Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Periprocedural myocardial necrosis remains a common complication of percutaneous coronary intervention (PCI).
The 2012 Third Universal Definition document defines a myocardial infarction (MI) associated with PCI as elevation of troponin values above five times the 99th percentile of upper reference limit (URL) in patients with normal baseline values or a rise of troponin values above 20% if the baseline values are elevated and are stable or falling.
Strategies to prevent periprocedural MI (PMI) include pharmacologic and mechanical approaches.
The primary pharmacologic interventions that have achieved significant success in preventing PMI include aggressive antiplatelet and statin therapy.
Mechanical approaches include the use of embolic protection devices (EPDs) in the setting of saphenous vein graft (SVG) intervention, carotid stenting, and transcatheter aortic valve replacement (TAVR).
The use of routine cerebral embolic protection during TAVR remains controversial, with some evidence to suggest benefit.
The contemporary definition of periprocedural myocardial infarction (PMI) is based on the rise and fall of biomarkers—such as total creatine kinase (CK), creatine kinase MB (CK-MB), and troponin—after percutaneous coronary intervention (PCI) in addition to clinical, electrocardiography (ECG), and imaging evidence of myonecrosis.
The incidence of PMI varies according to the type of assayed biomarker (CK-MB, troponin I [Tn I], or troponin T [Tn T]) and the preset threshold for diagnosis.
Larger PMIs are infrequent and usually follow angiographically documented complications, such as side-branch closure or no-reflow phenomenon, but smaller and more common PMIs often follow apparently uncomplicated procedures.
The primary underlying mechanisms of PMI are side-branch occlusions and distal embolization into the downstream microcirculation of the PCI-related vessel, with platelet aggregation/activation playing a significant role in subsequent myonecrosis.
Risk factors for development of PMI include acute presentation, heightened systemic inflammation, and advanced coronary and/or noncoronary atherosclerotic disease. Atheroablation devices (directional or rotational) are associated with higher rates of PMI, followed by stents and then balloon angioplasty.
PMI is associated with increased late mortality, and the association is more robust when the CK-MB or troponin levels exceed five times the upper limit of normal (ULN).
Potent antiplatelet therapies (intravenous [IV] glycoprotein [GP] IIb/IIIa inhibitors, and/or oral thienopyridine inhibitors) decrease the incidence of PMI, especially in high-risk procedures.
Pretreatment with statins reduces the incidence of PMI because of their antiinflammatory effects.
Embolism protection devices (EPDs) include distal occlusive balloons, filter devices, and proximal flow occlusion/reversal systems; all aim to prevent embolized debris produced at the angioplasty site from reaching the distal microvascular bed.
Clinical trials have demonstrated that using an EPD during vein graft PCI leads to a significant reduction in PMI. However, newer data suggests they may not be uniformly beneficial, particularly for certain lesion subsets.
Several randomized trials failed to show any benefit of an EPD in the setting of PCI for acute myocardial infarction (MI), thus highlighting the complexity of the mechanisms of myonecrosis and injury in those settings.
Good evidence suggests that EPDs reduce cerebral embolism during carotid stenting, but no prospective randomized trials based on clinical end points have been undertaken to confirm that benefit.
Comparison of carotid stenting with the use of embolic protection with carotid endarterectomy demonstrates similar outcomes in patients with asymptomatic disease.
The exact role of EPDs during transcatheter aortic valve replacement (TAVR) is yet to be defined. The relationship between imaging evidence for cerebral embolism, or volume of embolic particulate debris captured in devices, with clinical stroke and cognitive decline remains unclear.
Given the explosive growth of TAVR, keen interest persists in the development and application of new cerebro-protective devices.
Periprocedural myocardial necrosis remains the most common complication of PCI. Such myonecrosis can range from a clinically silent minor elevation of cardiac enzymes to a major MI with short- and/or long-term consequences. With advances in pharmacologic therapies and in interventional technology, the incidence of early major adverse cardiac events (MACEs)—such as large MI and death—has fallen to less than 3%, even in complex multivessel PCI. The reduced incidence of these complications can be attributed in large part to the role of coronary stents in treatment of abrupt closure and the aggressive antiplatelet therapies more commonly utilized over the last three decades. This improvement in outcomes is remarkable considering the ever-increasing number and complexity of patients and lesions treated with PCI today compared with 20 to 30 years ago. However, the frequency with which any periprocedural myonecrosis is detected has increased, primarily because of the development and widespread adoption of sensitive biomarkers of myocardial damage. As such, the exact definition and clinical significance of the periprocedural release of cardiac markers are topics for active debate, both in real-world practice and in clinical trial settings.
