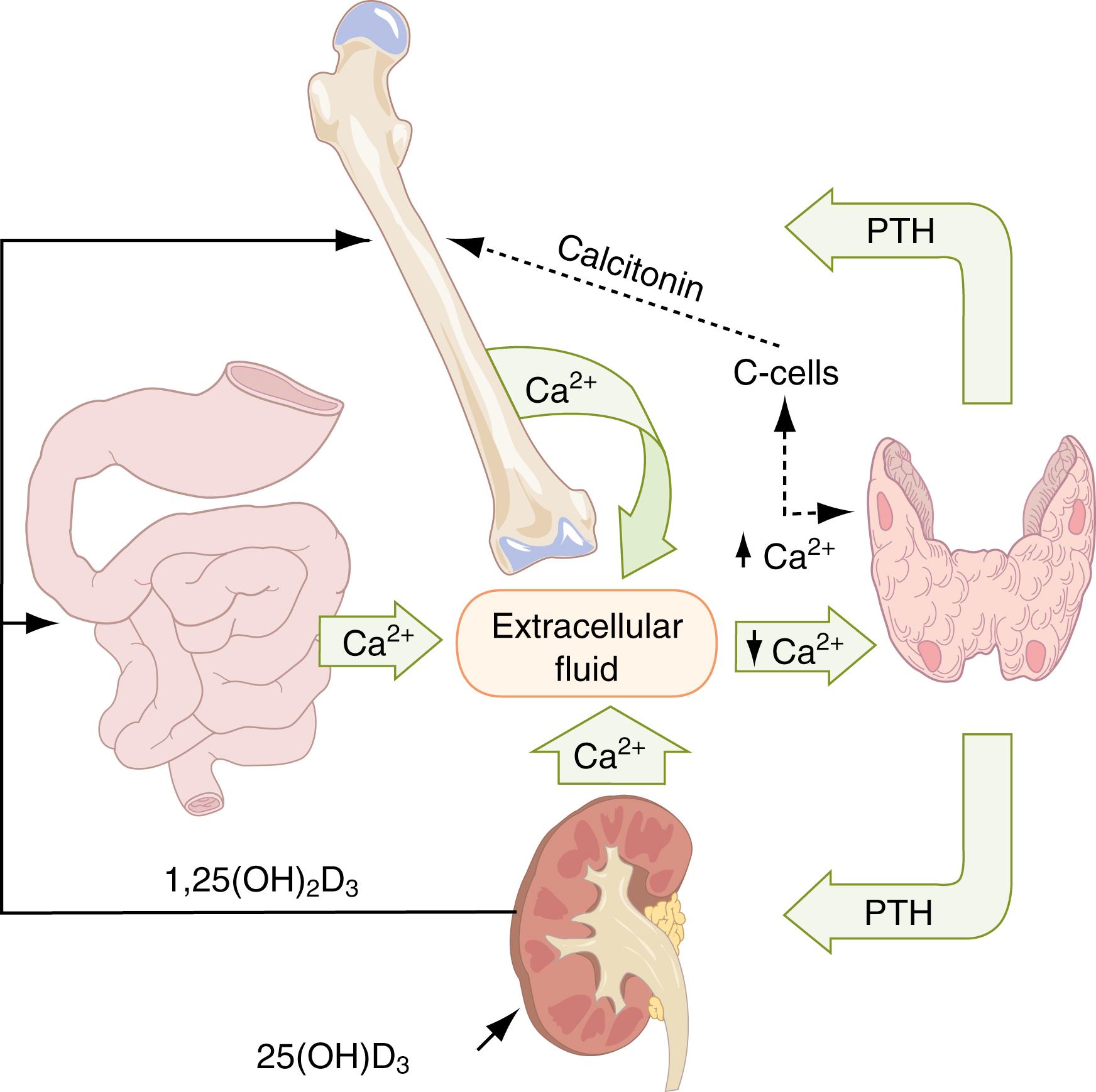
The plasma concentration of calcium, phosphate, and magnesium is dependent on the net balance of bone mineral deposition and resorption, intestinal absorption, and renal excretion. The principal hormones regulating these processes are PTH, calcitonin, and 1,25-dihydroxyvitamin D. Much of bone metabolism reflects the body’s effort to maintain serum calcium and phosphorus levels.
