Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors thank Dr. Sabato Sorrentino for editorial assistance.
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
Coronary angiography consists of the visualization of the coronary anatomy under fluoroscopy, facilitated by direct injection of contrast media into the epicardial coronary arteries through a catheter advanced from a peripheral artery to the aortic root and into the coronary ostia.
The history of coronary angiography starts in the 19th century with the discovery of x-rays by Roentgen in 1895. One month later, Haschek and Lindenthal injected a mixture of calcium carbonate in the blood vessels of an amputated hand and were able to visualize the vascular bed using a roentgenogram. Meanwhile, Andre Cournand and Dickinson Richards at Columbia University performed the first experiments on cardiac catheterization in animals, which led to the description of heart hemodynamics and the application to humans of crucial techniques and principles, such as the Fick method to measure cardiac output and pressure manometry (see Chapter 22 ). Forssmann performed the first human cardiac catheterization on himself in 1928, advancing a catheter through an antecubital vein into his right atrium, and acquired roentgenograms to document it.
Selective coronary angiography was first attempted in 1958 by Mason Sones, who cannulated a right coronary artery with a catheter inserted through a brachial artery. In the 1960s, angiographic studies for the determination of coronary artery disease (CAD) were performed in extremely ill patients in the few tertiary care centers in the United States with the necessary resources. Coronary angiography remained a purely diagnostic technique until 1977, when Gruentzig performed the first percutaneous transcatheter coronary angioplasty (see Classic References, Ryan). In the early 1990s the field of coronary angiography entered a period of explosive growth, such that by 2010, an estimated 1,029,000 inpatient diagnostic cardiac catheterization procedures and 954,000 inpatient percutaneous coronary intervention (PCI) procedures (see Chapter 41 ) were performed per year in the United States alone. Recent years have seen rapid development and maturation of the field, with continuous introduction of new materials, techniques, and innovations for coronary angiography and intracoronary interventions.
Despite the availability of noninvasive imaging techniques such as computed tomographic coronary angiography (CTCA) and magnetic resonance coronary angiography (MRCA) that allow visualization of the coronary anatomy without the risks related to an invasive percutaneous procedure (see Chapter 19, Chapter 20 ), selective coronary angiography remains the gold standard to determine the extent of CAD because it is the only technique that can simultaneously provide both functional and anatomic information for the estimation of ischemic burden of CAD. Although coronary angiography technique is well established, it is important to keep in mind that it is an invasive procedure with potential complications. Therefore, indications for coronary angiography are clearly defined in the current American Heart Association and American College of Cardiology (AHA/ACC) clinical practice guidelines. , This chapter reviews the indications for coronary angiography, the basic technique, and interpretation of angiographic images, with an overview of the available intravascular imaging techniques.
Selection of candidates for invasive coronary angiography is based on the pretest probability of CAD, which is estimated on the basis of the clinical evaluation of the patient, the patient’s clinical presentation, and the results of noninvasive diagnostic testing such as electrocardiography, echocardiography, blood tests, stress test, and CTCA or MRCA if performed , (see Chapters 15, 16, and 18 to 20). Current guidelines and indications for coronary angiography by clinical presentation are summarized in Chapter 59, Chapter 60, Chapter 61 ( eTable 21.1 ). ,
| CLASS I | CLASS IIA | CLASS IIB | CLASS III |
|---|---|---|---|
| Stable CAD | |||
|
|
|
|
| ACS—UA and NSTEMI | |||
|---|---|---|---|
|
|
|
|
| ACS—STEMI | |||
|---|---|---|---|
|
|
|
|
| Significant troponin increase Diagnostic ST or T wave changes GRACE score >140 Diabetes mellitus Reduced LV function (ejection fraction <40%) Early postinfarction angina Recent PCI Prior CABG Intermediate to high GRACE risk score |
In patients with low pretest probability of CAD, first-line noninvasive assessment of cardiovascular risk is necessary to decide whether to proceed to coronary angiography. Traditionally, stress test findings can be defined as low, intermediate, or high risk, which are associated with a cardiac mortality of less than 1%, 1% to 3%, and greater than 3% per year, respectively. For patients with intermediate-risk pretest probability, coronary angiography may be considered, whereas for patients with high-risk pretest probability, angiography should be performed without delay and with no need for further testing. Patients presenting with acute coronary syndrome (ACS), unstable angina (UA), or non–ST-segment elevation myocardial infarction (NSTEMI) with hemodynamic instability or who are at high clinical risk (as determined by the presence of any of the risk factors listed in eTable 21.2 ) should undergo early invasive evaluation. For hemodynamically stable UA/NSTEMI patients without high clinical risk, a delayed invasive strategy may be justified, although an initially noninvasive risk stratification may be practiced outside the United States. Patients presenting with ST-segment elevation myocardial infarction (STEMI) should generally undergo urgent invasive intervention as soon as possible after symptom onset. Patients with delayed cases may be treated conservatively, as described in other chapters.
In 2012 the appropriate use criteria (AUC) for diagnostic coronary angiography were released. This and a more recent focused update document provide a classification schema for procedures into appropriate, may be appropriate, and rarely appropriate care based on specific criteria. The proportion of “inappropriate” nonacute PCI has been reduced overall. Clinical indications for PCI are beyond the scope of this chapter, but the AUC for diagnostic catheterization are mentioned here to highlight the appropriate selection of patients referred for coronary angiography for diagnostic purposes, since coronary angiography itself may be an unnecessary invasive procedure that could trigger an inappropriate coronary intervention in some cases. The rate of angiographically normal or minimally diseased coronary arteries in patients undergoing elective procedures is approximately 39%. In particular, the use of coronary angiography and PCI in asymptomatic patients is uncertain. A recent study showed that among a sample of 300,000 patients receiving coronary angiography in the United States, 25% were asymptomatic at the time of the elective coronary angiography. Furthermore, the rate of angiographic procedures in asymptomatic patients directly correlated with the number of inappropriate PCI procedures performed. Therefore, strategies to verify the correct referral of patients for diagnostic coronary angiography are required to avoid unnecessary procedures, reduce health care costs, and prevent the therapeutic cascade that may lead from diagnostic angiography to inappropriate PCI.
There are no absolute contraindications to coronary angiography listed in the clinical practice guidelines. However, specific conditions should be taken into account when weighing risks and benefits of the procedure. Based on the patient’s cardiovascular risk and the clinical presentation, a decision should be made whether to avoid or postpone the procedure or proceed with coronary angiography using prophylactic measures to reduce the probability of periprocedural complications. Relative contraindications that should be taken into account are known anaphylactoid reaction to contrast media, moderate to severe kidney impairment, decompensated heart failure and pulmonary edema that prevent the patient from lying down during the procedure, uncontrolled hypertension, active infection, coagulopathy, and gastrointestinal bleeding. In addition, coronary angiography requires the use of radiation to visualize the wires and catheters advanced through the blood vessels and to obtain images of the coronary arteries. Therefore, pregnant women should not undergo angiography unless strictly necessary and on exhaustive explanation of the risks related to radiation exposure, medications, and contrast media for both the mother and the fetus. The presence of comorbidities that can increase the risk of complications should be critically considered before referring patients for coronary angiography.
Complications during coronary angiography are rare, occurring in approximately 2% of patients, with serious complications such as cerebrovascular accident (CVA), or stroke, or myocardial infarction (MI) accounting for less than 1% of all patients. Mortality rate is lower than 0.1%. Complications during PCI are more common (see Chapter 41 ). Table 21.1 lists complications that may be encountered during coronary angiography.
| Complication | Risk (%) |
|---|---|
| Mortality | 0.11 |
| Myocardial infarction | 0.05 |
| Cerebrovascular accident | 0.07 |
| Arrhythmias | 0.38 |
| Vascular complications | 0.43 |
| Contrast agent reaction | 0.37 |
| Hemodynamic complications | 0.26 |
| Perforation of heart chamber | 0.03 |
| Other complications | 0.28 |
| Total of major complications | 1.70 |
Although rare, the most common complications are allergic reactions to contrast, vascular complications, and worsening of kidney function (see next section). Vascular complications at the access site include hematoma, pseudoaneurysm, aneurysm, and dissection. The risk of a vascular complication increases with the diameter of the sheath used, age of the patient, and degree of local calcifications. Iatrogenic coronary dissection or perforation occurs infrequently but is potentially life-threatening and could require urgent coronary stenting ( Fig. 21.1 ). Ventricular and atrial arrhythmias are relatively common. Intracoronary injection of contrast media itself can induce arrhythmias. In particular, during injection of contrast media into the right coronary artery (RCA), one should take care to avoid deep cannulation of the RCA and injection of contrast media directly into the conus branch because this can result in ventricular fibrillation (VF). In addition, when performing ventriculography, the mechanical stress of the catheter on the ventricular walls can trigger ventricular arrhythmias ranging from isolated premature ventricular complexes (PVCs) to runs of ventricular tachycardia (VT). Usually, these arrhythmias are self-resolving with catheter relocation and do not require medical intervention. Embolic events are rare but can occur and may involve the coronary arteries, central nervous system, or peripheral arteries. Highly calcific axillary or subclavian arteries can increase the likelihood of embolization.
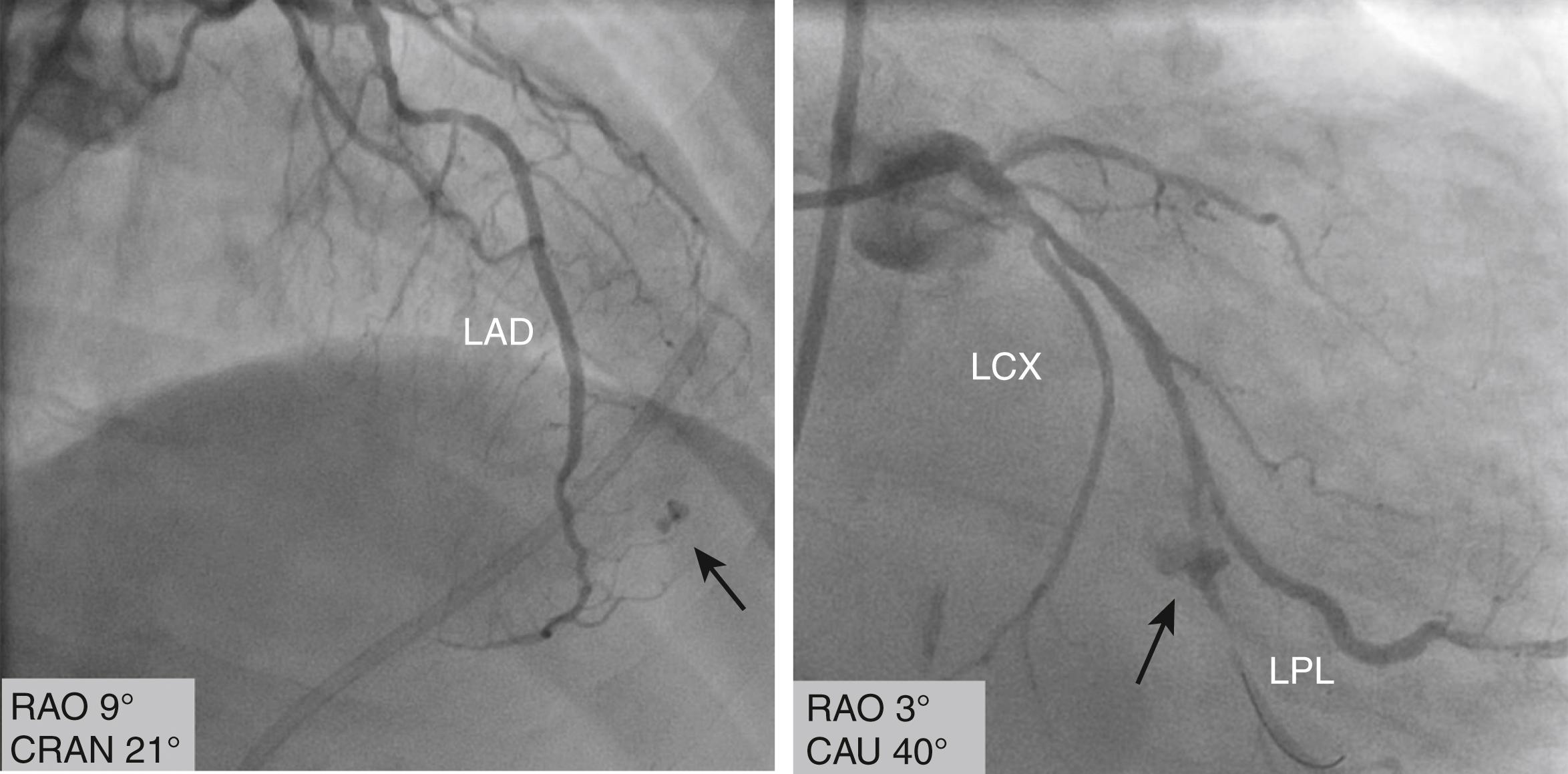
In addition, advanced age, diabetes mellitus, emergency coronary angiography, prior stroke, renal failure, and congestive heart failure (CHF) have been reported as risk factors for periprocedural stroke. Infections are exceptionally rare in immunocompetent patients, and prophylactic antibiotic therapy is not usually required. Bleeding is usually minor, except when precipitated by vascular complications. In general, the use of anticoagulation during diagnostic angiography should be dosed based on the length of the procedure, weight of the patient, and presence of comorbidities such as kidney impairment to avoid the risk of bleeding when the sheath is removed from the access site. Use of radial access rather than femoral access has significantly reduced the rate of vascular and bleeding complications (see Chapter 41 ).
