Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Errors and variables in the preanalysis stage can affect test results.
Patient variables include physical activity, diet, age, sex, circadian variations, posture, stress, obesity, smoking, and medication.
Strict adherence to proper technique and site selection can minimize collection variables such as hemolysis, hemoconcentration, clots, and other causes for sample rejection or erroneous results.
Blood collection containers are color coded based on additive or preservative, and each is suitable only for specific tests. Failure to use the proper tubes or filling tubes in the wrong sequence can produce erroneous results.
Blood collection staff must be adequately trained in safety and confidentiality issues.
Blood, urine, and other body fluid constituents can change during transport and storage. The extent of these changes varies by analyte.
The most common reasons for specimen rejection are clotted blood for hematology or coagulation tests; insufficient volume in a tube for coagulation tests; and hemolysis, icterus, and lipemia in serum or plasma that can interfere with chemistry testing.
Preanalysis refers to all the complex steps that must take place before a sample can be analyzed. Over the years, a series of studies identified that 32% to 75% of all testing errors occur in the preanalytic phase ( ; Stahl et al., 1998; ; ; ), and technologic advances and quality assurance procedures have significantly reduced the number of analytic-based errors. This has exposed the preanalysis stage as a major source of residual “error” and/or variables that can affect test results. Preanalytic factors include patient-related variables (diet, age, sex, etc.), specimen collection and labeling techniques, specimen preservatives and anticoagulants, specimen transport, and processing and storage. Potential sources of error or failure in this process include improperly ordered tests, sample misidentification, improper timing, improper fasting, improper anticoagulant/blood ratio, improper mixing, incorrect order of draw, and hemolyzed or lipemic specimens. The most frequent preanalytic errors include improperly filling the sample tube, placing specimens in the wrong containers or preservatives, and selecting the incorrect test ( ). Table 3.1 lists the most common errors associated with specimen collection, including those that occur before specimen collection (e.g., patient ID error), during collection (e.g., incorrect tube or order of draw), and after collection (e.g., improper transport or centrifugation).
| Phase | Error |
|---|---|
| Before Collection |
|
| During Collection |
|
| After Collection |
|
Errors in the preanalytic stage create extra work or additional investigation that may cause unnecessary procedures for patients and costs to the health care system ( ). Preanalytic issues have downstream impact on the use of laboratory resources, hospital costs, and overall quality of care. By some estimates, specimen collection errors cost the average 400-bed hospital about $200,000/year in recollection costs. The estimated average costs of a preanalytical error in North American institutions were estimated at $208, with costs representing between 0.23% and 1.2% of total hospital operating costs ( ). Proper collection technique is also essential to minimize injury to the phlebotomist and the patient. Treatment for an injury related to a traumatic needlestick can cost $500 to $3000, and poor technique can result in patient injury, such as nerve and arterial damage, subcutaneous hemorrhage, infection, and even death. The Centers for Disease Control and Prevention (CDC) estimates that 385,000 needlestick injuries occur per year (CDC, 2004). Many go unreported. This chapter discusses the preanalytic process, with special emphasis on the clinical impact of variables and sources of failure.
In preparing a patient for phlebotomy, care should be taken to minimize physiologic factors related to activities that might influence laboratory determinations. These include diurnal variation, exercise, fasting, diet, ethanol consumption, tobacco smoking, drug ingestion, and posture (Haverstick, 2015).
Diurnal variation. This may be encountered when testing for hormones, iron, acid phosphatase, and urinary excretion of most electrolytes, such as sodium, potassium, and phosphate ( ). Table 3.2 presents several tests affected by diurnal variations, posture, and stress.
