Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Mass spectrometry can identify and quantify analytes in human fluids and tissues.
Mass spectrometry is based on volatilizing samples and ionizing the analytes of interest, which can then be identified from their times of flight (TOF) or in electric fields based on their molecular masses and the relative masses of their molecular ion fragments.
New special techniques are now able to analyze patient samples by immediate direct sampling.
These techniques include matrix-assisted laser-desorption ionization (MALDI), paper spray ionization (PSI), desorption electrospray ionization (DESI), direct analysis in real time (DART), and coated-blade spray techniques.
As a result of these techniques and new, ultra-high-resolution mass spectrometers, mass spectrometry is able to analyze the presence and concentrations of many critical proteins, lipids, and small-molecule metabolites in patient samples.
Consequently, mass spectrometry is now being used to perform proteomic analysis of cells in different disease states and is able to identify proteins in disease-causing microorganisms—for example, bacteria—allowing for rapid identification of these organisms.
Mass spectrometry is now able to distinguish between normal and malignant tissue.
Recent innovations in mass spectrometry allow the size of the mass spectrometer to be greatly reduced so that it can be used to perform point-of-care tests, including a mass spectrometer that can be placed in operating rooms, allowing surgeons to sample tissue to determine if tissue margins contain malignant cells.
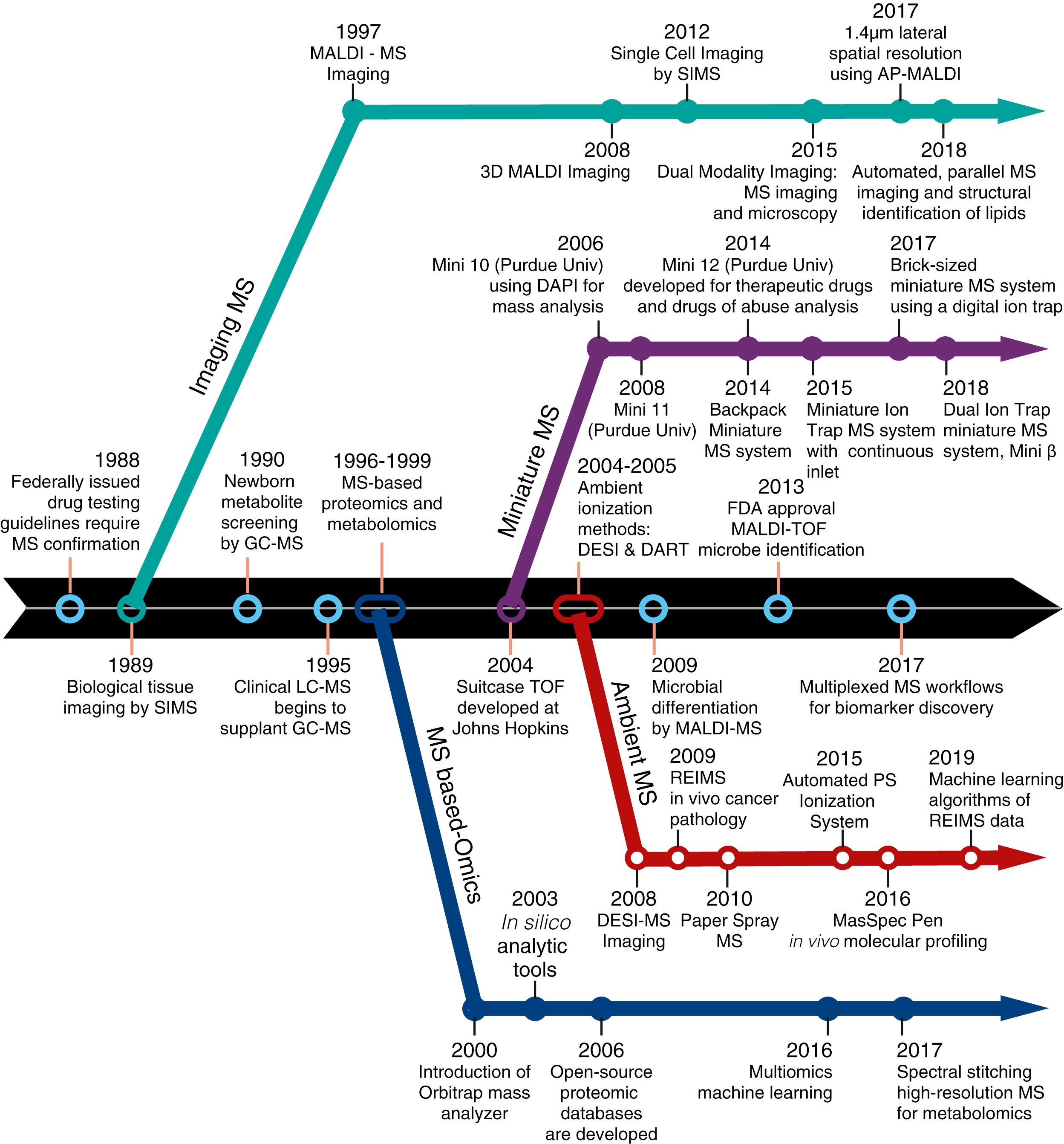
Clinical methods employing mass spectrometric techniques, termed clinical mass spectrometry , is a well established but relatively unknown field that encompasses a wide range of current medical diagnostics. Mass spectrometry provides undeniable merits in comparison with other analytic methods, such as structure identification, and qualitative and quantitative analysis in complicated samples. Many recent applications are focusing on biomedical research, such as proteomics, metabolomics, and drug development. In recent years, several significant advancements in mass spectrometry have allowed for the translation of these methods into clinical applications. Development of new instrumentation and novel methodologies have pushed the limits of mass spectrometry and increasingly expanded its applications in a clinical environment over a