Contrast-induced acute kidney injury (CI-AKI) is defined as an acute deterioration of renal function, defined as an increase in creatinine of 0.5 mg/dL or more or 25% or greater compared with baseline. It generally develops 24 to 72 hours after administration of an intravascular contrast agent in the absence of other identifiable causes (see Classic References, Goldenberg and Matetzky). This complication significantly impacts the duration of hospital stay and related health care costs. CI-AKI also has marked repercussions on short- and long-term morbidity and mortality. In particular, studies in patients with moderate to severe renal dysfunction (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m 2 ) undergoing coronary angiography or angioplasty show that the development of CI-AKI in such patients is a negative prognostic factor of clinical outcome both short and long term. The incidence of CI-AKI ranges from 2% in low-risk patients to 12% to 50% in patients with diabetes and known chronic kidney disease (CKD) (see Chapter 101, Chapter 31 ). The mechanisms of CI-AKI are only partially understood. Certainly, toxic damage caused by the passage of iodine molecules in the interstitial kidney is one of the causes. Another mechanism is related to the redistribution of flow in the kidney tissue secondary to contrast administration. In particular, after injection of contrast media, blood flow increases in the cortex and decreases in the medulla. Unfortunately, the medulla is particularly vulnerable to ischemic injury for the basal hypoxic condition (P o 2 = 20 mm Hg) because of high metabolic activity (e.g., sodium transporter channels). Therefore, blood flow reduction in the medulla after contrast injection further decreases oxygen tension, leading to endothelial dysfunction. Other important elements affecting kidney function are the physical and chemical characteristics of the contrast agents, in particular osmolality and viscosity. Contrast agents with a high osmolality and viscosity significantly increase hypoxemia and tubular stress. The downstream effect consists of an increase of free radicals, a reduction of nitric oxide (NO) bioavailability, and an increase in cellular death. ,
The risk of CI-AKI depends largely on baseline renal function. The eGFR is a valid index to describe the level of renal function. Patients with an eGFR value below 60 mL/min are at high risk of CI-AKI. However, eGFR is not able to identify subclinical or latent forms of renal dysfunction. Therefore, a careful assessment of CI-AKI risk is essential, particularly before interventional procedures that may require high contrast medium volume ( Fig. 21.2 ) (see Classic References, Mehran et al.). Risk of CI-AKI can be stratified using a risk score model that includes patients’ baseline and procedural characteristics.
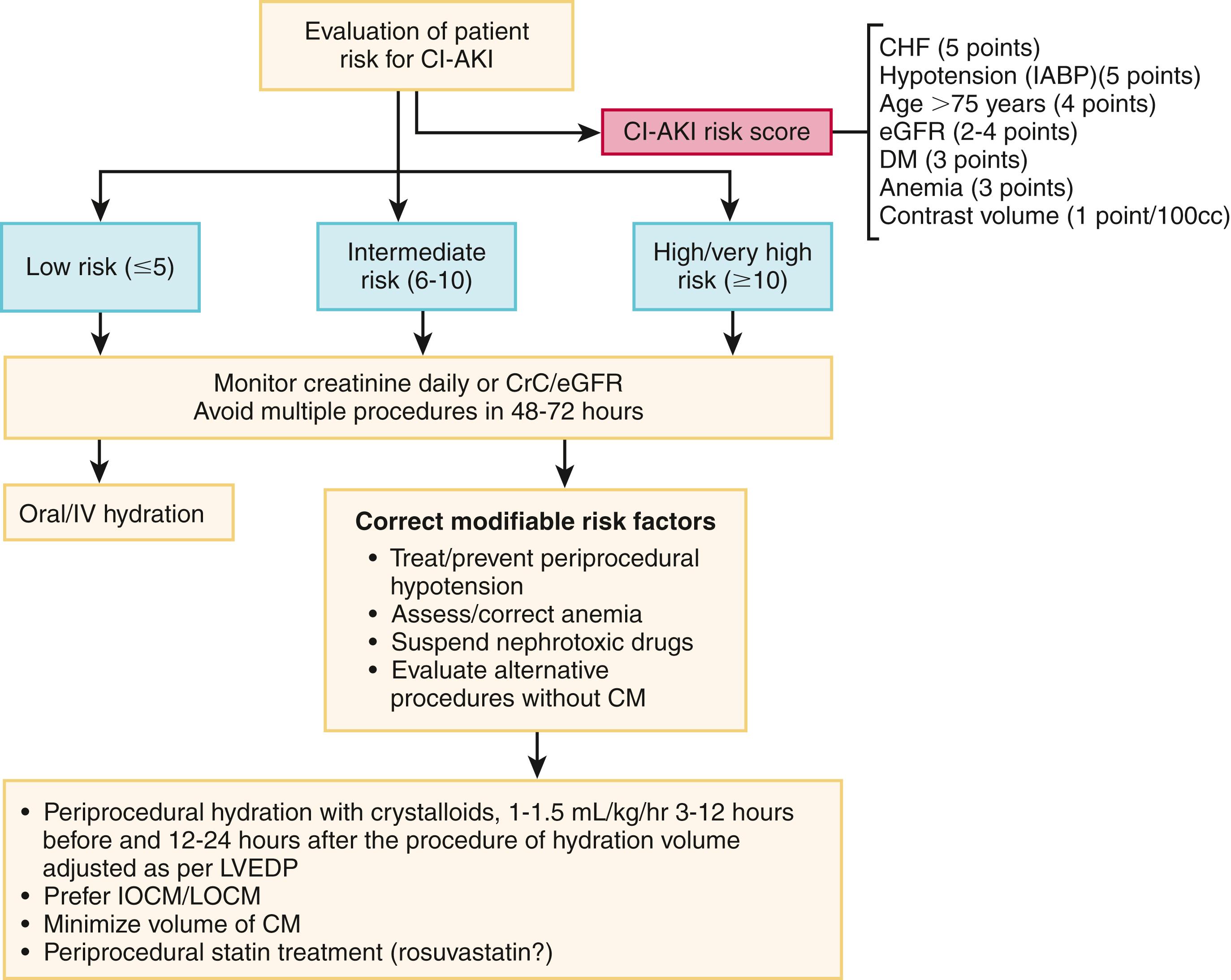
In high-risk patients, prevention is crucial and consists of pharmacologic and nonpharmacologic measures. Individual risk-benefit ratios should be carefully estimated for each patient, and the utility of an alternative noninvasive diagnostic test should be evaluated. If the use of contrast medium is necessary for diagnostic purposes, the volume used should be minimized, and the use of monomeric low- or iso-osmolality contrast agents is recommended. Hydration plays a pivotal role in reducing the incidence of CI-AKI. Depending on the clinical condition (e.g., CHF), the Contrast-Induced Nephropathy (CIN) Consensus Working Panel recommendations state that an infusion of 1.0 to 1.5 mL/kg/hr of isotonic saline solution, from 3 to 12 hours before until 6 to 24 hours after the procedure, is suitable to minimize the incidence of CIN. Recently, a clinical trial specifically investigated the efficacy and safety of a left ventricular (LV) end-diastolic pressure–guided hydration protocol with good results; thus filling pressure–guided fast hydration may be employed in the catheterization laboratory. Moreover, to obtain effective hydration, devices have been developed that balance the volume of infusion and fluids lost through diuresis. N -Acetylcysteine has been considered for the prevention of CI-AKI for years. In animal models of ischemia-reperfusion injury, the use of N -acetylcysteine significantly limited kidney damage mainly through its antioxidant properties. However, the efficacy of N -acetylcysteine in humans in clinical studies remains unclear, given the high heterogeneity in study protocols and populations. Similarly, some studies report that the use of isotonic sodium bicarbonate is associated with a higher reduction in the incidence of CI-AKI than saline solution. These findings were attributed to a potential reduction in the production of reactive oxygen species in the renal parenchyma. However, recent meta-analyses did not show superiority of sodium bicarbonate over saline solution. , For this reason, both N -acetylcysteine and sodium bicarbonate have minimal roles in the latest guidelines on prevention (i.e., no benefit) and the routine prevention of CIN in patients undergoing percutaneous coronary angiography and interventions.
Coronary catheterization may result in radiation-related injury, which although infrequent, may be potentially serious. Radiation injury may be deterministic (i.e., dose-dependent), which can present weeks after exposure, or stochastic, which is genetically determined and not dose-dependent. Stochastic injury can result in cancer, pregnancy complications, and inheritable diseases. Deterministic injury may result in skin injury, hair loss, and lens injury. However, the most common location of radiation-induced lesions in cardiac catheterization is the skin of the back, and common patterns include erythema, telangiectasia, and plaques. The sensitivity of the skin to radiation exposure is differentiated by site; areas at risk in decreasing order of sensitivity include anterior neck, antecubital and popliteal areas, flexor extremities, chest and abdomen, face, back, extensors, nape of the neck, scalp, palms, and soles. Although uncommon in contemporary practice, early reports from coronary catheterization indicate deep and extensive skin rashes and burns at the site of radiation exposure, some requiring skin grafting.
PCI procedures may result in 10-fold higher radiation exposure compared with diagnostic catheterization (see Chapter 41 ). An average PCI results in 150 times more exposure than a chest radiograph and five times the annual radiation exposure received as environmental background radiation. Measures used to assess patient dose include dose-area product (DAP; the absorbed dose multiplied by the area irradiated), air kerma (AK; k inetic e nergy r eleased per unit ma ss of air), and fluoroscopy time (FT), which are routinely measured and documented. All procedures should be performed using the ALARA (as low as reasonably achievable) principle. Exposure can be minimized in several ways: reduced FT and acquisition time, use of multiple angles rather than a single working camera position, reduced fluoroscopy dose, avoidance of high magnification, use of collimator beams and filters, avoidance of high angulation, and reduction in the flat-panel image detector as much as possible. For exposures of absorbed radiation greater than 5 Gy, patients should be advised to watch for areas of erythema; for those greater than 10 Gy, a medical physicist should be consulted to calculate the peak dose in 2 to 4 weeks; greater than 15 Gy is regarded as a hospital risk management event. Similarly, in the event that FT exceeds 60 minutes, physicians must be vigilant for late radiation effects.