| Cortisol | Peaks 4-6 am ; lowest 8 pm –12 am ; 50% lower at 8 pm than at 8 am ; increased with stress |
| Adrenocorticotropic hormone | Lower at night; increased with stress |
| Plasma renin activity | Lower at night; higher standing than supine |
| Aldosterone | Lower at night |
| Insulin | Lower at night |
| Growth hormone | Higher in afternoon and evening |
| Acid phosphatase | Higher in afternoon and evening |
| Thyroxine | Increases with exercise |
| Prolactin | Higher with stress; higher levels at 4 and 8 am and at 8 and 10 pm |
| Iron | Peaks early to late morning; decreases up to 30% during the day |
| Calcium | 4% decrease supine |
Exercise. Physical activity has transient and long-term effects on laboratory determinations. Transient changes may include an initial decrease followed by an increase in free fatty acids, and lactate may increase by as much as 300%. Exercise may elevate creatine phosphokinase (CK), aspartate aminotransferase (AST), and lactate dehydrogenase (LD) and may activate coagulation, fibrinolysis, and platelets (Garza & Becan-McBride, 2014). These changes are related to increased metabolic activities for energy purposes and usually return to preexercise levels soon after exercise cessation. Long-term effects of exercise may increase CK, aldolase, AST, and LD values. Chronic aerobic exercise is associated with lesser increases in plasma concentration of muscle enzymes such as CK, AST, alanine aminotransferase (ALT), and LD. Decreased levels of serum gonadotropin and sex steroid concentrations are seen in long-distance athletes, while prolactin levels are elevated ( ).
Diet. An individual’s diet can greatly affect laboratory test results. The effect is transient and is easily controlled. Glucose and triglycerides, absorbed from food, increase after eating ( ). After 48 hours of fasting, serum bilirubin concentrations may increase. Fasting for 72 hours decreases plasma glucose levels in healthy women to 45 mg/dL (2.5 mmol/L), while men show an increase in plasma triglycerides, glycerol, and free fatty acids, with no significant change in plasma cholesterol. When determining blood constituents such as glucose, triglycerides, cholesterol, and electrolytes, collection should be done in the basal state (Garza & Becan-McBride, 2014). Eating a meal, depending on fat content, may elevate plasma potassium, triglycerides, alkaline phosphatase, and 5-hydroxyindoleacetic acid (5-HIAA). Stool occult blood tests, which detect heme, are affected by the intake of meat, fish, iron, and horseradish, a source of peroxidase, causing a false-positive occult blood reaction ( ). In addition, consumption of bismuth-containing antacids such as Pepto-Bismol also renders false-positive results. Physiologic changes may include hyperchylomicronemia, thus increasing turbidity of the serum or plasma and potentially interfering with instrument readings.
Certain foods or diet regimens may affect serum or urine constituents. Long-time vegetarian diets are reported to cause decreased concentrations of low-density lipoproteins (LDLs), very-low-density lipoproteins (VLDLs), total lipids, phospholipids, cholesterol, and triglycerides. Vitamin B 12 deficiency can also occur unless supplements are taken ( ). A high-meat or other protein-rich diet may increase serum urea, ammonia, and urate levels. High-protein, low-carbohydrate diets, such as the Atkins diet, greatly increase ketones in the urine and increase the serum blood urea nitrogen (BUN). Foods with a high unsaturated-to-saturated fatty acid ratio may show decreased serum cholesterol, while a diet rich in purines will show an increased urate value. Foods such as bananas, pineapples, tomatoes, and avocados are rich in serotonin. When ingested, elevated urine excretion of 5-HIAA may be observed. Beverages rich in caffeine elevate plasma free fatty acids and cause catecholamine release from the adrenal medulla and brain tissue. Ethanol ingestion increases plasma lactate, urate, and triglyceride concentrations. Elevated high-density lipoprotein (HDL) cholesterol, γ-glutamyl transferase (GGT), urate, and mean corpuscular volume (MCV) have been associated with chronic alcohol abuse. Serum concentrations of cholesterol, triglycerides, and apoB lipoproteins are correlated with obesity. Serum LD activity, cortisol production, and glucose increase in obesity. Plasma insulin concentration is also increased, but glucose tolerance is impaired. In obese men, testosterone concentration is reduced ( ).
Stress. Mental and physical stresses induce the production of adrenocorticotropic hormone (ACTH), cortisol, and catecholamines. Total cholesterol has been reported to increase with mild stress, and HDL cholesterol to decrease by as much as 15% ( ). Hyperventilation affects acid-base balance and elevates leukocyte counts, serum lactate, or free fatty acids.