The definition of periprocedural myocardial infarction (PMI) is continuing to evolve with changing and improving biomarker assays and a better understanding of the prognostic significance of these events. Currently, commonly used definitions for the diagnosis of PMI are based on one of two documents: the Third Universal Definition of Myocardial Infarctions and the definition proposed by the Society of Cardiac Angiography and Interventions (SCAI) expert consensus document.
Both definitions are based on identifying the rise and fall of cardiac biomarkers following PCI in addition to corroborating clinical, ECG, imaging, and/or angiographic evidence of myonecrosis. They differ in the various thresholds considered enough to make the diagnosis and in how they indicate a clinically relevant change in prognosis. It is important to note that both definitions of PMI are complicated by earlier referral of acute coronary syndrome (ACS) and MI patients to the catheterization laboratory. Importantly, the prognostic implication of elevated biomarkers after PCI cannot be known unless the baseline level is taken into account. In patients with elevated biomarker levels at baseline, prognosis is more directly linked to the baseline or initial injury than to the postprocedural level. In fact, biomarker elevation before PCI is the most important determinant of long-term mortality. In those situations, detection of abnormal levels of cardiac markers after PCI may not necessarily be related to the procedure but are simply a reflection of the ongoing myonecrosis caused by the thrombotic event that led to the clinical presentation. In ACS patients, biomarker levels may rise after a normal initial sample, which commonly coincides with the time when angiography and PCI are performed. For these reasons, both definitions include specific criteria to extend the definition of PMI to those who were referred to PCI in the setting of ACS or ST-elevation myocardial infarction (STEMI).
The 2012 Third Universal Definition document proposed the following updated definition for PMI: an MI associated with PCI is arbitrarily defined by elevation of troponin values greater than five times the 99th percentile (upper reference limit [URL]) in patients with normal baseline values (≤99th percentile URL) or a rise of troponin values greater than 20% if the baseline values are elevated and are stable or falling. The required enzymatic criteria should be associated with (1) symptoms consistent with myocardial ischemia; (2) new ischemic ECG changes or new left bundle branch block (LBBB); (3) angiographic loss of patency of a major coronary artery or a side branch, a persistent slow- or no-flow state, or embolization; or (4) imaging demonstration of new loss of viable myocardium or new regional wall motion abnormality. PCI-related MI (type 4) is distinguished from spontaneous MI (type 1), secondary MI (type 2), and MI associated with sudden death (type 3) or coronary artery bypass grafting (CABG; type 5). A documented stent thrombosis is recognized as a type 4b MI, whereas an MI associated with restenosis greater than 50% is type 4c ( Table 30.1 ).
| Category | Description/Definition |
|---|---|
| Type 4a: MI related to PCI | MI associated with PCI is arbitrarily defined by elevation of cardiac troponin values greater than five times the 99th percentile URL in patients with normal baseline values or a rise of cardiac troponin values >20% if the baseline values are elevated and are stable or falling. In addition, one of the following features needs to be present:
|
| Type 4b: MI related to stent thrombosis | MI associated with stent thrombosis is detected by coronary angiography or autopsy in the setting of myocardial ischemia and with a rise and/or fall of cardiac biomarker values with at least one value above the 99th percentile URL. |
| Type 4c: MI related to restenosis | MI with evidence of 50% or more stenosis at coronary angiography or a complex lesion associated with a rise and/or fall of troponin values above the 99th percentile URL and no other significant obstructive CAD of greater severity following (1) initially successful stent deployment or (2) initially successful PTCA (diameter stenosis <50% at the end of the procedure). |
When a troponin value is elevated but less than or equal to five times the 99th percentile URL after PCI, and the troponin value was normal before the PCI, or when the troponin value is more than the 99th percentile URL in the absence of ischemic, angiographic, or imaging findings, the task force suggested that the term myocardial injury should be used.