From the perspective of occupational radiation exposure, operators should be cognizant of the need to wear protective personal equipment during catheterization procedures, including a lead apron, thyroid drape, lead eyeglasses, and dosimeters. Table height and distance from the x-ray source are important, and radiation risk decreases as the inverse square of distance from the source. Operators should also optimally position lead shields and skirts and should be compliant with use of radiation dosimeters for monitoring exposure to the whole body (chest) and eye. Novel dosimeters providing real-time monitoring and alerts can serve to decrease operator radiation exposure. Monitoring, reporting, and audit of radiation exposure can promote improved awareness and practice in the operator and catheterization laboratory staff. Systematic tracking of FT and radiation exposure is expanding through inclusion in the procedure report as well as in quality assurance databases (national/statewide).
Patients should receive a comprehensive explanation of the diagnostic angiographic procedure and of the coronary intervention potentially required. Risks of angiography should be discussed in-depth and weighed against both the clinical benefit and the risks related to refusal of the procedure. Patients are required to provide written informed consent before coronary angiography. Women of childbearing age should be questioned on their pregnancy status and advised on the additional risks of radiation exposure for pregnant women. A thorough medical history, including comorbidities, current medications, and allergies, needs to be obtained before the procedure. In the event of an emergency procedure, as with a STEMI presentation, a brief evaluation of the patient history with particular attention to known CKD and known allergies to contrast media should be obtained if possible. In patients with prior coronary artery bypass graft (CABG) procedure, a report stating the type, arterial or venous graft(s), and position of the graft(s) should be attained if available to facilitate the cannulation and subsequent imaging of the grafts. Patients may receive mild sedation with a benzodiazepine before the procedure according to the hospital standard practice. In case of hemodynamic instability or respiratory distress, anesthesiologist support might be necessary. In most patients, however, general anesthesia and deep sedation are unnecessary for coronary angiography. Conscious sedation with short-term agents such as midazolam or fentanyl is most common. Constant monitoring of the patient’s ECG, heart rate, blood pressure, respiratory rate, and oxygen saturation is required periprocedurally. A venous access line should be readily available for the infusion of fluids or medications. Local anesthesia with topical anesthetic cream or subcutaneous injection of 1% lidocaine or mepivacaine (0.5 to 1 mL for radial access and 2 to 5 mL for femoral access) should be performed in all patients before puncturing the peripheral artery and introducing the sheath. An adequate local anesthetic will not only make the patient more comfortable but, by reducing the pain during the arterial cannulation, also reduce the risk of peripheral artery spasm.
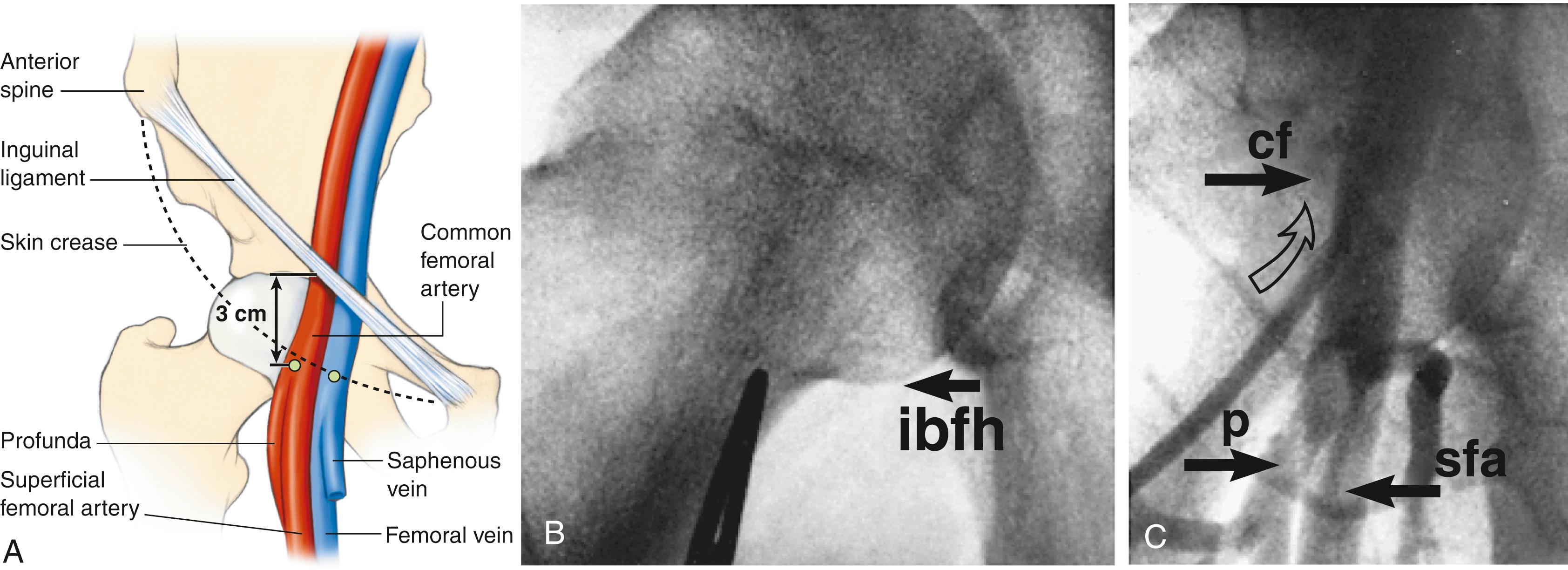
Possible access sites for coronary angiography are the femoral artery and the radial artery. Although the radial access approach is associated with fewer vascular and bleeding complications, femoral access is still commonly used in the United States. Femoral access allows for larger-diameter equipment that could be necessary in case of PCI. In addition, accessing from the femoral artery usually grants an easier advancement of the catheter to the aortic root due to the lack of tortuosity in the descending aorta. After disinfection and appropriate local anesthesia at the access site, the common femoral artery (CFA) is punctured with a base-metal needle approximately 1 cm below the inguinal line with a 45- to 60-degree angulation. In obese patients, the ideal puncture site is sometimes difficult to determine. The head of the femur, visualized under fluoroscopy, can be used as a landmark ( eFig. 21.1 ). Ultrasound can localize the common femoral artery and its bifurcation (see eFig. 41.4 for the use of ultrasound guidance for femoral access). Puncture should be performed with the needle leveled at half the head of the femur. Multiple punctures should be avoided to reduce the risk of bleeding and vascular damage. A J-tip flexible guide is inserted through the needle into the CFA. The needle is then removed and a sheath advanced around the wire into the artery ( eFig. 21.2 ). Once the sheath is fully advanced in the artery, the dilator and wire are removed, and the sheath is flushed with saline. Usually, a 6 French (6F) sheath (French units: F = 0.33 mm) is used for coronary angiography and coronary interventions. Verification of the correct position of the sheath in the vessel can be ascertained simply by drawing blood from the sheath.
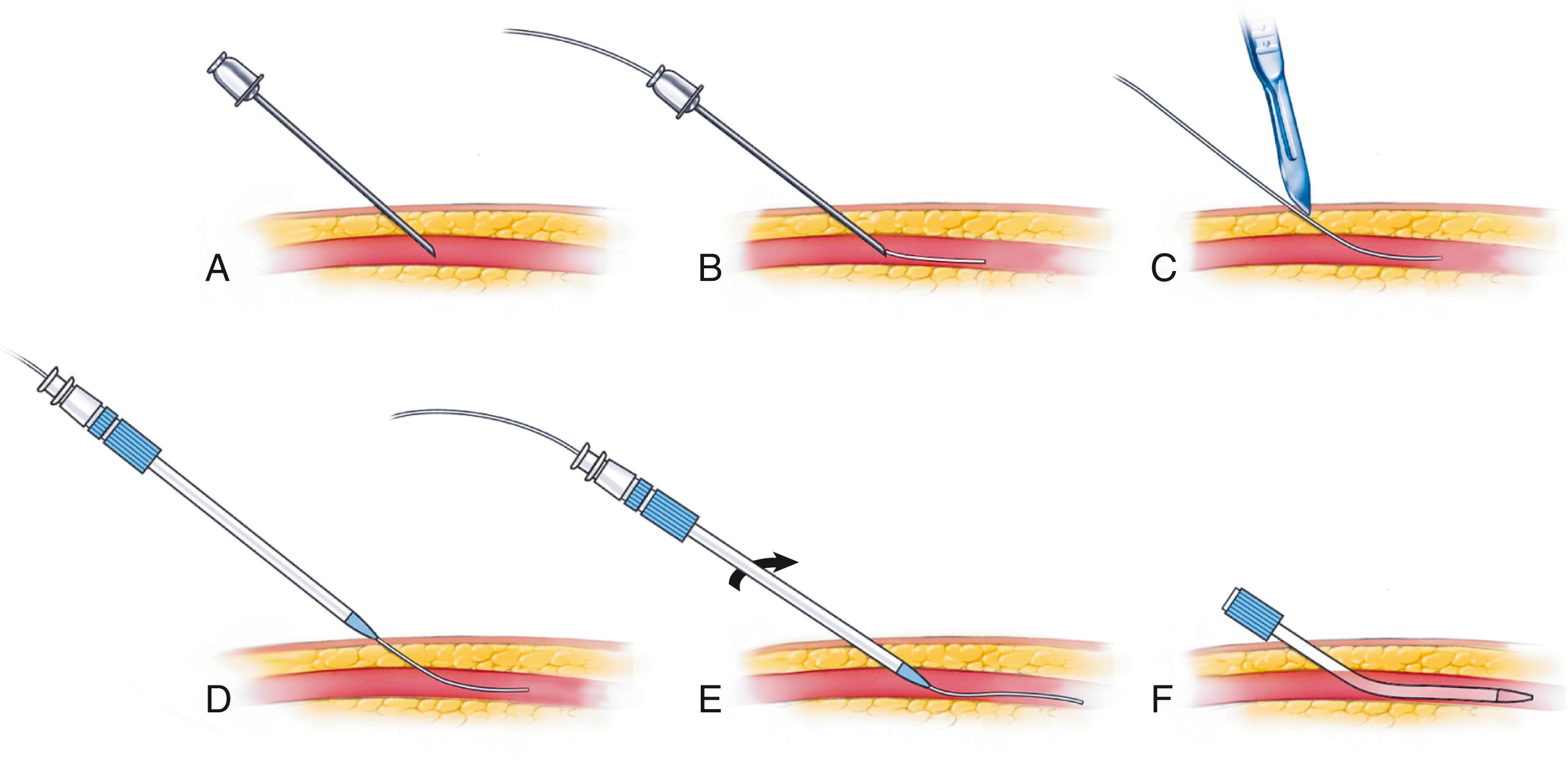
Radial access should always be considered first, before resorting to the femoral approach, especially for diagnostic coronary angiography. The procedure for the sheath insertion is similar to that described for the femoral artery. The modified Allen test is performed by applying pressure on both the ulnar and the radial artery of one wrist to occlude them while the patient keeps the hand elevated with the fist clenched for approximately 30 seconds. Once opened, the hand appears pale. The compression on the ulnar artery is then removed while pressure is maintained on the radial artery. If the ulnar artery supply to the hand is adequate, the color quickly returns to the hand and the test is normal. Conversely, if color does not return, the ulnar artery supply is insufficient, meaning that the radial artery supports the entire circulation of the hand. In this case the radial artery should not be punctured, because this may compromise the blood flow to the hand. This rule may be bypassed if an oximeter is placed in the thumb during radial artery occlusion, and resurgence of pulsation and oxygenation is documented after its initial disappearance (“Barbeau method”).