Posture. Posture of the patient during phlebotomy can have an effect on various laboratory results. An upright position increases hydrostatic pressure, causing a reduction of plasma volume and increased concentration of proteins. Albumin and calcium levels may become elevated as one changes position from supine to upright. Elements that are affected by postural changes are albumin, total protein, enzymes, calcium, bilirubin, cholesterol, triglycerides, and drugs bound to proteins. Incorrect application of the tourniquet and fist exercise can result in erroneous test results. Using a tourniquet to collect blood to determine lactate concentration may result in falsely increased values. Prolonged tourniquet application may also increase serum enzymes, proteins, and protein-bound substances—including cholesterol, calcium, and triglycerides—as the result of hemoconcentration when plasma water leaves the vein because of back pressure. After bed rest in the hospital, a patient’s hemoglobin (Hb) can decrease from the original admitting value enough to falsely lead a physician to suspect internal hemorrhage or hemolysis ( ). This effect can be amplified by intravenous fluid administration. Patients should be advised to avoid changes in their diet, consumption of alcohol, and strenuous exercise 24 hours before having their blood drawn for laboratory testing.
Age. Age of the patient has an effect on serum constituents. Young defines four age groups: newborn, childhood to puberty, adult, and elderly adult ( ). In the newborn, much of the Hb is Hb F, not Hb A, as seen in the adult. Bilirubin concentration rises after birth and peaks at about 5 days. In cases of hemolytic disease of the fetus and newborn (HDFN), bilirubin levels continue to rise. This often causes difficulty in distinguishing between physiologic jaundice and HDFN. Infants have a lower glucose level than adults because of their low glycogen reserve. With skeletal growth and muscle development, serum alkaline phosphatase and creatinine levels, respectively, also increase. The high uric acid level seen in a newborn decreases for the first 10 years of life and then increases, especially in boys, until the age of 16 ( ). Most serum constituents remain constant during adult life until the onset of menopause in women and middle age in men. Increases of about 2 mg/dL (0.05 mmol/L) per year in total cholesterol and 2 mg/dL (0.02 mmol/L) per year in triglycerides until midlife have been reported. The increase in cholesterol seen in postmenopausal women has been attributed to a decrease in estrogen levels. Uric acid levels peak in men in their 20s but do not peak in women until middle age. The elderly secrete less triiodothyronine, parathyroid hormone, aldosterone, and cortisol. After age 50, men experience a decrease in secretion rate and concentration of testosterone, and women have an increase in pituitary gonadotropins, especially follicle-stimulating hormone (FSH) ( ).
Sex. After puberty, men generally have higher alkaline phosphatase, aminotransferase, creatine kinase, and aldolase levels than women; this is due to the larger muscle mass of men. Women have lower levels of magnesium, calcium, albumin, Hb, serum iron, and ferritin. Menstrual blood loss contributes to the lower iron values ( ).
Tobacco smokers have high blood carboxyhemoglobin levels, plasma catecholamines, and serum cortisol. Changes in these hormones often result in decreased numbers of eosinophils, while neutrophils, monocytes, and plasma free fatty acids increase. Chronic effects of smoking lead to increased Hb concentration, erythrocyte (red blood cell [RBC]) count, MCV, and leukocyte (white blood cell [WBC]) count. Increased plasma levels of lactate, insulin, epinephrine, and growth hormone and urinary secretion of 5-HIAA are also seen. Vitamin B 12 levels may be substantially decreased and have been reported to be inversely proportional to serum thiocyanate levels. Smoking also affects the body’s immune response. Immunoglobulin A (IgA), IgG, and IgM are lower in smokers, and IgE levels are higher. Decreased sperm counts and motility and increased abnormal morphology have been reported in male smokers when compared with nonsmokers ( ).
On occasion, when there is a problem finding a vein for phlebotomy, the specimen may be hemolyzed as the result of sheer forces on the RBCs. Hemolysis can also be caused by using a needle that is too small, pulling a syringe plunger back too fast, expelling the blood vigorously into a tube, shaking or mixing the tubes vigorously, or performing blood collection before the alcohol has dried at the collection site. A recent emergency department study concluded that reduced hemolysis is associated with straight stick, antecubital location, shorter tourniquet time (less than 60 seconds) and larger gauge for IV draws ( ). Smaller volume and smaller vacuum blood collection tubes can also reduce hemolysis ( ). Hemolysis is present when the serum or plasma layer is pink. Hemolysis can falsely increase blood constituents such as potassium, magnesium, iron, LD, phosphorus, ammonium, and total protein (Garza & Becan-McBride, 2014). Table 3.3 shows changes in serum concentrations (or activities) of selected constituents caused by lysis of RBCs.