Calcium is the fifth most common element and is the most prevalent cation in the human body. A healthy adult contains approximately 1 to 1.3 kg of calcium, 99% of which is in the form of hydroxyapatite in the skeleton. The remaining 1% is contained in the extracellular fluid (ECF) and soft tissues. Additionally, less than 1% of the skeletal content of calcium is in bone fluid and exchanges freely with the ECF ( ).
Serum (plasma) calcium exists in three distinct forms: free or ionized calcium, which is the physiologically active form, accounting for approximately 50% of the total serum calcium; complexed calcium, which is bound tightly to a variety of anions, including bicarbonate, lactate, phosphate, and citrate, accounting for approximately 10%; and plasma protein-bound calcium, accounting for approximately 40%. Both ionized calcium and the calcium complexes are freely dialyzable. Approximately 80% of the protein-bound calcium fraction is associated with albumin.
Because ionized calcium binds to negatively charged sites on the protein molecules, there is competition with hydrogen ions for binding sites on albumin and other calcium-binding proteins, and its binding is pH dependent. Although total serum calcium levels may remain unchanged, the relative distribution of the three forms is altered as a result of pH changes in ECF. Alkalosis promotes increased protein binding, with a subsequent decrease in free calcium, whereas acidosis decreases protein binding, causing an increase in free calcium levels. Because calcium is bound to proteins, total calcium levels are also altered by plasma protein concentration.
In addition to its obvious importance in skeletal mineralization, calcium plays a vital role in such basic physiologic processes as blood coagulation, neural transmission, plasma buffering capacity and enzyme activity, and in the maintenance of normal muscle tone and excitability of skeletal and cardiac muscle. It is an activator of intracellular signal transduction processes and is essential for DNA and RNA biosynthesis. Calcium is also involved in glandular synthesis and in regulation of exocrine and endocrine glands, as well as in the preservation of cell membrane integrity and permeability, particularly in terms of sodium and potassium exchange.
The average dietary intake of calcium for most adults in the United States is approximately 15 to 20 mmol/day (600 to 800 mg/day), most of which is derived from milk or other dairy products. The National Osteoporosis Foundation recommends that all adults have a daily intake of at least 1200 mg of elemental calcium with diet plus supplements. Lactating females and postmenopausal females not given exogenous estrogen therapy should probably have at least 1500 mg/day ( ).
Calcium is absorbed in the duodenum and upper jejunum via an active transport process. Less than half of dietary calcium is absorbed in adults. However, calcium absorption increases during periods of rapid growth in children, in pregnancy, and during lactation. It decreases with advancing age. Absorption also decreases at doses greater than 500 mg, suggesting that split doses may be more effective if supplemental calcium is consumed. The major stimulus to calcium absorption is vitamin D (see later discussion). Calcium absorption is also enhanced by growth hormone, an acid medium in the intestines, and by increased dietary protein. The ratio of calcium to phosphorus in the intestinal contents is also important, because a ratio greater than 2:1 results in the formation of insoluble calcium phosphates and tends to inhibit calcium absorption. Phytic acid, derived from various cereal grains, can also form insoluble calcium compounds, as can dietary oxalate and fatty acids. Cortisol and excessive alkalinity of the intestinal contents are both inhibitory to calcium absorption.
Estimates of daily calcium excretion in sweat vary widely—from 15 mg to more than 100 mg. The loss can greatly exceed this range during extreme environmental conditions. The major net loss of calcium occurs via urinary excretion and varies between 2.5 and 10 mmol/day (100 and 200 mg/day). In normal individuals, wide variations in dietary calcium intake have little effect on urinary calcium. Urinary calcium excretion is enhanced by hypercalcemia, phosphate deprivation, acidosis, and glucocorticoids. Parathyroid hormone (PTH), certain diuretics, and probably vitamin D diminish urinary calcium excretion. The physiology of calcium, its regulating hormones, and alterations of calcium homeostasis in disease have been extensively reviewed ( ; ).