When both radial arteries are acceptable access sites, the patient’s right, closer to the operator, is preferred for technical reasons. However, the left subclavian artery may be less tortuous than the innominate artery. The ideal puncture site is 1 to 2 cm proximal to the radial styloid with the wrist slightly hyperextended. After local anesthesia, usually 0.5 to 1 mL of 1% lidocaine, the needle is advanced angled 30 to 45 degrees to the skin until a flashback of blood is visualized. A straight-tip wire is gently inserted through the needle. After removing the needle, a 5F or 6F sheath is inserted in the radial artery over the wire. A small incision 1 mm long can be made on the skin to facilitate advancement of the sheath. Because the radial artery is extremely vasoactive, the risk of spasm is high, especially in women; therefore, as soon as access is obtained, an intra-arterial spasmolytic agent such as nitroglycerin (100 to 200 μg) or verapamil (2.5 mg) diluted into 10 mL of saline should be administered. A hydrophilic-coated sheath can further reduce the likelihood of spasm and regional pain. To prevent thromboembolic events and radial artery occlusion, weight-adjusted unfractionated heparin (UFH), 40 to 70 U/kg up to 5000 U, is administered either intravenously or intra-arterially.
Radial access appears associated with fewer periprocedural events and should be preferred whenever possible. It should be noted, however, that the axillary-subclavian axis can be tortuous and calcific, particularly in elderly patients, and it can therefore be technically difficult to advance the catheter to the aortic root. Brachial access is very uncommon, but unlike radial access, it avoids the small-caliber arteries in the forearm and therefore may be required in the event that radial access is not available or fails. Brachial access can be obtained with a percutaneous or cutdown approach. On the other hand, there is no alternative blood supply to the forearm in case of closure.
Coronary angiography is an invasive procedure based on the intravascular advancement of angiographic guidewires and catheters from a percutaneous access using the Seldinger technique. After a valved sheath is inserted into the access site artery (see Access Sites), a flexible metallic J-tipped guidewire is inserted through the sheath and advanced slowly under fluoroscopic imaging through the arterial axis until the aortic root is reached. A fluid-filled catheter is then advanced over the angiographic guidewire, while the wire itself is maintained in place. Once the catheter is in the aortic root, the wire is fully extracted from the sheath, and the catheter is flushed and connected to the contrast media injection apparatus. Under fluoroscopic imaging, and with the help of small injections of contrast, the coronary ostium is engaged with the tip of the catheter. At this point, the x-ray tube is positioned appropriately (see Projection), and angiographic images are obtained while injecting contrast directly into the cannulated coronary artery.
There are several types of diagnostic catheters, characterized by differing lengths, diameters, and shapes. In general, catheters are composed of an external layer, which is not thrombogenic or lubricious, and by a lubricious inner layer. These two layers include a fine metallic core required to confer stability, improve maneuverability, and reduce the risks of kinking. Lengthwise, the catheter is divided into three parts: hub, body, and tip. Through a female Luer-Lok, the hub connects the catheter to the contrast injection system and facilitates the catheter grip and rotation with winged tips. The body , mostly strong and rigid, transmits to the tip the movements impressed on the hub by the operator. The tip can be divided, starting from the distal end, into three curves: primary, secondary, and tertiary, which allows the best possible fit to the aortic root curvature. The size of the catheter is another important characteristic. Compared with guiding catheters used for PCI (see Chapter 41 ), diagnostic catheters have a thicker wall, which considerably reduces their internal lumen. The 5F catheter allows an optimal balancing between contrast flow and satisfactory catheter manipulation, particularly for the radial approach. Catheter length can vary from 80 to 110 cm (32 to 44 inches), depending on the anatomic characteristics and the access site (radial, brachial, or femoral). However, the standard length for adult left-heart catheterization by both the radial and the femoral approach is 100 cm (40 inches), while 80 cm is suitable for brachial access.
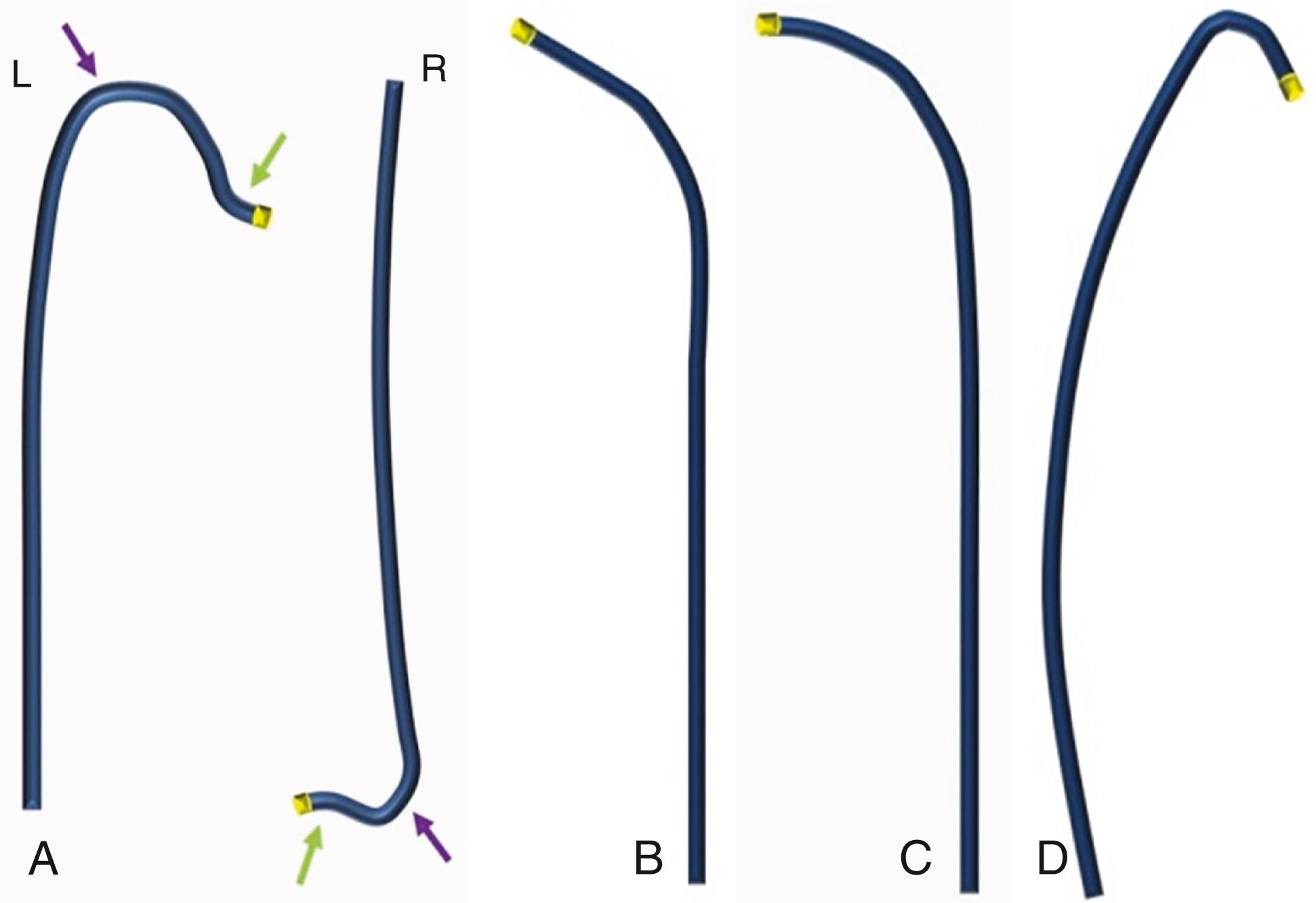
Among the diagnostic catheters, the most commonly used are the Judkins and the Amplatz catheters. Judkins catheters can be used both for the femoral and for the right/left radial approach. A preformed left Judkins (JL) presents a primary curve of 90 degrees and a secondary curve of 180 degrees, whereas the right Judkins (JR) presents a primary curve of 90 degrees and secondary curve of 30 degrees ( Fig. 21.3 ). Since the JL is preformed, after removing the angiographic guide, it automatically engages the ostium of the left coronary artery (LCA). The JR, in contrast, once positioned in the right coronary sinus, requires a clockwise rotation to engage the ostium of the RCA from any vascular approach. In both JL and JR catheters, the distance between the primary and secondary curves (termed arm ) is variable; for example, JL4 has a 4.2-cm length arm, and JL5 and JL6 have 5.2- and 6.2-cm-long arms, respectively (see Fig. 21.3 ). Catheter selection depends on the approach (radial or femoral), the height of the patient, and the aortic diameter and curvature. For example, when using a femoral access, the JL4 is the most adaptable catheter for the LCA, whereas for the radial access, the JL3.5 catheter may be more suitable. Moreover, the presence of a dilated aortic root or the anatomy of particularly tall patients (>180 cm [72 inches]) may increase the length required between the primary and secondary curves and might require the selection of a catheter with a longer arm. In addition to their conventional use, JR catheters may be used for saphenous vein graft (SVG) and left internal mammary artery graft study through femoral and left radial approach.
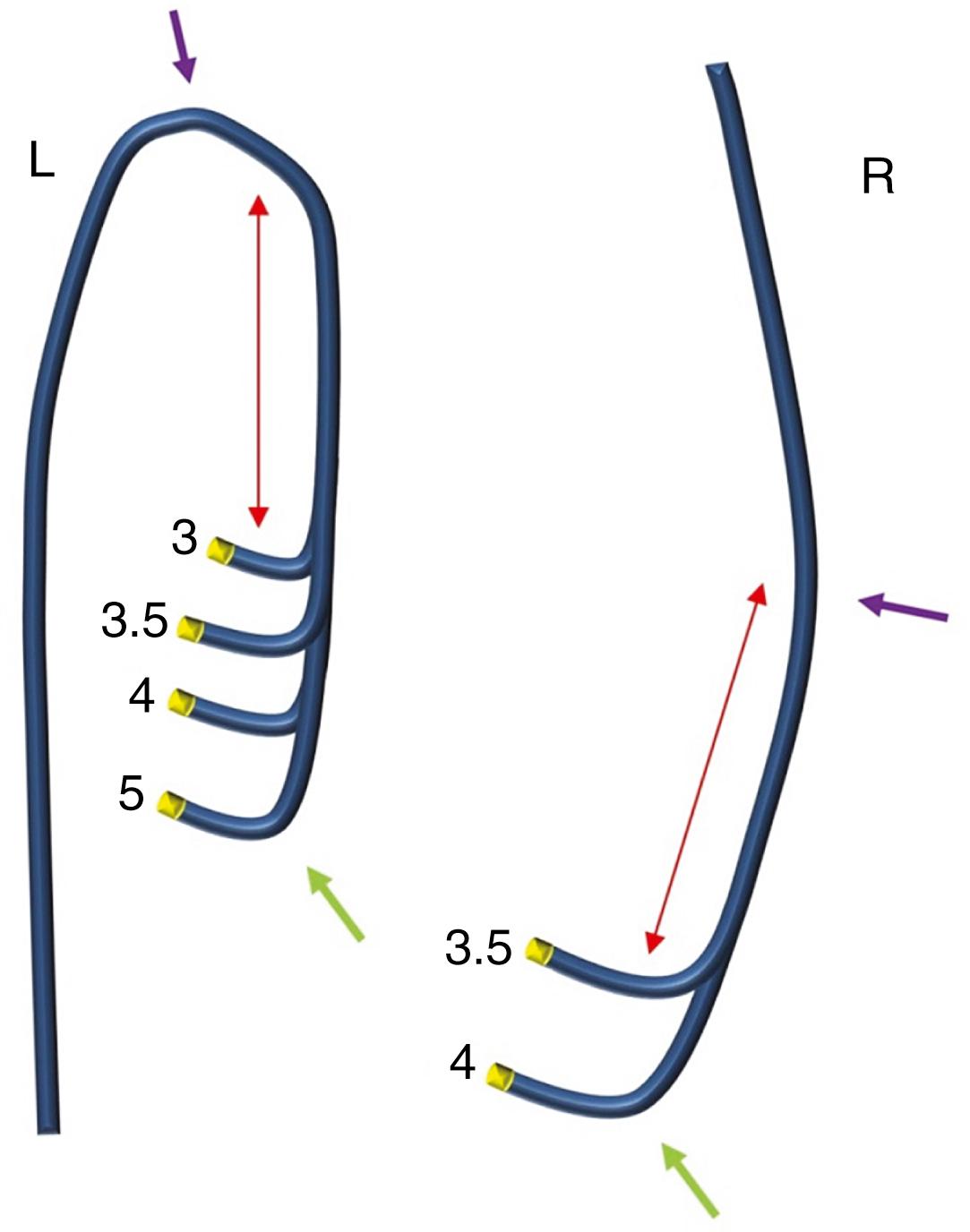
Amplatz catheters for the LCA (AL) and RCA (AR) represent a valid alternative to Judkins catheters ( Fig. 21.4 ). The available lengths and sizes are the same as for the Judkins catheters, but the tip morphology of the left Amplatz (AL) catheter differs, allowing for easier coronary engagement in specific settings, such as short left main ostium, separate ostium of circumflex (Cx)–left anterior descending (LAD) artery branches, and RCA with anterior-high origin. Conversely, the right Amplatz (AR) catheter allows engagement of RCAs with inferior orientation. Amplatz catheters may also be used with confidence for the study of SVGs. Multipurpose (MP) catheters present a single bend (MPA 1 and 2 have a 45- to 60-degree primary curve, while MPB 1 and 2 have approximately an 80-degree primary curve) and may be used for the cannulation of coronary ostia that are difficult to reach with other catheters, as well as for engagement of SVGs. Internal mammary artery (IMA) catheters have a high angulated primary curve tip (80 degrees) to facilitate the engagement of the IMA through either the femoral or the radial approach. These catheters can also be used to engage the upward-pointing RCA (see Fig. 21.4 ). It should be specified that the catheters just described are the ones most frequently used to perform diagnostic coronary angiography. Additional catheter types are available, although less frequently used, in case of specific coronary anatomic variables.