Notably, the universal definition states that troponin is the preferred biomarker, the prognostic significance of which is less well validated than CK-MB in the setting of post-PCI myonecrosis. A large body of literature has demonstrated that post-PCI CK and CK-MB elevations have serious adverse prognostic implications, even in absence of pathologic Q-waves. Three meta-analyses to examine the prognostic impact of periprocedural elevated CK-MB have confirmed a proportionate increase in early and late mortality with rising values. The use of abnormal troponin assays to diagnose type 4a MI is supported by some datasets, although supporting evidence is not as wide ranging as has been demonstrated on the basis of abnormal CK-MB. Troponin is a particularly sensitive biomarker used ubiquitously for risk stratification in patients with ACS, but its widespread adoption has by itself increased the incidence of spontaneous MI and PMIs by 40% to 50%. In contrast to its predictive value in type 1 MI (ACS), existing data on peri- or post-PCI troponin elevation do not establish a clear association with an adverse prognosis. To make the definition more clinically relevant, the biomarker threshold for PMI was raised from greater than three times the 99th percentile URL in the earlier version of the Universal Definition document to greater than five times the 99th percentile URL in the most recent one. Nonetheless, the more recently recommended threshold remains arbitrary, and critics argue that any diagnosis of PMI should be associated with meaningful, well-defined prognostic significance.
To address the limitations of the universal definition of PMI discussed above, an expert consensus group from the SCAI proposed an alternate definition of clinically relevant MI after PCI, defined as “an event associated with a worsened prognosis.” To this end, the document recommended that CK-MB be the preferred biomarker to assess clinically relevant PMI, defined as a CK-MB 10 or more times the ULN. A lower threshold (≥5 × ULN) may be accepted in the patient in whom new pathologic Q-waves in two or more contiguous leads (or new, persistent LBBB) develop after PCI, although further study is required to validate this threshold. If CK-MB assays are not available, the consensus document suggests a troponin level of 70 or more times the ULN to diagnose a type 4a MI. The authors selected this high troponin threshold so that the prognostic implication would be comparable to that associated with a CK-MB elevation of 10 or more times the ULN.
The following recommendations were made to diagnose post-PCI MI in ACS patients in whom the baseline level has not returned to normal: (1) in patients with elevated troponin (or CK-MB) in whom the biomarker levels are stable or falling, a new CK-MB elevation by an absolute increment of 10 or more times the ULN (or ≥70 × ULN for troponin) from the previous nadir level should be evident; (2) in patients with elevated troponin (or CK-MB) in whom the biomarker levels have not been shown to be stable or falling, there should be a further rise in CK-MB or troponin beyond the most recently measured value by an absolute increment of 10 or more times the ULN in CK-MB or 70 or more times the ULN in troponin plus new ST-segment elevation or depression plus signs consistent with a clinically relevant MI, such as new-onset or worsening heart failure or sustained hypotension.
Although substantial debate surrounds the clinical significance of minor biomarker elevation after PCI, there is solid imaging evidence that biomarker release following PCI corresponds to irreversible myocardial injury, both qualitatively and quantitatively.
One study had 48 patients undergo cardiac magnetic resonance imaging (CMRI) before and after PCI to detect newly developed late gadolinium enhancement (LGE) as evidence of procedure-related myonecrosis. Findings were correlated with serum troponin levels recorded 24 hours after the index PCI. Troponin elevation above the ULN was noted in 37% (14 patients), all with evidence of LGE in the target vessel territory. A linear correlation was also apparent between the troponin level at 24 hours after PCI and the mass (in grams) of newly hyper-enhanced myocardium, both on the early post-PCI scan and on delayed 8-month scans, thus confirming the correlation between periprocedural biomarker release and irreversible myocardial damage ( Fig. 30.1 ).
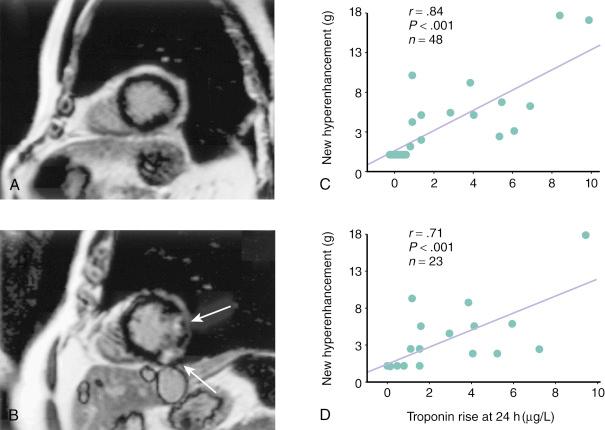
In addition to confirming the irreversible nature of the myocardial injury, the location of MR hyperenhancement in relation to the PCI target segment may give insight into the pathophysiologic mechanism underlying PMI. When the hyperenhancement is visualized in proximity of the treated segment, side-branch occlusion (SBO) is the more likely explanation. However, in cases in which the myocardial injury/damage is downstream from the treated segment, PMI can be best explained by distal microembolization and adverse platelet and inflammatory reactions in the microcirculation.