Ionized calcium concentration of the ECF is kept constant within a narrow range of approximately 1.25 mmol/L ( Fig. 16.2 ). It is the ionized calcium concentration of the ECF that is the primary determinant of the hormonal influences that exert effects on ECF calcium levels. These effects are sometimes achieved at the expense of bone integrity. Adjustment of the ionized calcium concentration of the ECF is achieved mainly by the actions of PTH and active 1,25-dihydroxyvitamin D 3 (1,25[OH] 2 D 3 ); calcitonin plays a smaller, yet significant, role. The principal target organs of these hormones are bone, kidneys, and intestines. When plasma-ionized calcium concentration decreases, the parathyroid glands sense the change via a membrane-bound calcium sensor receptor (CaSR) and secrete PTH immediately. Although PTH has no direct effect on osteoclasts, it stimulates osteoblasts and their precursors to produce RANKL (the receptor activator of nuclear factor κB ligand). This substance, a member of the tumor necrosis factor superfamily, activates its receptor, RANK, which is expressed on osteoclasts and their precursors. This, in turn, promotes osteoclast formation and activity and prolongs osteoclast survival by suppressing apoptosis ( ). This explains why bone formation and bone resorption are coupled in normal bone physiology. The resorption of bone matrix releases calcium and phosphate into the ECF. At the same time, PTH also acts on the kidney to stimulate increased urine phosphate excretion and some calcium reabsorption in the distal nephron, returning the ionized calcium concentration to normal. It has been suggested that sufficient action of 1,25(OH) 2 D 3 is mandatory for these steps to work appropriately. The kidney is almost exclusively responsible for this vitamin D activation ( ). Calcitonin may play a role in the regulating process, although its significance in humans is controversial. Other hormones that affect calcium metabolism but whose secretions are not primarily affected by changes in plasma calcium and phosphate include thyroid hormone, growth hormone, adrenal glucocorticoids, and gonadal steroids.
Total calcium measurements include protein-bound calcium and ionized calcium; alternatively, ionized calcium alone can be measured. The total calcium measurement is easier to perform in the laboratory, but this result must be interpreted in clinical context. For example, patients with malignancies often exhibit hypoalbuminemia, a condition that may result in misleadingly low total calcium levels. When this occurs, the total calcium level (expressed in mg/dL) can be corrected with the following equation:
An albumin of about 4.4 is typically used as the normal value in the previous formula. This corrected value is a more accurate assessment of the patient’s calcium status. Because albumin is the primary protein that binds calcium, variations in this protein are clinically significant. Only a small percentage of calcium binds to other proteins, such as γ-globulins. Therefore, clinical states such as hypogammaglobulinemia are unlikely to drastically alter total calcium levels.
Although many total calcium procedures have been reported, only three methods are commonly used: colorimetric analysis with metallochromic indicators, atomic absorption spectrometry (AAS), and indirect potentiometry. Total calcium is most widely measured by spectrophotometric determination of the colored complex when various metallochromic indicators or dyes bind calcium. Orthocresolphthalein complexone (O-CPC) and arsenazo III are the most widely used indicators. The structures of both of these dyes are shown in Chapter 28 . O-CPC reacts with calcium to form a red color in alkaline solution, which is measured at near 580 nm. Interference by magnesium ions is reduced by the addition of 8-hydroxyquinoline. Arsenazo III reacts with calcium to form a calcium-indicator complex, usually measured at near 650 nm. The stable reagent exhibits high specificity for calcium at slightly acidic pH. These reactions are also discussed in Chapter 28 .
AAS is the reference method for determining calcium in serum. Despite its greater accuracy and precision compared with other methods, very few laboratories continue to use AAS for routine determination of total calcium. This may be because laboratories performing large numbers of sample determinations rely on automated methods that are not widely available for this technique. In addition, the level of equipment maintenance required in this technique is difficult for high-volume laboratories.