| Constituent | Ratio of Concentration (or Activity) in RBC to Concentration (or Activity) in Serum | Percent Change of Concentration (or Activity) in Serum after Lysis of 1% RBC, Assuming a Hematocrit of 0.50 |
|---|---|---|
| Lactate dehydrogenase | 16:1 | +272.0 |
| Aspartate aminotransferase | 4:1 | +220.0 |
| Potassium | 23:1 | +24.4 |
| Alanine aminotransferase | 6.7:1 | +55.0 |
| Glucose | 0.82:1 | –5.0 |
| Inorganic phosphate | 0.78:1 | +9.1 |
| Sodium | 0.11:1 | –1.0 |
| Calcium | 0.10:1 | +2.9 |
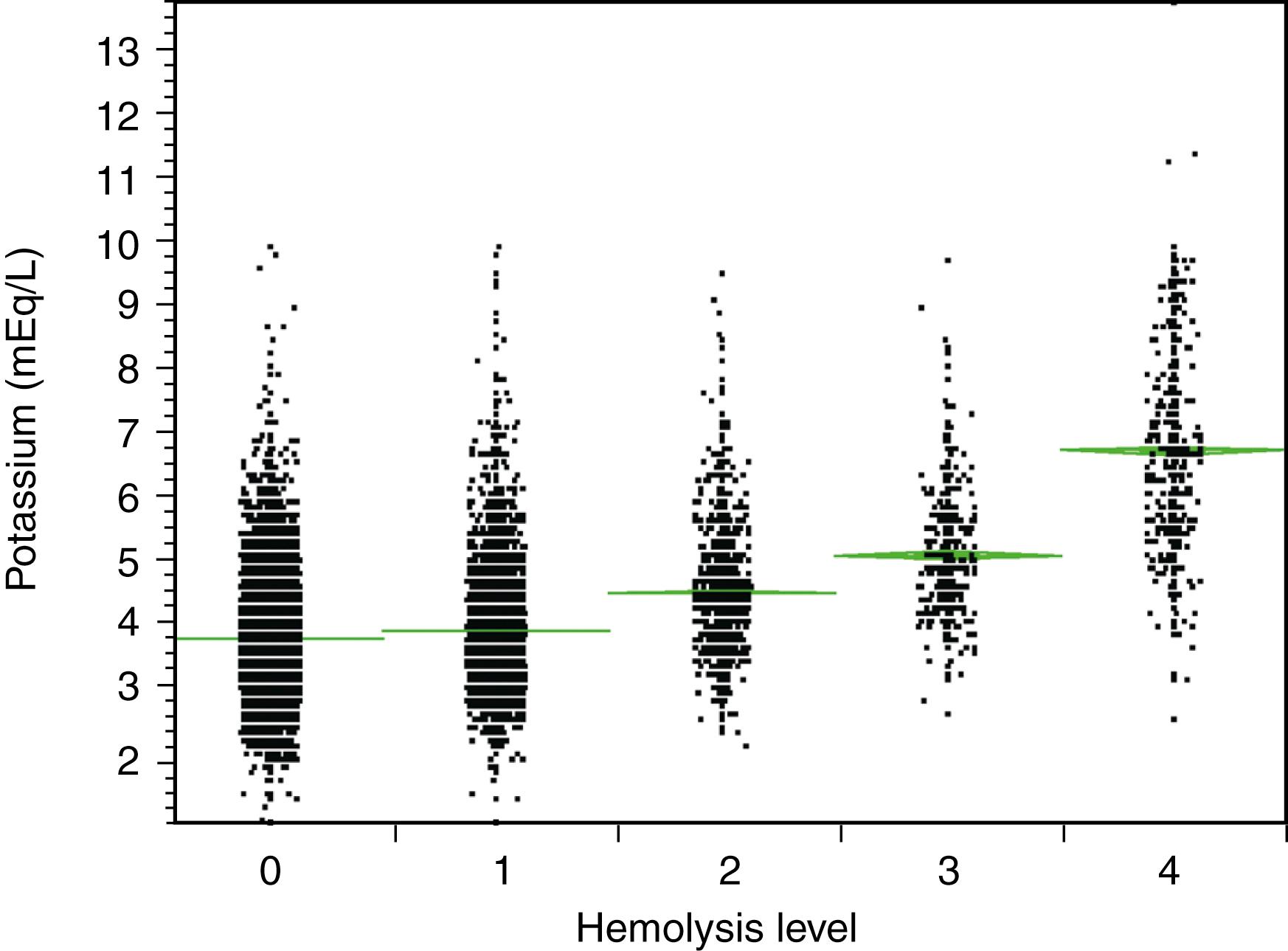
Because of the extremely important role of potassium in cardiac excitation, elevations due to hemolysis can be problematic, especially for emergency department patients who are at risk of hemolysis during frantic blood collection. The relationship between level of hemolysis and potassium (as determined on a Siemens ADVIA 1650 chemistry analyzer [Siemens Healthcare Diagnostics, Deerfield, IL]) in serum and plasma specimens is shown in Figure 3.1 . Even with no hemolysis, the range of potassium concentrations can be broad in a combination of healthy and sick individuals. Low levels of hemolysis cause only minor elevations, but very strong hemolysis can raise the potassium level by 2 to 3 mEq/L into a critical range.