As with its definition, the reported incidence of PMI has varied widely from one published report to another ( Table 30.2 ). This variation can be attributed to several factors, but the predominant predictors are the choice of biomarker assayed and the threshold value used to diagnose PMI.
| Reference | n | Type of PCI | Biomarker Definition of PMI | Incidence of PMI (%) |
|---|---|---|---|---|
| Harrington et al. | 1012 | PTCA DCA |
CK-MB × 2 ULN | 3.8 10.3 |
| Abdelmeguid et al. | 4664 | PTCA, DCA | CK 2–5 × ULN | 2.6 |
| Ellis et al. | 8409 | PCI | CK-MB >8.8 | 17.2 |
| Ghazzal et al. | 15,637 | PCI | CK 1–2 × ULN | 4.6 |
| CK >3 × ULN | 1.6 | |||
| Simoons et al. | 5025 | PTCA | CK-MB 1–3 × ULN | 13.2 |
| Ioannidis et al. | ||||
| Roe et al. | 2384 | PCI | CK-MB 1–3 × ULN | 21.3 |
| CK-MB 3–5 × ULN | 6.0 | |||
| CK-MB 5–10 × ULN | 7.1 | |||
| CK-MB >10 × ULN | 9.5 | |||
| Stone et al. | 7147 | PTCA Stent Ablation Ablation + stent |
CK-MB >4 | 25.1 34.4 37.8 48.8 |
| Natarajan et al. | 1128 | PCI | Tn I >0.5 | 16.8 |
| Nallamothu et al. | 1157 | PCI | Tn I 1–3 × ULN | 16.0 |
| Tn I 3–5 × ULN | 4.6 | |||
| Tn I 5–8 × ULN | 2.0 | |||
| Tn I ≥ 8 × ULN | 6.5 | |||
| Cavallini et al. | 3494 | PCI | Tn I >0.15 | 44.2 |
| CK-MB >5 | 16.0 | |||
| Testa et al. | 7578 | PCI | Tn >99% URL | 28.7 |
| Tn >3 × URL | 14.5 |
In a randomized trial of PCI and CABG for triple-vessel disease, 75% of PCI and 100% of CABG patients had biomarker level elevation despite successful revascularization. Use of ultrasensitive troponin resulted in nearly all such patients reaching criteria for a type 4a MI, whereas only about 15% were classified as such using CK-MB. In other studies composed of less complex patients, an appreciable elevation of cardiac troponin above the ULN following PCI was still noted in about 40% to 50%. In one meta-analysis of 15 studies that included more than 7500 patients with normal baseline troponin levels, troponin elevation occurred in 29% of the procedures. When applying the older universal definition of PMI (any troponin elevation >3 × URL), the incidence of PMI is 14.5%. In a patient series that excluded patients with initially positive markers, the average incidence of PMI using CK-MB, troponin T, and troponin I greater than the ULN was 23%, 23%, and 27%, respectively.
When the incidence of PMI is reported for a consecutive series of patients undergoing PCI (irrespective of their clinical condition after the procedure), it is invariably higher than in other series, in which biomarkers were assayed only in patients who developed certain symptoms or signs of ischemia. This is the result of detection of a fairly larger proportion of clinically silent events, with small-magnitude biomarker release. The American College of Cardiology (ACC)/American Heart Association (AHA) PCI guidelines update published in 2011 recommended that for those patients who have signs or symptoms suggestive of MI during or after PCI, or for asymptomatic patients with significant persistent angiographic complications, e.g., large SBO, flow-limiting dissection, no-reflow phenomenon, or coronary thrombosis, CK-MB and/or troponin should be measured (class I recommendation). A class IIb recommendation is proposed for routine measurement of cardiac biomarkers in all patients after PCI. Using a lower biomarker cutoff value to define PMI increases its epidemiologic incidence. Other important factors that contribute to the heterogeneity of the conclusions of the various published series include the widely disparate baseline and procedural characteristics in the studied populations, inclusion or exclusion of patients with antecedent MI, and the timing of blood sampling.
According to the distribution of hyperenhancement indicative of acute injury, MR myocardial imaging in patients who develop biomarker release after PCI reveals two different types of PMI. In the more commonly seen distal type of PMI, hyperenhancement is in the distal distribution downstream from the treated segment. In the proximal type of PMI, the injury is primarily detected adjacent to the treated segment. Proximal PMI is usually linked to flow impairment in a side branch arising from the treated segment, whereas the more commonly seen distal PMI results from microvascular obstruction in the distribution of the artery subjected to PCI.