In indirect potentiometry, an electrode selective for calcium measures a sample that is also measured against a sodium-selective electrode, and calcium concentrations are proportional to the difference in potential between the electrodes.
Instruments with calcium-selective electrodes (see Chapter 28 ) provide accurate, precise, and automatic determinations of free (ionized) calcium. Calcium ion-selective electrodes (ISEs) consist of a calcium-selective membrane enclosing an inner reference solution of CaCl 2 , AgCl, and other ions, as well as a reference electrode. ISEs are discussed in Chapters 4 and 28. In Chapter 28 , use of ISEs to measure total calcium is also discussed.
The reference interval for total calcium in normal adults ranges between 8.8 and 10.3 mg/dL (2.20 and 2.58 mmol/L). Serum is the preferred specimen for total calcium determination, although heparinized plasma is also acceptable. Citrate, oxalate, and ethylenediaminetetraacetic acid (EDTA) interfere with commonly used methods. Other factors that have been reported to interfere with the colorimetric methods include hemolysis, icterus, lipemia, paraproteins, and magnesium.
The reference interval for ionized (free) calcium in normal adults is 4.6 to 5.3 mg/dL (1.16–1.32 mmol/L). Whole blood, heparinized plasma, or serum may be used. Specimens should be collected anaerobically, transported on ice, and stored at 4°C to prevent loss of carbon dioxide (CO 2 ) and glycolysis and to stabilize pH (because pH changes alter the ionized calcium fraction). Proper collection technique is important to ensure accurate ionized calcium results; a tourniquet left on too long can lower pH at the site of collection and falsely elevate levels.
The reference interval for urinary calcium varies with diet. Individuals on an average diet excrete up to 300 mg/day (7.49 mmol/day). Urine specimens should be collected with appropriate acidification to prevent calcium salt precipitation.
The total body phosphorus content in normal adults is around 700 to 800 g. Approximately 80% to 85% is present in the skeleton; the remaining 15% is present in the ECF in the form of inorganic phosphate and intracellularly in the soft tissues as organic phosphates such as phospholipids, nucleic acids, and adenosine triphosphate (ATP). The skeleton contains primarily inorganic phosphate, predominantly as hydroxyapatite and calcium phosphate.
In blood, organic phosphate is located primarily in erythrocytes, with the plasma containing mostly inorganic phosphate. Approximately two-thirds of blood phosphorus is organic, while only about 3 to 4 mg/dL of the total of 12 mg/dL represents the inorganic form. Inorganic phosphate in serum exists as both divalent (HPO 4 2− ) and monovalent (H 2 PO 4 − ) phosphate anions, both of which represent important buffers. The ratio of H 2 PO 4 − :HPO 4 2− is pH dependent and varies between 1:1 in acidosis, 1:4 at pH of 7.4, and 1:9 in alkalosis. Approximately 10% of the serum phosphorus is bound to proteins; 35% is complexed with sodium, calcium, and magnesium; and the remaining 55% is free. Only inorganic phosphorus is measured in routine clinical settings.
In addition to its role in the skeleton, phosphate has important intracellular and extracellular functions. Phosphate is an important constituent of nucleic acids in that both ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) represent complex phosphodiesters. In addition, phosphorus is contained in phospholipids and phosphoproteins. It forms high-energy compounds (ATP) and cofactors (nicotinamide adenine dinucleotide phosphate [NADPH]) and is involved in intermediary metabolism and various enzyme systems (adenylate cyclase). Phosphorus is essential for normal muscle contractility, neurologic function, electrolyte transport, and oxygen carrying by hemoglobin (2,3-diphosphoglycerate).