Another special case where pseudohyperkalemia can occur is in patients with extremely high blast counts in acute- or accelerated-phase leukemias. Those blasts can be fragile and may lyse during standard phlebotomy, releasing potassium. In contrast, specimens with very high WBC counts that are collected gently can show pseudohypokalemia when potassium is taken up by highly metabolically active leukemic cells along with glucose. Such specimens can be transported on ice to slow this enzymatically mediated uptake.
Normally, platelets release potassium during clotting; thus serum has a slightly higher value of potassium than plasma from the same individual. This difference is accentuated when the platelet count is extremely elevated.
To avoid problems with hemoconcentration and hemodilution, the patient should be seated in a supine position for 15 to 20 minutes before the blood is drawn ( ). Extended application of the tourniquet can cause hemoconcentration, which increases the concentrations of analytes and cellular components. When blood collection tubes that contain various anticoagulants/additives are used, it is important to follow the proper order of draw and to thoroughly mix an anticoagulated tube of blood after it has been filled. Failure to mix a tube containing an anticoagulant will result in failure to anticoagulate the entire blood specimen, and small clots may be formed. Erroneous cell counts can result. If a clot is present, it may also occlude or otherwise interfere with an automated analyzer. It is very important that the proper anticoagulant be used for the test ordered. Using the wrong anticoagulant will greatly affect the test results.
Icteric or lipemic serum provides additional challenges in laboratory analysis. When serum bilirubin approaches 430 mmol/L (25 mg/L), interference may be observed in assays for albumin (4-hydroxyazobenzene-2-carboxylic acid [HABA] procedure), cholesterol (using ferric chloride reagents), and total protein (Biuret procedure); the bromcresol green method is less susceptible to bilirubin interference (see Chapter 28 ). Artifactually induced values in some laboratory determinations result when triglyceride levels are elevated (turbidity) on the basis of absorbance of light of various lipid particles. Lipemia occurs when serum triglyceride levels exceed 4.6 mmol/L (400 mg/dL). Inhibition of assays for amylase, urate, urea, CK, bilirubin, and total protein may be observed (see Chapter 28 ). To correct for artifactual absorbance readings, “blanking” procedures (the blank contains serum but lacks a crucial element to complete the assay) or dual-wavelength methods may be used. A blanking process may not be effective in some cases of turbidity, and ultracentrifugation may be necessary to clear the serum or plasma of chylomicrons.
In addition to the preanalytic variables discussed earlier, there is a variety of special conditions and interferences that may impact sample analysis.
A variety of substances can interfere with immunoassays; this, in turn, can lead to the misinterpretation of a patient’s results. For example, biotin supplements (which have increased in usage in recent years) can create analytic interference in biotin-based immunoassays (Holmes et al., 2017). Dietary supplementation with over-the-counter biotin has recently been recognized to interfere with a multitude of commercial immunoassays, causing either falsely low or falsely high results. The United States Food and Drug Administration (FDA) issued a warning on November 28, 2017, regarding clinically significant errors due to biotin interference. Examples include a falsely low troponin result leading to missed diagnosis of heart attack and hormone errors.