The JL4.0 coronary catheter is used most often to engage the LCA ( Fig. 21.5 ). The catheter is advanced over the guidewire until it reaches the aortic root. There, the catheter is rotated clockwise to direct it toward the left sinus of Valsalva. Once in position, the wire is removed, and the catheter regains its primary bent and should engage the ostium of the LCA. When the ascending aorta is dilated or the aortic arch is unfolded, advancement of the JL4.0 or JL5.0 might be necessary. If the tip of the JL catheter advances beyond the ostium of the LCA without engaging the ostium, the catheter can be advanced farther until the tip enters the left sinus and the catheter body assumes an acute angle. At that point, prompt withdrawal of the catheter should allow the tip to “pop” into the ostium of the LCA.
The RCA is cannulated in the left anterior oblique (LAO) position (see later, Angiographic Projections). Once the JR or modified Amplatz catheter reaches the aortic root, it must be rotated clockwise to engage the vessel. The height of the catheter during the rotation may need to be adjusted by gently withdrawing the catheter to engage the ostium.
In patients with prior CABG, cannulation might be challenging because the locations of graft ostia are more variable, even when surgical clips or ostia markers are used. Whenever possible, the number, type, and course of the bypass grafts should be obtained before the procedure.
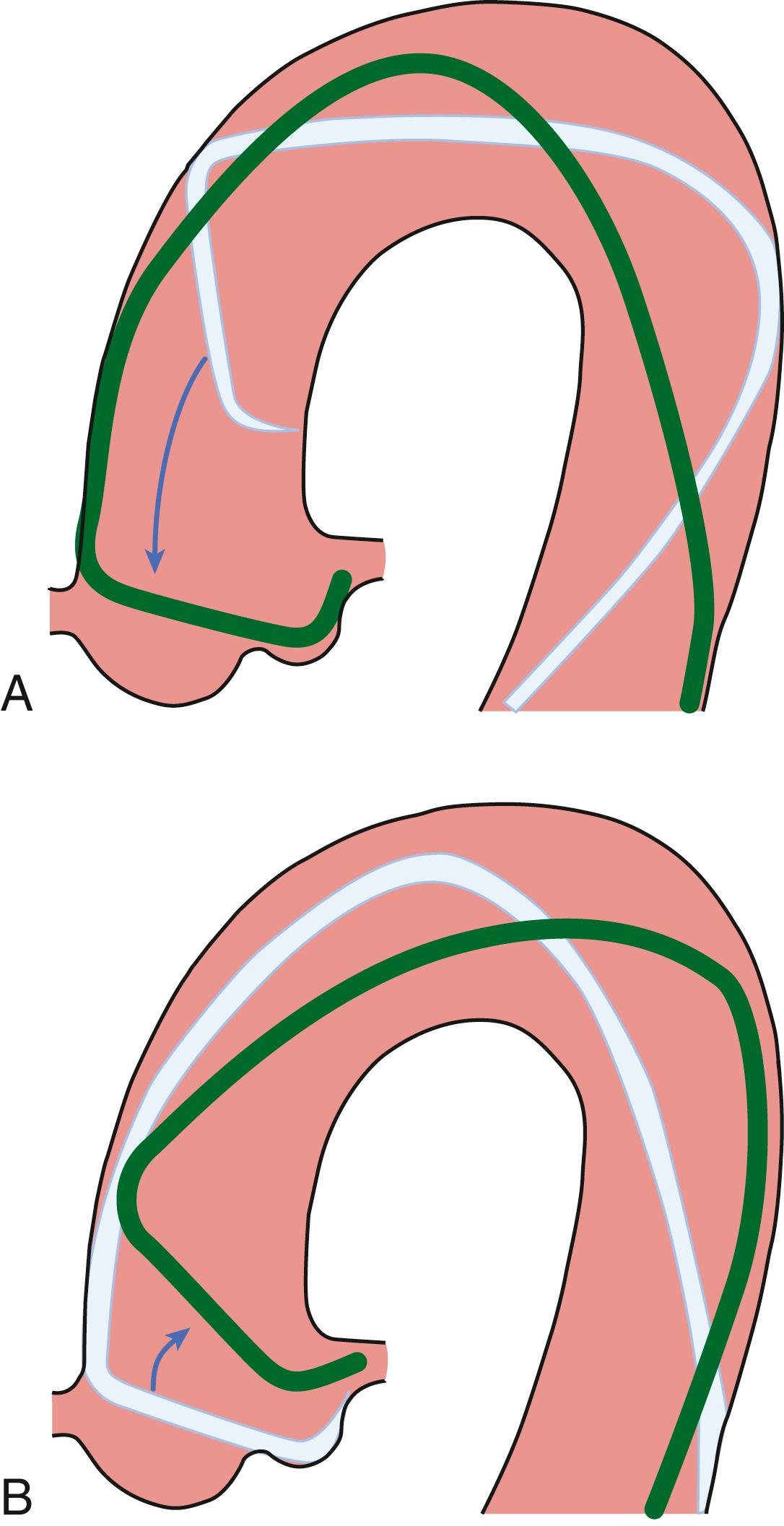
SVGs from the aorta to the distal RCA or posterior descending artery (PDA) originate from the right anterolateral aspect of the aorta approximately 5 cm (2 inches) superior to the sinotubular ridge. SVGs to the LAD artery (or diagonal branches) originate from the anterior portion of the aorta approximately 7 cm superior to the sinotubular ridge ( Fig. 21.6 ). SVGs to the obtuse marginal branches arise from the left anterolateral aspect of the aorta 9 to 10 cm superior to the sinotubular ridge. In most patients, all SVGs can be engaged with a single catheter, such as a JR4.0 or a modified Amplatz right 1 or 2.
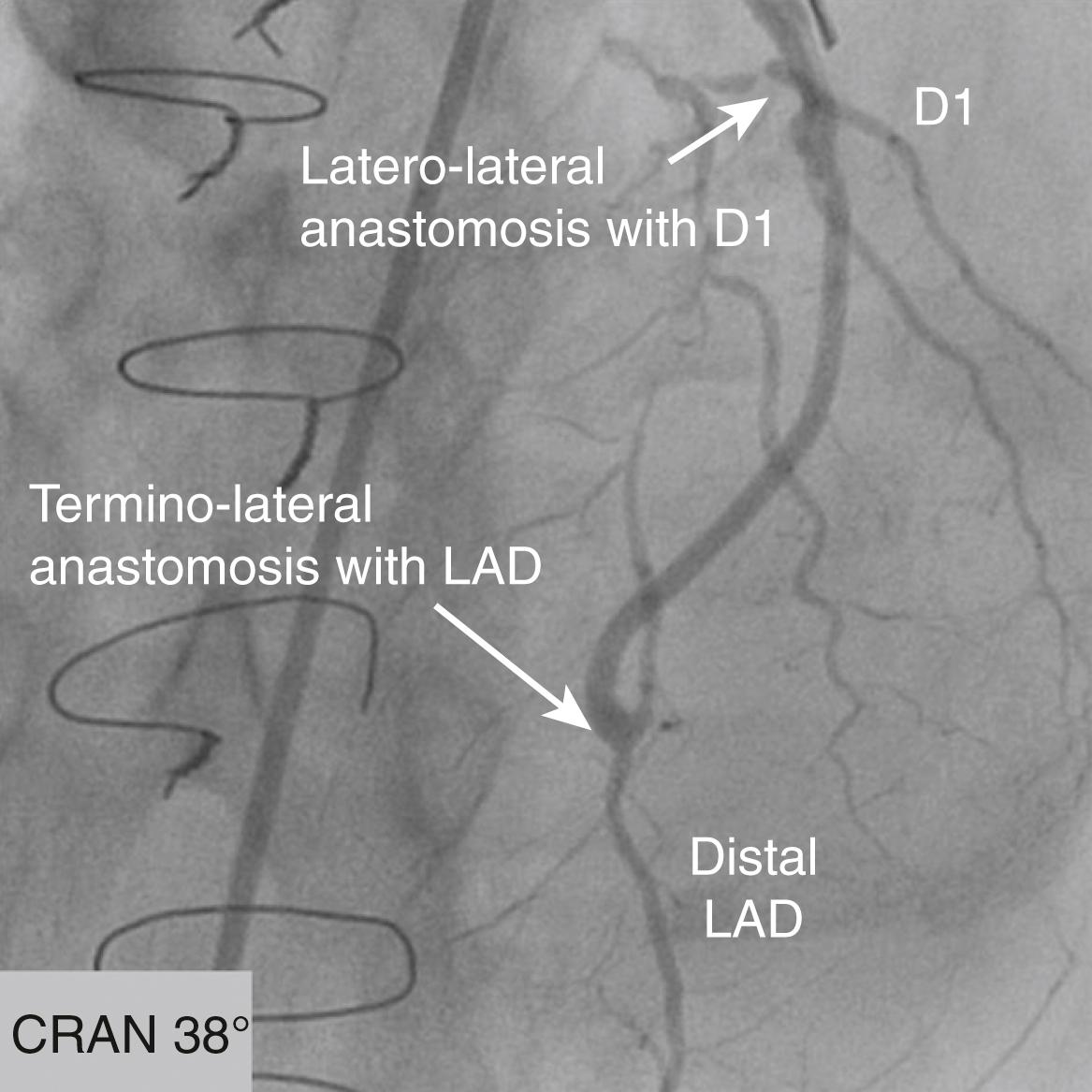
Viewed in the LAO projection, the catheter should be rotated anteriorly from the leftward position as it is rotated in a clockwise direction. This movement should be repeated with the catheter at various heights in the ascending aorta, 5 to 10 cm above the sinotubular ridge, and with various degrees of rotation. Small test injections of contrast media can be used to verify that the catheter is in the SVG. If the graft is occluded, usually it is possible to visualize a “stump” during contrast injection. The surgical clips can be used to verify that all the grafts have been visualized. If one or more SVGs cannot be visualized, it can be useful to perform an ascending aortogram (preferably in biplane) to visualize all SVGs and their course to the coronary arteries. When visualizing an SVG, it is important to evaluate the ostium and the anastomotic site for irregularities or stenosis. It is also important to evaluate the flow distal to the anastomosis. Sequential grafts are those that supply two different epicardial branches in a side-to-side fashion (for the more proximal epicardial artery) and terminate in an end-to-side anastomosis (for the more distal epicardial artery). A Y graft is characterized by a proximal anastomosis in an end-to-side fashion to another saphenous vein or arterial graft, with two distal end-to-side anastomoses to the two epicardial grafts from these two grafts. It should be noted that with severe calcifications of the ascending aorta, the SVG could depart from the descending aorta to reach lateral wall branches.