Most blood phosphate is derived from diet, but some is derived from bone metabolism. Phosphorus is present in virtually all foods. The average dietary intake for adults is about 800 to 1400 mg, most of which is derived from dairy products, cereals, eggs, and meat. About 60% to 80% of ingested phosphate is absorbed in the gut, mainly by passive transport. However, there is also an active energy-dependent process, which is stimulated by 1,25(OH) 2 D 3 . Serum calcium and phosphorus generally maintain a reciprocal relationship. Phosphorus is freely filtered in the glomerulus. More than 80% of the filtered phosphorus is reabsorbed in the proximal tubule, and a small amount is reabsorbed in the distal tubule. Proximal reabsorption occurs by passive transport coupled to sodium (Na–P cotransport). Phosphorus intake and PTH mainly regulate this cotransport. Phosphorus restriction increases reabsorption, and intake decreases it. PTH induces phosphaturia by inhibition of Na–P cotransport. The effect is exerted mainly in the proximal tubule. The hormone binds to specific receptors in the basolateral membrane, resulting in the activation of two pathways—the adenylate cyclase/cyclic adenosine monophosphate/protein kinase A and the phospholipase C/calcium/protein kinase C systems—both of which are involved in inhibition of Na–P cotransport ( ).
Although PTH lowers serum phosphate, serum levels of phosphate are increased by administration of vitamin D and growth hormone. Vitamin D increases intestinal absorption and renal reabsorption of phosphorus. Growth hormone is a main regulator of skeletal growth. Its presence in the bloodstream reduces renal excretion of phosphates, thereby increasing serum levels.
Recently, a regulation cascade comprising a hormone, fibroblast growth factor-23 (FGF-23), an enzyme termed PHEX (phosphate-regulating gene with homologies to endopeptidases) thought to be involved in the metabolism of FGF-23, and a protein (matrix extracellular glycoprotein [MEPE]) has been elucidated ( ). This cascade is involved in phosphate homeostasis. FGF-23 is normally produced by osteocytes and osteoblasts as well as in marrow pericytes, thymus, and lymph nodes. However, current data support that most of FGF-23 is derived from bone in response to phosphate levels ( ) and provide the first evidence of an independent hormonal regulation of phosphate levels. FGF-23 is also involved in the regulation of the amount of vitamin D and dietary phosphate absorbed by the intestines ( ). Elevated levels of FGF-23 result in hyperphosphaturia, primarily by inhibiting sodium-dependent phosphate resorption channels. FGF-23 also inhibits intestinal phosphorus absorption by inhibiting 25OH-vitamin D 1-α-hydroxylase in the renal proximal tubules ( ). Mutations involving FGF-23, PHEX, and MEPE have been implicated in phosphate wasting by the kidneys and have been associated with various mineralization abnormalities, including hereditary hypophosphatemic rickets, hyperphosphatemic familial tumoral calcinosis, and kidney stones ( , ).
Most commonly used methods for determination of inorganic phosphate are based on the reaction of phosphate with ammonium molybdate to form phosphomolybdate complex (see Chapter 45 ). Direct ultraviolet (UV) measurement of the colorless unreduced complex by absorption at 340 nm, as originally described by Daly and Ertinghausen in 1972, has been adapted for use on most of the automated analyzers. Alternatively, the phosphomolybdate complex can be reduced by a wide variety of agents (e.g., aminonaphtholsulfonic acid, ascorbic acid, methyl- p -aminophenol sulfate, ferrous sulfate) to produce molybdenum blue, which can be measured at 600 to 700 nm. The formation of phosphomolybdate complex is pH dependent, and the rate of its formation is influenced by protein concentration. Measurements of unreduced complexes have the advantages of being simple, fast, and stable. An enzymatic method has also been described whereby phosphorus undergoes successive enzymatic reactions catalyzed by glycogen phosphorylase, phosphoglucomutase, and glucose-6-phosphate dehydrogenase. The NADPH produced can be quantitated fluorometrically or spectrophotometrically. The reaction takes place at neutral pH, thus permitting the measurement of inorganic phosphorus in the presence of unstable organic phosphate.
Serum is preferred because most anticoagulants, except heparin, interfere with results and yield falsely low values. Phosphorus levels are increased by prolonged storage with cells at room temperature. Hemolyzed specimens are unacceptable because erythrocytes contain high levels of organic esters that are hydrolyzed to inorganic phosphate during storage and thus yield elevated levels. Additional interferences are created by icterus and lipemia. False elevations in phosphorus levels can also occur in the setting of dysproteinemia associated with monoclonal gammopathy of uncertain significance, multiple myeloma, or Waldenstrom’s macroglobulinemia ( ).