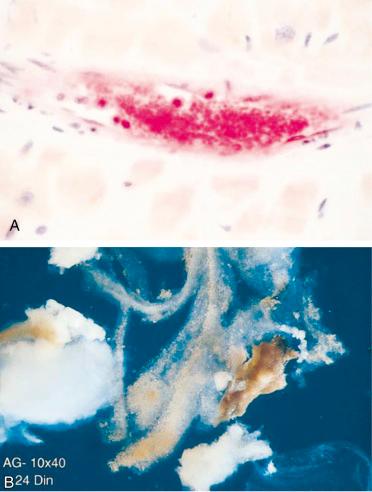
Although distal embolization associated with endothelial injury has been recognized for years, the importance of this phenomenon in relation to PCI was not fully appreciated until the last decade. Significant distal embolization can cause no-reflow phenomenon after PCI in large part as a result of microvascular dysfunction, because evidence of myocardial ischemia and reduced antegrade coronary flow are present in the absence of an occlusive epicardial stenosis or side-branch compromise. In a report of patients undergoing PCI for non-STEMI (NSTEMI), those with a postprocedural troponin I elevation were significantly more likely to have reduced tissue-level perfusion than those without a troponin I elevation. Platelet aggregates have been identified in the distal microcirculation and in atherosclerotic debris retrieved from arteries downstream from the site of angioplasty using filter devices ( Fig. 30.2 ).
Clinically, intravascular ultrasound (IVUS) studies provided further insight into the relationship between embolization of plaque material and PMI. Prati and coworkers examined the relationship between change in plaque volume before and after stenting and the degree of CK-MB release in 54 patients. In patients with unstable angina, the reduction in plaque volume was more significant; more importantly, however, such reduction significantly correlated with CK-MB release even after adjusting for other variables that influence PMI. A more recent and sophisticated analysis of 62 patients undergoing complex PCI by Porto and colleagues demonstrated a significant association between the change in target lesion plaque area by IVUS and the mass of myonecrosis assessed by hyperenhancement on MR imaging after PCI. The authors also correlated impaired microvascular flow (thrombolysis in myocardial infarction [TIMI] perfusion grade 0 or 1) with MR evidence of hyperenhancement downstream from the treated segment, hence suggesting that particulate matter from the atherosclerotic plaque disrupted by angioplasty drifts downstream and leads to microvascular obstruction and myonecrosis. With the higher resolution of frequency domain optical coherence tomography (OCT), correlations were made between the morphologic features of plaque and subsequent risk of PMI. In a study of 50 patients undergoing single vessel stenting and OCT imaging before and after PCI, a thin-cap fibroatheroma (TCFA) pre-PCI was defined as a lipid-rich plaque (two or more quadrants of lipid core) with a fibrous cap ≤65 μm. TCFA was more frequently seen in those who developed type 4a MI (76% vs. 41%, P = .017). The association was statistically significant and independent of other variables that predicted the development of type 4a MI.
Platelet activation plays a critical role in the development and perpetuation of coronary microvascular obstruction following PCI. By definition, the interventional devices used to treat an epicardial stenosis will result in a break in the endothelial surface and a release of debris into the coronary bloodstream. The exposed intraplaque contents stimulate platelet activation and aggregation at the site of the PCI and probably also in the downstream microvasculature. Thus, the platelet aggregates that plug the microcirculation not only cause mechanical obstruction but also lead to detrimental biochemical responses due to their interaction with the injured endothelium, the neutrophils, and more platelets. The release of vasoactive substances such as serotonin and endothelin-1 from the activated platelets and the injured endothelium lead to intense microvascular vasoconstriction, which accentuates the ischemic injury and resultant myonecrosis. The odds of developing PMI, in a cohort of 151 patients presenting for nonurgent PCI, increased threefold if they were found to be aspirin resistant before the procedure.
A PCI-related infarction presenting with ST-segment elevation is caused by acute and total occlusion of a relatively sizable epicardial coronary branch. This is most commonly the result of abrupt closure of a branch or acute stent thrombosis. On occasion, embolization of large thrombus or atheroma may occlude the distal vessel and cause STEMI; such embolization is more common in degenerated saphenous vein graft (SVG) interventions than in native vessel interventions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here