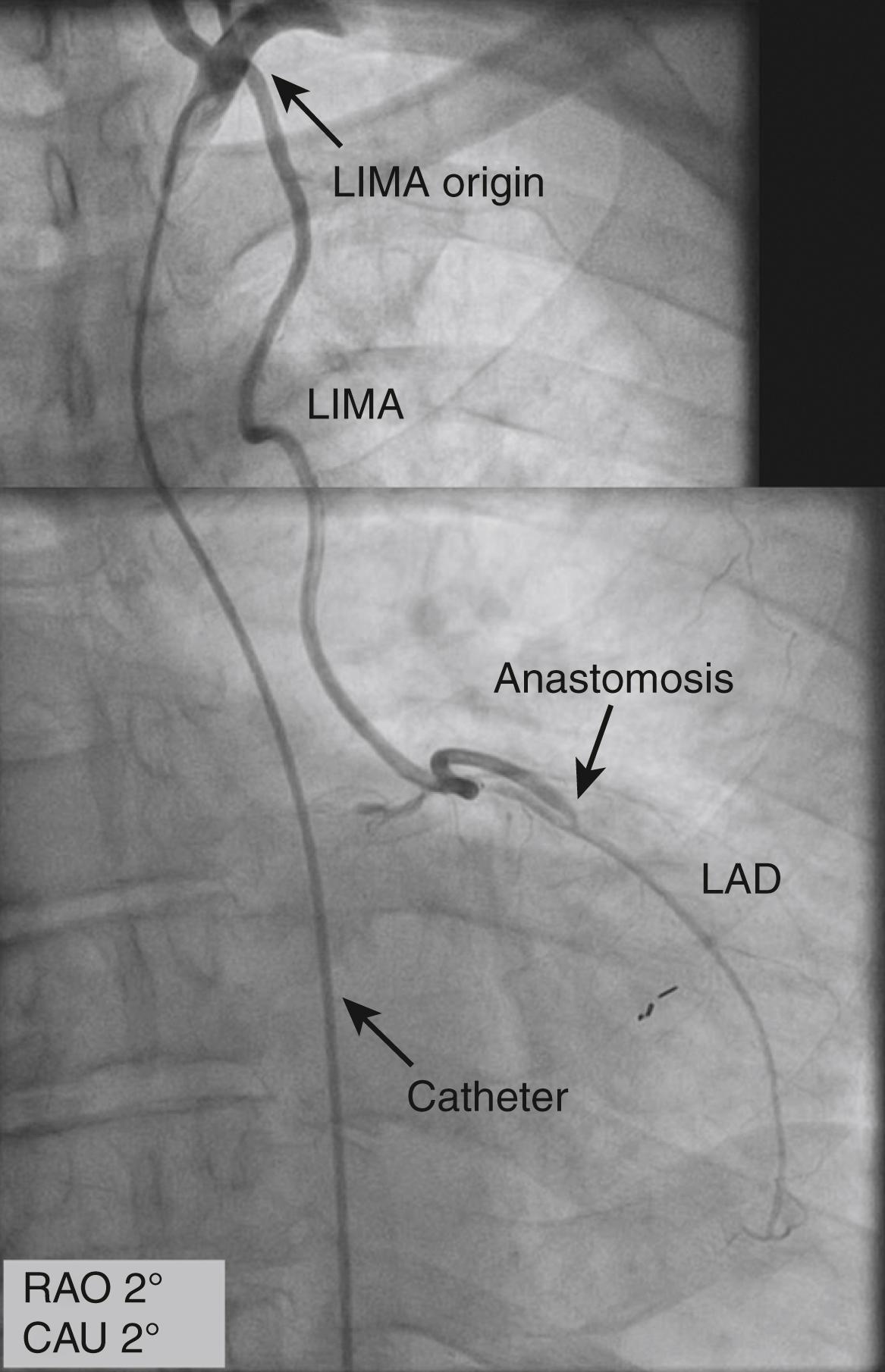
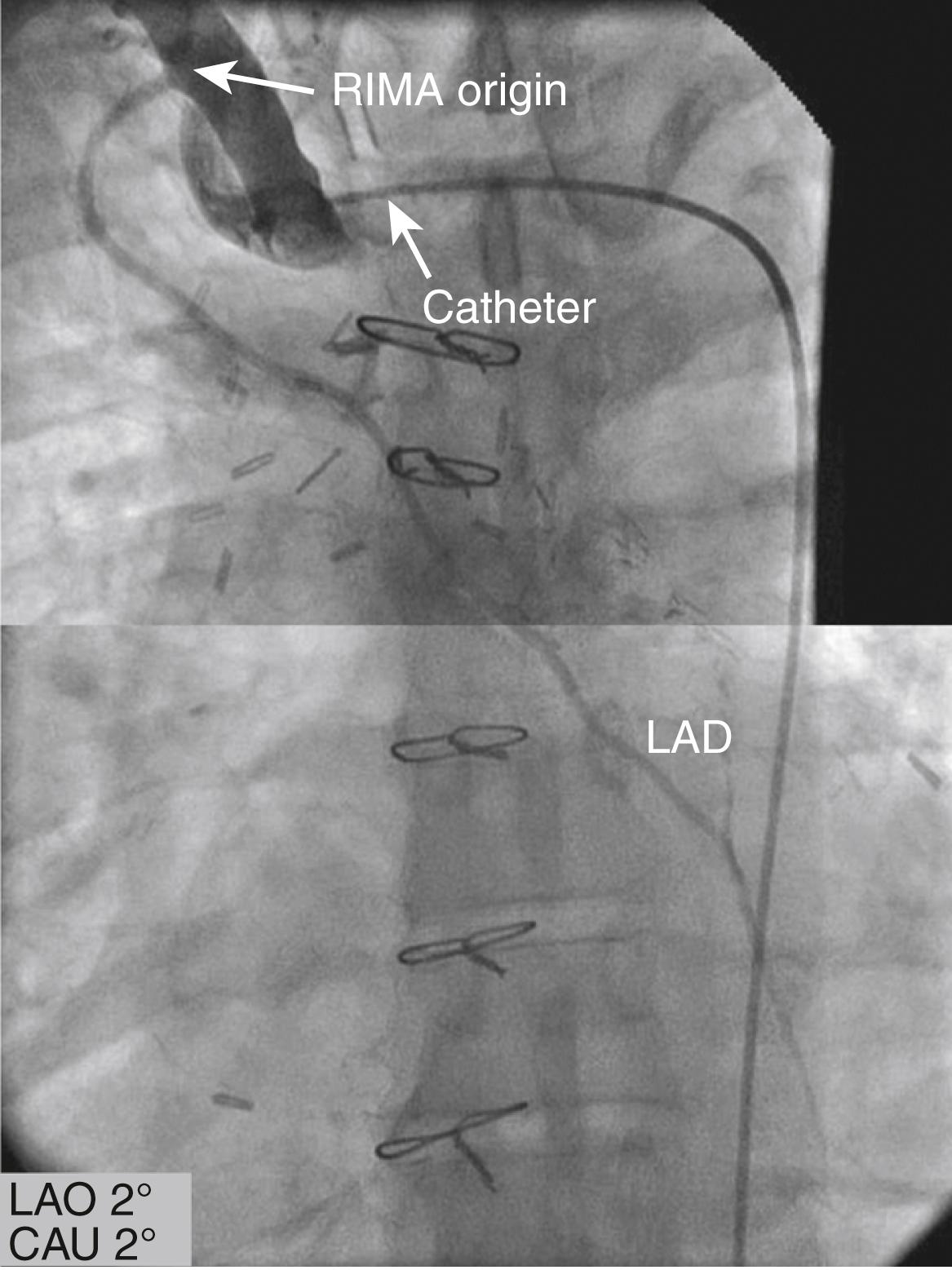
The left IMA (LIMA) can be cannulated with a specially designed J-tip IMA catheter. The catheter is advanced into the aortic arch distal to the origin of the left subclavian artery, then rotated counterclockwise and gently withdrawn with the tip pointing in a cranial direction, allowing entry into the left subclavian artery. The right anterior oblique (RAO) or anteroposterior (AP) projections can be used to visualize the IMA ( Fig. 21.7 and eFig. 21.3 ). For the right IMA (RIMA), first the innominate artery is entered with the guidewire in the LAO projection, then the IMA catheter is advanced to a point distal to the expected origin of the RIMA. The catheter is withdrawn slowly in the LAO view and rotated to cannulate the RIMA. Small injections of contrast are used to assess the position and the cannulation of the IMA. If the IMA cannot be selectively engaged and arteriography of the subclavian artery can be used, this usually allows for the opacification of all or most of the IMA, although weak ( eFig. 21.4 ). The IMA can also be visualized with semiselective contrast injection; to avoid injury of the ostium, the catheter can simply be oriented toward the IMA without cannulating it. The correct orientation can be obtained by advancing a guidewire in the IMA to stabilize the position of the catheter during injection.