relatively short period of time, as summarized in Figure 5.1 . Currently, mass spectrometry is playing a progressively important role in medical diagnostics, especially for biomarker research, toxicology, and newborn metabolite screening. This chapter will go over the principles of mass spectrometry and emphasize its application as a comprehensive system for clinical and biological analyses.
A summary of necessary terminology for the principles of mass spectrometry, criteria, and field of clinical analysis is provided in this section to assist in understanding clinical mass spectrometry.
Mass spectrometry focuses on the molecular weight of each specific component within a sample. Molecules are expressed in terms of atomic mass units (amu) or Daltons (Da). Both terms are interchangeable and are scaled by 1/12th to the reference carbon isotope,
, defined at exactly 12 amu. The following equalities summarize the relation of amu, Da, and kg:
Mass-to-charge ratio (m/z) comes from dividing the relative mass ( m ) in terms of amu or Da to the number of charges ( z ). The charge number is an integer value indicated in multiples of the elementary charge. Most positive ions or negative ions correspond to the loss or addition of a single electron, respectively. In most cases, these ions directly reflect the relative mass. However, large molecules, such as proteins, have multiple sites that can hold a charge, forming multiply charged ions. It should be noted that multiply charged molecules have apparent m/z values that represent only a fraction of its actual mass. A mass spectrometer separates the ions by m/z, which is plotted in the x-axis of a mass spectrum against their relative abundance (y-axis).
Absolute quantitation by mass spectrometry requires an internal standard (IS) to correlate signal intensity to concentration. The IS is usually a compound that is similar but not the same as the species of interest in the sample. In most cases, this entails a deuterated form where several hydrogens have been replaced with deuterium. The similarity to the species of interest minimizes significant differences in ionization to the target species. Further detail on absolute quantitation is provided in the following section.
The sample matrix refers to all other components besides the species of interest. In the case of biological samples—such as blood, urine, or saliva—this encompasses a wide range of proteins, lipids, and other molecules that can easily confound mass analysis.