In normal adults, serum phosphorus varies between 2.8 and 4.5 mg/dL (0.89 and 1.44 mmol/L). Higher phosphorus levels occur in growing children (between 4.0 and 7.0 mg/dL, or 1.29 and 2.26 mmol/L). Serum phosphate is best measured in fasting morning specimens because of diurnal variation, with higher levels in the afternoon and evening as well as a reduction in serum phosphate after meals. Levels are influenced by dietary intake, meals, and exercise.
Magnesium is the fourth most abundant cation in the body after calcium, sodium, and potassium; it is the second most prevalent intracellular cation. The normal body magnesium content in an adult is approximately 1000 mmol, or 22.66 g, of which 50% to 60% is in bone; the remaining 40% to 50% is in the soft tissues. One-third of skeletal magnesium is exchangeable and probably serves as a reservoir for maintaining a normal extracellular magnesium concentration.
Only 1% of total body magnesium (TBMg) is in extracellular fluid. In serum, about 55% of magnesium is ionized or free magnesium (Mg ++ ), 30% is associated with proteins (primarily albumin), and 15% is complexed with phosphate, citrate, and other anions. The interstitial fluid concentration is approximately 0.5 mmol/L. In cerebrospinal fluid (CSF), 55% of the magnesium is free or ionized, and the remaining 45% is complexed with other compounds ( ).
Approximately 99% of TBMg is in bone matrix or is intracellular. About 60% of this total is within bone matrix; the other 40% is within skeletal muscle, within blood cells, or in the cells of other tissues. Intracellular magnesium concentration is approximately 1 to 3 mmol/L (2.4–7.3 mg/dL). Within the cell, magnesium is compartmentalized, and most of it is bound to proteins and negatively charged molecules; approximately 80% of cytosolic magnesium is bound to ATP. Significant amounts of magnesium are found in the nucleus, mitochondria, and endoplasmic reticulum. Free magnesium accounts for 0.5% to 5.0% of the total cellular magnesium; it is the fraction that is probably important as a cofactor supporting enzyme activity.
Magnesium is essential for the function of more than 300 cellular enzymes, including those related to the transfer of phosphate groups, all reactions that require ATP, and every step related to the replication and transcription of DNA and the translation of messenger RNA. This cation is also required for cellular energy metabolism and has an important role in membrane stabilization, nerve conduction, ion transport, and calcium channel activity. In addition, magnesium plays a critical role in the maintenance of intracellular potassium concentration by regulating potassium movement through the membranes of the myocardial cells. Thus, magnesium deficiency can result in a variety of metabolic abnormalities and clinical consequences, including refractory plasma electrolyte abnormalities (especially depressed potassium) and cardiac arrhythmias, most often observed after stress such as cardiac surgery ( ).
TBMg depends mainly on gastrointestinal (GI) absorption and renal excretion. The average dietary intake of magnesium fluctuates between 300 and 350 mg/day, and intestinal absorption is inversely proportional to the ingested amount. The factors controlling the intestinal absorption of magnesium remain poorly understood.
The kidney is the principal organ involved in magnesium regulation. Renal excretion is about 120 to 140 mg/24 hours for a person on a normal diet. Approximately 70% to 80% of plasma magnesium is filtered through the glomerular membrane. Tubular reabsorption of Mg ++ is different from that for other ions because the proximal tubule has a limited role and 60% to 70% of the reabsorption of Mg ++ takes place within the thick ascending loop of Henle ( ). Even though the distal tubules reabsorb only 10% of the filtered Mg ++ , they are the major sites of magnesium regulation. Many factors, both hormonal and nonhormonal (e.g., parathyroid hormone, calcitonin, glucagon, vasopressin, magnesium restriction, acid-base changes, potassium depletion), influence both Henle’s loop and distal tubule reabsorption. However, the major regulator of reabsorption is the plasma concentration of Mg ++ itself. Increased Mg ++ concentration inhibits loop transport, whereas decreased concentration stimulates transport, regardless of whether or not there is magnesium depletion. The mechanisms appear to be regulated by the Ca ++ /Mg ++ -sensing receptor, located on the capillary side of the thick-ascending-limb cells, which senses the changes in Mg ++ ( ). Other factors that may play a role in magnesium regulation include calcium concentration and rate of sodium chloride reabsorption.