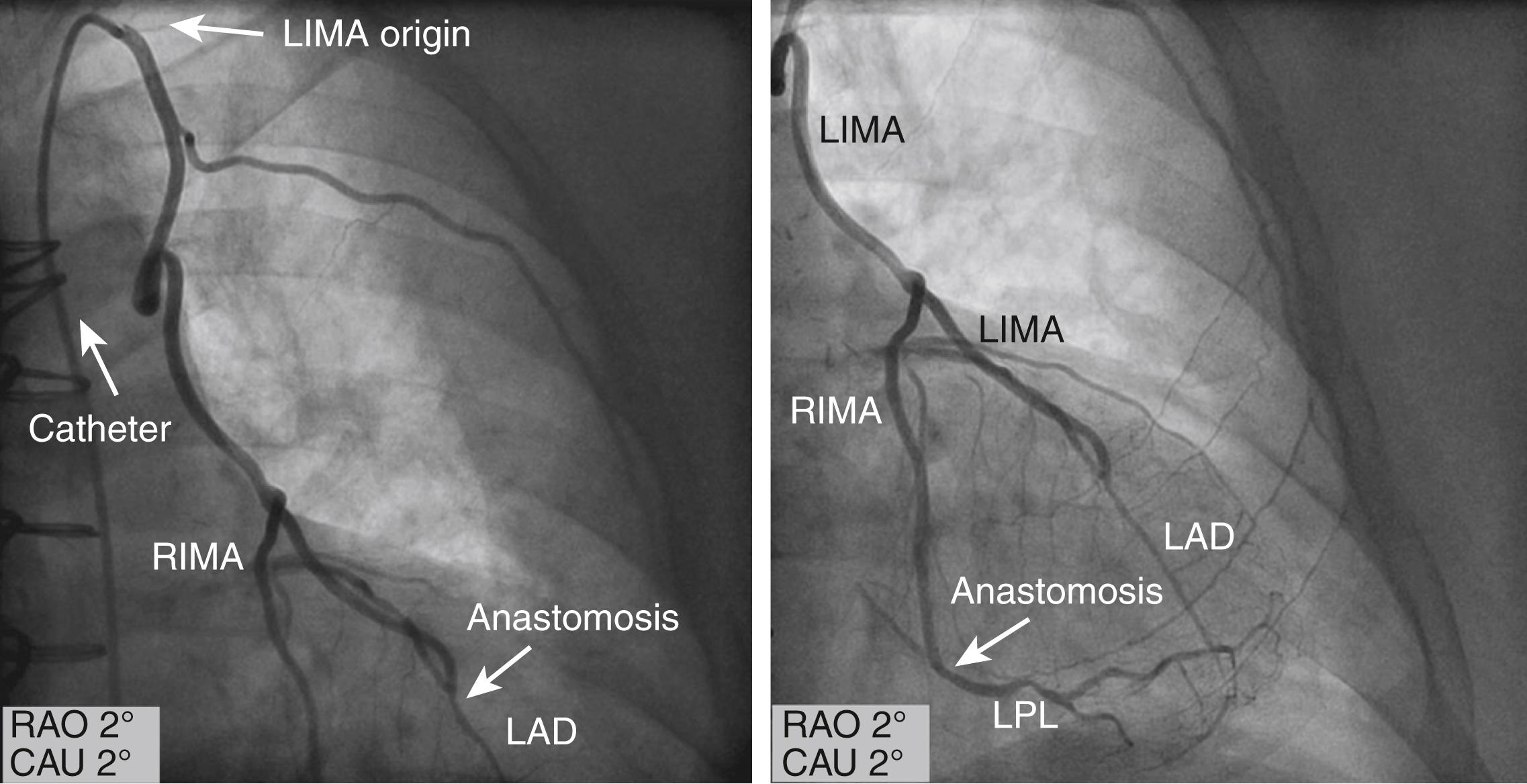
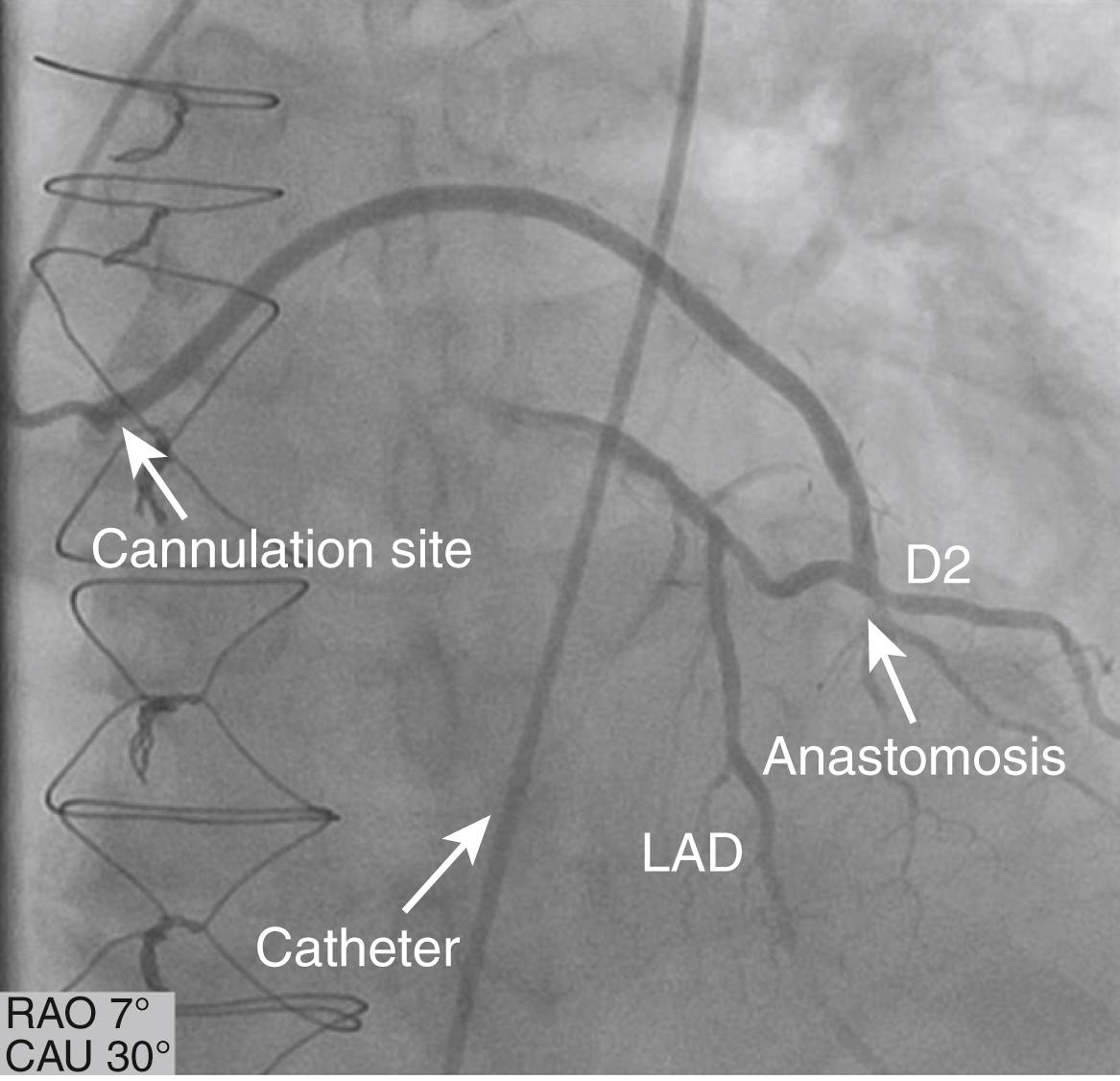
Radial artery (RA) grafts represent the most popular arterial grafts after the LIMA and RIMA. Similar to SVGs, radial grafts require a double anastomosis, one on the aorta and one on the coronary vessel. Because of potential early spasm, RA grafts were abandoned in the 1970s and 1980s. In the 1990s, however, this procedure was rediscovered, and with specific surgical techniques and pharmacologic prophylaxis, it has safely been used with good short- and long-term results ( eFig. 21.5 ).
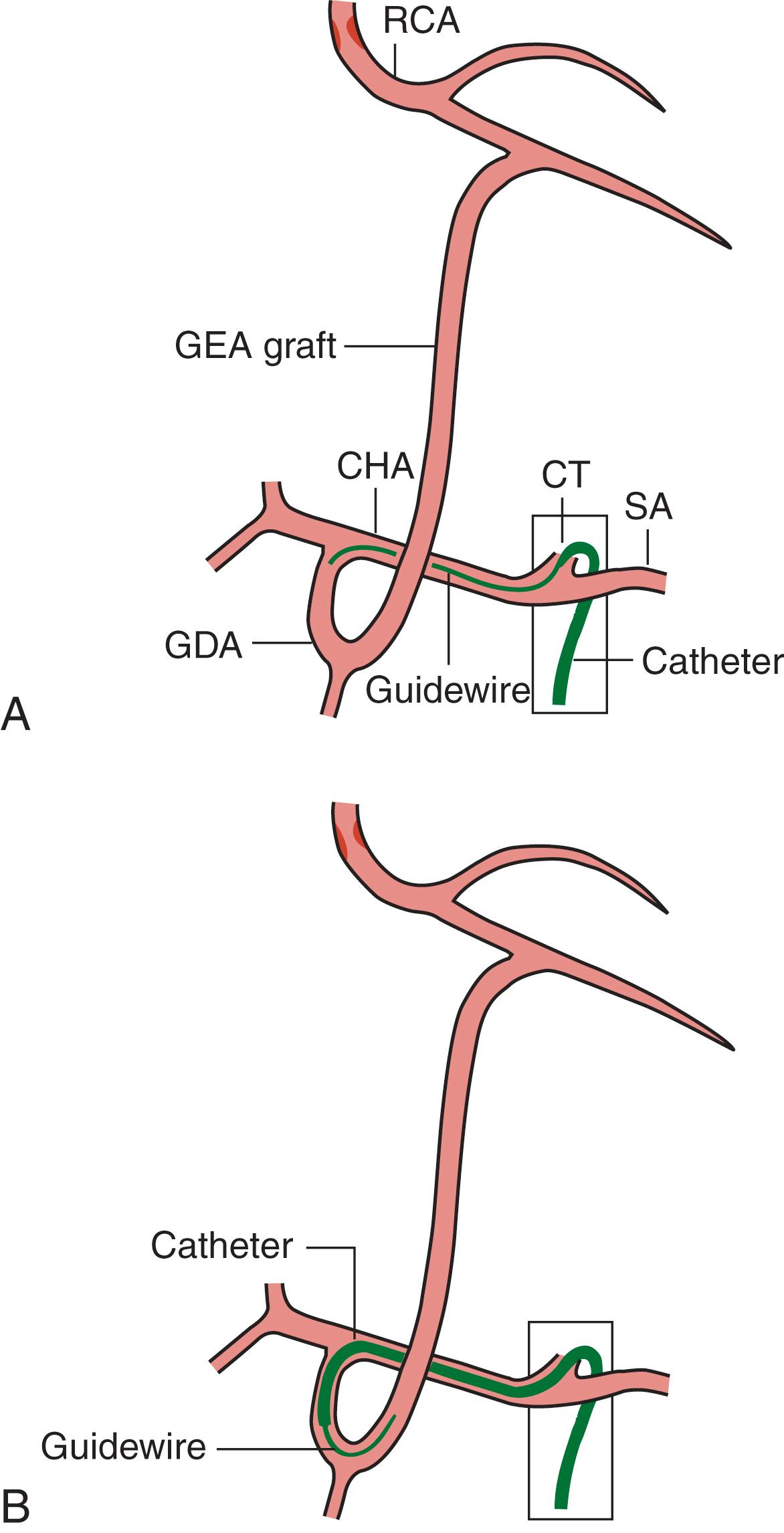
Rarely the right gastroepiploic artery (GEA) can be used for CABGs. To cannulate the GEA, first a special catheter called the “cobra” is inserted into the common hepatic artery. Next, a hydrophilic-coated guidewire is advanced to the gastroduodenal artery and then to the right GEA. The cobra catheter is then exchanged for an MP or JR catheter, which is used for the selective cannulation of the GEA ( Fig. 21.8 ).
Left ventriculography provides important information about volumes, global and segmental function, and anatomic abnormalities such as ventricular septal defect, ventricular thrombus, and valvular dysfunction. However, it is not routinely performed in the current era given the continuous evolution of noninvasive technologies such as echocardiography, CT, or MRI and concerns for complications and contrast volume. Incomplete ventricular opacification with hand-injection of up to 10 cc of contrast through a JR catheter has been become popular as a method to verify an already known normal LV function based on earlier noninvasive studies.
Since the physiologic high pressure developed during each cycle in the left ventricle, the operator should inject a rather high volume of contrast agent in a rather short time for an effective opacification. Accordingly, 6F to 8F catheters with multiple lateral holes are the best option since the single end-hole catheter could be unstable during the high-pressure injection, thus increasing the risk of arrhythmias or inadequate ventricle opacification. The pigtail catheter, including multiple side holes and a “pigtail-like” end-configuration, is frequently used for several reasons. First, usually, the pigtail catheter easily crosses the aortic valve, either directly or by prolapsing across the valve leaflets. Second, the loop shape keeps the end-hole of the catheter away from the cardiac wall, thus decreasing the risk of endocardium trauma, intramyocardial ventricular staining, and arrhythmias. Third, the simultaneous delivery of the contrast agent along the numerous side holes allows a correct opacification of the left ventricle and a further stabilization of the catheter. The pigtail catheter is available with a preformed straight shaft or with a 145- to 155-degree angled shaft, allowing a central position also for that ventricle having an accentuate angle between the aortic root and long axis of the chamber. Crossing the aortic valve requires careful movements, despite the safety profile of the pigtail catheter, and watchful rhythm observation since VT occurrence may require immediate wire/catheter manipulations and even retraction ( eFig. 21.6 ). A common approach is advancing the pigtail close to the aortic valve with a 0.035-inch J-tip guidewire up to the end of the straight catheter section and rotate the catheter to achieve a “6” shape on a RAO projection. Then the catheter should be pushed against the aortic plane to obtain a U-shape curve, and following a deep inspiration or under pullback and clockwise rotation, the tip of the catheter usually falls into the left ventricle. An alternative to the pigtail could be the Halo-type catheters, constituted by a perpendicular helix, inwardly and upwardly directed, and a single end-hole, thus decreasing the risk of ectopic beats and allowing a superior pressure measurement when necessary (e.g., hypertrophic cardiomyopathy). Other catheters can be used to better suit the anatomic variability and facilitate the crossing through the aortic valve. The Judkins catheter for the RCA may be more appropriate for the small aortic roots, whereas the AL or multipurpose catheters might be used to cross bicuspid valves or stenotic aortas. In severe aortic valve stenosis, straight rather than J-tipped guidewires are used, including the straight Glidewire, which may afford faster crossing but also requires greater care to avoid microperforations. Of note, regardless of the type of catheter used to cross the valve, it may need to be exchanged over a 0.035-inch J-tip guidewire with a Pigtail catheter for the following indications: (1) the complete ventriculography via power-injector, (2) detailed hemodynamic assessment of stenosis (dual-lumen pigtail) particularly with excessive femoral pressure amplification of any significant aortoiliac arterial stenosis, (3) if an end-hole catheter is specifically required (e.g., Halo or multipurpose) for slow pull-back hemodynamic measurements, and (4) for advancement of the stiff pigtail-end guidewires to the ventricular apex, as necessary for aortic valve interventions. For optimal ventriculography technique, the catheter should be in midcavity and the RAO projection selected. Table 21.2 and Fig. 21.9 summarize settings and other structures that can be evaluated. Both qualitative and quantitative assessment requires at least two free extra-beats periods since the evaluation of ectopic or post ectopic beats will over- or underestimate the ventricular function and regurgitation entities as well.