The reason for this interference is assay configuration in which molecular sandwich formation is either blocked or enhanced excessively by high concentrations of biotin. In one such competitive binding assay design, analyte (e.g., thyrotropin, thyroid-stimulating hormone [TSH]) in test blood sample binds to reagent capture antibody, thereby displacing reagent detector analyte (labelled with chemiluminescent molecule). The capture antibody is coupled to biotin (i.e., biotinylated), which, in turn, binds to streptavidin coated on magnetic beads. After washing off unbound constituents, the amount of analyte from the sample is inversely proportional to the signal from bound detector molecule. If the patient sample has a very high level of free biotin, it will bind to the streptavidin and block binding of the capture antibody to the beads. The resulting signal erroneously looks as if the patient sample had a very high level of the analyte. Other assay configurations can result in erroneously low values.
Daily recommended allowance for biotin is 0.03 mg, but supplements sold for benefit of hair, skin, and nails may have up to 20 mg of biotin. For treatment of multiple sclerosis, up to 300 mg per day may be recommended. These extremely high doses of biotin can lead to concentrations up to 1200 ng/mL in blood, which is very likely to interfere with susceptible immunoassays. A study of various assays from the same manufacturer showed false reductions in high-sensitivity troponin T, TSH, and follicle-stimulating hormone but false elevations in triiodothyronine and vitamin D from biotin, indicating that each assay must be evaluated individually for its particular susceptibility to interference ( ). The prevalence of biotin supplementation was 7.7% in outpatients surveyed by questionnaire; by direct measurement, 7.4% of emergency department patients were found to have concentrations of biotin at or above levels known to interfere with one manufacturer’s immunoassays ( ). One recommendation for patients taking biotin supplements is to stop taking them for 48 to 72 hours prior to scheduled blood tests to allow the water-soluble biotin to clear from their bodies (Charles et al., 2019). Additional laboratory measures include having backup methods known to be free of biotin interference and to communicate to clinicians through result comments that specific assays are susceptible to biotin interference ( ).
Endogenous substances, human antianimal species, or autoantibodies can interfere with the reaction between analyte and reagent antibodies. Manufacturers usually add blocking agents to immunoassay reagents to inhibit or neutralize the interference ( ). Immunoassays use antibodies derived from a variety of species—for example, mouse antibody. Human antimouse antibodies (HAMAs), also referred to as heterophiles, can arise following antigenic stimulation from therapeutic mouse monoclonal antibodies that are administered to alter immune responses (e.g., anti–T cell antibody), to bind and remove toxic levels of drugs (e.g., digoxin), or to attack tumors. Some individuals with HAMAs have no history of therapeutic exposures but could conceivably have had incidental exposure to mouse proteins through contaminated food or other environmental sources. The effect of HAMAs in immunoassays can be to cross-link capture and signal antibodies in a sandwich that mimics true antigen ( ). For example, an immunoassay for TSH that has separate antibodies against α and β subunits might yield an astonishingly high false-positive result in a euthyroid person with HAMA. In this case, the other thyroid function tests could be completely normal. The presence of HAMA can be confirmed by direct measurement (usually sent to a reference laboratory) and can also be inferred by adsorption of the HAMA onto special tubes coated with mouse antibodies, followed by repeat measurement of the analyte to look for reduction in signal strength in the treated specimen ( ). Monoclonal antibodies with specific molecular targets are rapidly moving into clinical practice. One example is emicizumab, which has application as a coagulation factor VIII inhibitor bypass agent as it is a humanized bispecific monoclonal antibody that links activated coagulation factor IX and factor X, which replicates the action of factor VIII ( ). Its initial use is for hemophilia A patients who have developed resistance (i.e., inhibitor) to factor VIII infusion. Future use might extend to all hemophilia patients due to its effectiveness and relative ease of administration by subcutaneous injection. Unfortunately, the binding of emicizumab to factors IX and X also occurs in activated partial thromboplastin time (APTT)–based assays for factor VIII levels and for inhibitor assays even at very low levels of this monoclonal antibody. Because of this interference, the APTT cannot be used to monitor patients receiving emicizumab. One possible solution to this interference is to include in the assay for factor VIII specially prepared anti-idiotype monoclonal antibodies to neutralize any emicizumab present ( ).