Matrix effect is the effect of other components in a sample excluding the analyte of interest. In most cases, this is associated with a lowered specificity and sensitivity due to an excess of nonrelevant compounds in the mass spectrometer.
Reference laboratories are laboratory sites that perform analysis that is not commonly done in the hospital or other local laboratories. In most cases, these sites are far away from hospitals or other health care buildings and require long-term biosafe transport to analyze patient samples. These laboratories do not have to use high-throughput methods and analyze patient samples as needed.
Clinical laboratories are sites that are located within or nearby hospitals. Clinical laboratories can vary greatly depending on location, such as acute-care hospitals or medical centers. These laboratories maintain mostly automated instrumentation to run all routine clinical exams at a high volume. For low-volume tests or confirmations, samples are referred to reference laboratories.
Point-of-care settings are environments in which health care is administered. This can include hospitals, intensive care centers, and surgery rooms. Rapid, simple tests are conventionally administered here to obtain preliminary information on whether more in-depth analysis is needed for diagnostics. In most cases, examinations at this setting are to make the initial diagnosis and collect patient samples for analysis in clinical or reference laboratories.
Clinical tests are evaluated based the level of false positives or false negatives that occur in a controlled patient sample set. False positives refer to samples that are returned as positive by the clinical test but are negative. False negatives are samples that are returned as negative but are positive. In terms of clinical test efficacy, false positives prove the least amount of risk and can be mitigated with consecutive tests. However, false negatives are extremely problematic, especially in diseases that require early intervention.
Resolution refers to the ability of a mass analyzer to separate two nearby peaks. In mass spectrometry, this is defined through the following equation:
where R refers to resolution, M is the mass of the peak, and ΔM is resolving power. In mass spectrometry, resolving power is defined through several methods. The peak width definition calculates the resolving power to be equal to the width of the peak measured at a fraction of the peak height. In most cases, this is defined to be at 50%, otherwise called full-width at half maximum (FWHM) . The valley definition is defined as the minimum distance between two overlapping peaks that form a valley (lowest value of signal) at a specified fraction of peak height. In most cases, this refers to either 10% or 50% loss of peak height.
Reproducibility is the ability for the same sample to be analyzed with the same or statistically similar outcomes. This is measured using criteria such as relative signal deviation and evaluated through large-volume clinical samples.
A measure of variability during repeats of the same sample. Relative standard deviation (RSD) values are calculated using the standard deviation and weighted to the average sample mean. These values are provided in percentages.
The lower bounds of an analyte that can be quantified or detected by mass spectrometry.
Mass spectrometry is an analytic technique that outlines the composition of a sample. This is shown by a mass spectrum, where the x-axis represents the m/z and the y-axis is its relative abundance within the sample. Mass spectrometry systems comprise several major components: (1) an ion source, (2) mass analyzer, and (3) detector. In the last several decades, each of these components has undergone advancements that either improved on existing instruments or realized novel hybrid systems that enable unique mass spectrometric analyses. Current research using mass spectrometry has slowly begun to transition from instrumentation into biological applications, translating research-based analytic techniques for high-throughput clinical use.
Before mass analysis, sample extraction and separation are critical steps for highly sensitive and specific analysis. Especially with clinical samples such as blood, urine, or even biopsy tissues, these matrices are immensely complex, containing numerous proteins, lipids, and salts. This step is commonly done to remove unwanted matrices and provide a preliminary separation, preventing detector overloads. However, solid samples such as biopsy tissue sections do not require extraction or separation and rely solely on the mass spectrometer for specificity. Afterwards, the three basic components of a mass spectrometer work sequentially to ionize, identify, and detect ions: the ion source, mass analyzer, and ion detector. Depending on the product from sample extraction—whether it is solid, liquid, or gas—the sample inlet must be adjusted accordingly to promote effective and comprehensive sample ionization.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here