In magnesium deficiency, serum levels decrease, which leads to reduced urinary excretion. Later, bone stores of magnesium are affected as the process of equilibration with bone stores takes place over several weeks.
Because serum contains only about 1% of TBMg, it may not accurately reflect total stores. In general, a low serum level indicates deficiency and a high level indicates adequate stores. However, the most common result—a normal level—should be interpreted with caution because it does not exclude an underlying deficiency. The most accurate assessment of magnesium status is generally considered to be the loading test, wherein magnesium is given intravenously. Magnesium-deficient individuals retain a greater proportion of the load and excrete less in the urine than normal individuals ( ). However, the test is not commonly used because it is difficult to administer.
Serum is preferred over plasma for magnesium determination because anticoagulant interferes with most procedures. Serum magnesium is usually measured by photometry. The reference method for total magnesium is AAS. Most clinical laboratories use a photometric method on an automated analyzer. These methods use metallochromic indicators or dyes that change color upon selectively binding magnesium from the sample. Some of the chromophores used include calmagite, methylthymol blue, formazan dye, and magon (see Chapter 28 ). In the calmagite photometric method, which is the one most commonly used, calmagite, whose structure is shown in Chapter 28 (see Fig. 28.2 ), forms a colored complex with magnesium in alkaline solution. This complex is stable for over 30 minutes, and its absorbance at 530 to 550 nm (depending on the indicator) is directly proportional to the magnesium concentration in the specimen aliquot. Some of these measurements are affected by increased serum bilirubin levels, which can result in a significant underestimation of Mg in the sample.
Ionized magnesium can be measured with magnesium ISEs that have been incorporated into several commercial clinical analyzers ( ). These ISEs employ neutral carrier ionophores that are selective for Mg ++ . However, in addition to Mg ++ , these ISEs measure Ca ++ , thus requiring a chemometric correction to calculate the true free magnesium levels in the sample. Studies have shown significant differences in the measured ionized magnesium on different analyzers that were attributed to interference from free calcium in the sample as well as to insufficient specificity and lack of standardization of the calibrators ( ; ). Further improvements in the method for ISEs for ionized magnesium will improve the performance and increase the availability of Mg ++ determination in the clinical laboratory.
As with ionized calcium determinations, ionized magnesium measurements are affected by pH. The rate of change of ionized magnesium measurements is not as significant as that seen in ionized calcium determinations. Changes in magnesium in relation to alterations of pH are similar to those in ionized calcium, although less well characterized. With an increase in pH, ionized magnesium is decreased; with a decrease in pH, it is increased ( ). There is a potential for discrepancies between free and total magnesium levels in the setting of alcoholism, diabetes mellitus, cardiovascular disease, and even pregnancy ( ).
The reference interval for serum total magnesium in normal adults ranges between 0.75 and 0.95 mmol/L (1.7 and 2.2 mg/dL, or 1.5 and 1.9 mEq/L). There appear to be no significant sex or age differences. Erythrocyte magnesium is about three times that of serum. The magnesium concentration in CSF is 2.0 to 2.7 mg/dL (1.0–1.4 mmol/L). The reference interval for ionized magnesium depends on the analyzer used for its measurement and varies from 0.44 to 0.60 mmol/L ( ).
The three principal hormones regulating mineral and bone metabolism are PTH, 1,25(OH) 2 D 3 , and calcitonin. PTH and 1,25(OH) 2 D 3 are the primary hormones that exert an effect; calcitonin is less prominent in the cycle that maintains mineral metabolism. In addition, the metabolic effects of calcitonin are less well understood.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here