| Settings | Suggested Projection | Structures Observed | |
|---|---|---|---|
| Left Ventricle | Flow rate 10-15 mL/sec Total contrast volume 30-45 mL Pressure limit 750-1200 psi 0- to 0.5-second rise |
30-degree right anterior oblique and 0-degree cranial angulation |
|
| 60-degree left anterior oblique and 25-degree cranial angulation |
|
||
| Right Ventricle | Flow rate 8-10 mL/sec Total contrast volume 20-30 mL Pressure limit 750 psi |
30-degree right anterior oblique and 0-degree cranial angulation |
|
| Anteroposterior view |
|
Complications related to left ventriculography are as follows: (1) cardiac arrhythmias (both supraventricular and ventricular) often requiring dynamic repositioning, (2) microembolization, (3) intramyocardial contrast staining, (4) contrast associated issues, including nephropathy or high volume load in end-stage heart failure or dialysis patients.
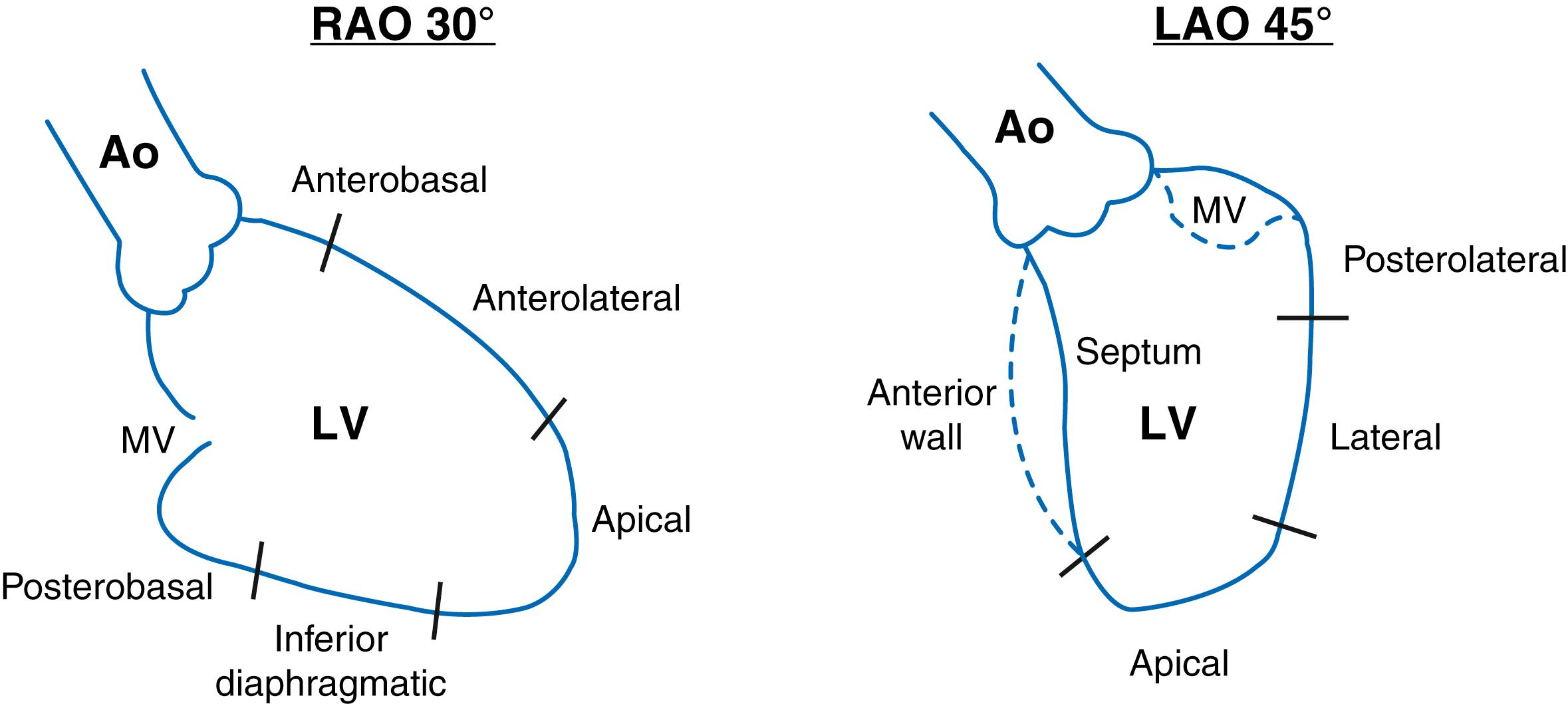
The wall motion pattern is graded from normokinesis, hypokinesis, akinesis, and dyskinesis. Anterobasal, anterolateral, apical, diaphragmatic, and inferobasal segments can be evaluated in RAO projection; lateral, posterolateral, apical septal, and basal septal are viewed in LAO projection. Ejection fraction can be determined qualitatively with a visual estimation or quantitatively assessing end-diastolic and end-systolic contours (centerline method).
The degree of mitral regurgitation can be scored by Sellers classification (see Classic References, Sellers et al.).
Trivial (+1): A minimal jet with a brief and incomplete atrial opacification during systole, rapidly clearing during each cycle without atrial enlargement.
Mild (+2): A moderate opacification of the left atrium with each cycle, clearing with the subsequent beats. The atrium is less opacified than the left ventricle, usually with preserved dimensions.
Moderate (+3): A complete opacification of the left atrium, equal intensity to ventricular opacification. There is delayed atrial clearing over several cycles and a significant enlargement of the left atrium.
Severe (+4): A complete and immediate opacification of the left atrium, even denser than the ventricle. The left atrium is typically severely enlarged and opacification of pulmonary veins may be visible.
Dos Santos first described aortography in 1929 by a direct abdominal aorta puncture. Ascending aortography, as practiced by Sones, is indicated to assess the following: (1) aortic valve regurgitation, (2) dimensions, (3) aortic coarctation, (4) sub- or supravalve aortic stenosis, (5) shunts, and (6) identification of bypass grafts.
The standard approach of LAO 30-degree projection allows the best view of ascending aorta, aortic arch, the innominate artery, and the left subclavian and carotid arteries; RAO is preferred for aortic valve evaluation and related interventions. The typical set up for aortic injection is flow rate 15 to 20 mL/sec, volume of the contrast agent 30 to 45 mL, rate of rising 0 to 0.5 s, and pressure limit 750 to 1000 psi.
The less traumatic side-hole pigtail catheter should be preferred for complete opacification. After tight connection of the catheter to the power injector with high-pressure tubing, the operator should ensure any air bubbles are removed from the injector system. The loop of the pigtail catheter must not be in Valsalva sinus and the operator should avoid iatrogenic regurgitation minimizing catheter-valve contact.
Aortic regurgitation may be trivial, mild, moderate, or severe depending on ventricular opacification after the third cycle following contrast injection.
Trivial or grade 1 (1+): minimal regurgitation jet with a brief and incomplete left ventricle opacification during diastole and fast clearance of the contrast agent.
Mild or grade 2 (+2): regurgitation jet causing a moderate ventricular opacification, which less dense than in the ascending aorta and is cleared within one to two cardiac cycles.
Moderate or grade 3 (+3): regurgitation jet causing complete ventricular opacification within two cycles, as dense as in the ascending aorta and with delayed clearing from the ventricle over several cycles, often associated with dilated left ventricle.
Severe or grade 4 (+4): complete and immediate opacification of the left ventricle, denser than observed in the ascending aorta.
The optimal position is the midcavity that allows only few ventricular premature beats. Right ventriculography is indicated to assess right-to-left ventricular shunts, right ventricle dimensions or dysplasia, abnormalities of the RV outflow tract (RVOT), and pulmonary stenosis or global and segmental ventricular function. However, it is not valuable for assessing tricuspid regurgitation due to the presence of the catheter across that valve. A multiple-hole pigtail might be used. The 7F Berman balloon-directed multiple-sidehole catheter is frequently used. The septum and RVOT can be evaluated using an AP cranial or AP lateral projections. Typically, 20 to 30 mL of contrast material is injected at 8 to 10 mL/sec (but if the ventricle is severely dilated, the volume could be increased up to 40 to 50 mL at 12 to 18 mL/sec).
Since the introduction of intravascular contrast agents (ICAs) in the 1950s, clinical practice has become increasingly dependent on their use, particularly as the use of computed tomography (CT) and cardiac catheterization procedures has expanded markedly in recent years. All currently used ICAs are classified on the basis of their physical and chemical structure, specifically osmolality, iodine content, ionization in solution, and viscosity ( eTable 21.3 ). The most useful classification in clinical practice divides available ICAs into high-osmolar contrast agents (HOCAs), low-osmolar contrast agents (LOCAs), and iso-osmolar contrast agents (IOCAs). The HOCAs have an osmolality four to five times higher than blood (300 Osm). LOCAs have an osmolality twice as high as blood. The latest-generation IOCAs have the same osmolality as blood. Ionic high-osmolality ICAs were the first class of ICA used. However, the high-osmolality and calcium-chelating proprieties often resulted in heart rhythm disorders (sinus bradycardia, atrioventricular blocks, QRS prolongation, long QT, ST-T, giant T-wave inversion, and extremely rarely, VT and VF) and altered LV contractility. Therefore, in recent decades, new-generation ICAs have been developed with low osmolality and neutral chemical characteristics that allow a significant reduction of adverse events. In large cohort studies, the incidence of all types of adverse reactions to contrast was approximately 12% with a high-osmolality agent, compared with only 3% with a low-osmolality ICA (see Classic References, Katayama et al.). For this reason, LOCAs and IOCAs are now considered the safest ICAs to use for vascular diagnostic procedures.
| Generic Name | Osmolality Range (mOsm/kg H2O) | Viscosity Range (cP or mPa-s ∗ ) 37°C | Ionicity | |
|---|---|---|---|---|
| High osmolarity | Diatrizoate/meglumine Diatrizoate/sodium ( ionic compounds ) |
1500-2000 | 4.1-10.5 | Ionic |
| Iothalamate ( ionic compound ) | 600-1400 | 1.5-4 | ||
| Low osmolarity | Ioxaglate ( ionic compound ) | 600 | 7.5 | |
| Iodipamide | 664 | 5.6 | ||
| Iohexol | 322-844 | 1.5-10.4 | Nonionic | |
| Iopamidol | 413-796 | 3.0-9.4 | ||
| Iopromide | 330-770 | 1.5-10 | ||
| Ioversol | 502-792 | 3-9 | ||
| Ioxilan | 610-721 | 5.1-8.1 | ||
| Iso-osmolarity | Iodixanol | 270-320 | 6.3-11.8 |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here