Another instance of interference from therapeutic monoclonal antibody is daratumumab, which is approved as a treatment for multiple myeloma and is used increasingly for B-cell malignancies ( ). Although CD38 is present on plasma cells and B-cell proliferations, it is also present on RBCs. Accordingly, daratumumab shows panreactive interference with reagent panel RBCs and acts as an antibody to a high-prevalence antigen in blood bank compatibility procedures ( ). This interference can mask the presence of other significant antibodies. Procedures to work around daratumumab interference include use of dithiothreitol to break disulfide bonds in CD38 and inactivate that antigen (but it can also degrade other antigens). Other strategies to neutralize the anti-CD38 activity include use of soluble CD38 fragments and anti-idiotype antibody against the monoclonal antibody of daratumumab.
Common biochemical analytes—such as electrolytes, small molecules, enzymes, and so on—are generally distributed in the water phase of plasma or serum. Consequently, specimens with reduced water phase due to hyperproteinemia (e.g., from very high concentrations of a myeloma protein) or hyperlipidemia (e.g., high chylomicron content) can have reduced content of those solvent analytes even though other properties, such as ionic activities in those specimens, may be within normal physiologic range. This phenomenon is termed the solvent exclusion effect , referring to the exclusion of water and small molecules in the aqueous phase when more volume within a specimen is occupied by protein or lipid that excludes water. The content of small molecules per volume is the osmolarity (which is the measurement that can be erroneous), whereas the physiologically important aspect, such as ionic activities, is the osmolality. If excess lipids are the cause, they may be removed by ultracentrifugation. If interference is due to excess protein, an alternative mode of analysis, such as ion-selective electrode in undiluted specimen, can be employed to yield correct electrolyte activity (i.e., equivalent of osmolality).
Matrix effects from very high or very low concentrations of proteins and other constituents may be problematic when dealing with other body fluids, especially when the specimens are highly viscous or otherwise atypical. In those situations, it may be necessary to qualify results in the report to indicate the site of the body fluid and possible limitations in accuracy of measurement.
Laboratory manipulations of nucleic acids are susceptible to interferences at various stages, including specimen collection and processing. Introduction of inhibitory substances and contamination with false-positive signals are among the significant interferences. Blood specimens for nucleic acid testing are generally collected into EDTA anticoagulant to inhibit enzymes that might break them down. Heparin is a poor choice for anticoagulant in this application because it can be coextracted with DNA and inhibits DNA polymerase in polymerase chain reactions (PCRs). Hemin from hemolysis in plasma or serum can also inhibit DNA polymerase. RNA is labile in blood or tissues; thus these specimens must be stored appropriately by rapid freezing in liquid nitrogen if the extraction will be delayed.
Extraction of nucleic acids from clinical specimens such as plasma (e.g., for viral load measurement), blood cells (e.g., for genetic testing), or tissues (e.g., for analyzing mutations in tumors) entails lysing cells and separating nucleic acids from proteins and lipids. Reagents for extraction include salts, proteases, and phenol-chloroform to denature the substances complexed with nucleic acids. This process must be optimized for specimen type to recover high-quality nucleic acids with good quantitative yield. Care must be taken to avoid contamination of specimens with target nucleic acids from other specimens or with amplified targets from specimens that have been analyzed previously in that vicinity. Accordingly, laboratories practicing nucleic acid amplification, especially PCR, should have separate preamplification, amplification, and postamplification areas with strict rules about personnel movements between them.
Analytic methods that are based on oxidation–reduction reactions may be influenced positively or negatively by ingested substances such as ascorbic acid (vitamin C). This interference is observed in chemical testing of serum on automated analyzers ( ) and can also occur in urine testing for glucose (positive interference for reducing substance method; negative interference with enzymatic method). In stool testing for occult blood, peroxidases from meats (myoglobin) or vegetables (horseradish) in the diet can yield a false-positive result with guaiac-based methods, as can topical iodine or chlorine used as a disinfectant.
Drugs can have unanticipated reactions with the reagents intended for specific chemical tests. The list of potential interfering drugs is extremely long, and some methods for a particular analyte may be strongly affected, whereas other methods may not be affected at all. A voluminous compendium of drug interactions has been developed by Dr. Donald S. Young ( ). In addition to assisting with recognition of potential interferences, this source can be used to evaluate a different method that is unaffected by a particular drug to confirm the accuracy of measurement in cases of suspected interference. These interferences are separated into those whose effects are manifested directly in the assay in vitro and those that are due to drug actions in vivo, whereby physiologic functions are changed (e.g., prolonged prothrombin time [PT] with Coumadin, lower potassium in blood with some diuretics).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here