Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Occurring more frequently than ruptured abdominal aortic aneurysm, aortic dissection is the most common catastrophe involving the aorta. Because major aortic branch occlusion can complicate the clinical presentation of patients with acute dissection and thus mimic many other acute medical and surgical problems, a high index of clinical suspicion is necessary to diagnose this life-threatening condition early. The lethal nature of acute aortic dissection mandates prompt medical, interventional, or surgical treatment. In the chronic phase, aortic dissection involving the descending thoracic aorta is responsible for a substantial proportion of thoracic and thoracoabdominal aortic problems requiring surgical repair.
Although a consensus exists today regarding the need for emergency surgical treatment of essentially all patients with acute type A aortic dissection, the optimal management of patients with aortic dissection involving the descending thoracic aorta—medical only versus catheter interventional and medical versus surgical and medical—remains controversial. Most patients with acute type B dissections are treated medically. A complication-specific approach is favored by most authorities, reserving surgical replacement or endovascular stent grafting of the descending thoracic aorta for patients with complicated dissections, including rupture, ischemia of vital organs, persistent pain, uncontrollable arterial hypertension, or sizable dilation of the false lumen. On the other hand, we and other groups have also advocated consideration of early surgical treatment for carefully selected patients with uncomplicated acute type B dissection who are young and are otherwise good surgical candidates, including those with Marfan syndrome or other connective tissue disorders, in an attempt to lower the long-term risk of dissection-related complications and aortic reoperation. Since the late 1990s, emergency thoracic endovascular aortic repair (TEVAR) for patients with complicated acute type B aortic dissection has played a major role (see Chapter 72 ), occasionally supplemented by catheter interventional flap fenestration and true lumen bare metal stenting to relieve distal malperfusion.
The first surgical attempt to treat acute aortic dissection complications was described in 1935 by Gurin and associates, who used surgical iliac artery fenestration to correct dissection-related lower extremity ischemia. In 1955, DeBakey and colleagues initiated the modern era of surgical management of aortic dissection by introducing graft replacement of the dissected thoracic aorta. Subsequently, DeBakey introduced the use of cardiopulmonary bypass during clamping of the descending thoracic aorta. Wheat and associates, in 1965, recommended medical treatment with use of pharmacologic antihypertensive drugs for aortic dissection involving the descending thoracic aorta. The introduction of percutaneous interventional techniques in the early 1990s (e.g., fenestration of the dissection flap and stenting of aortic branches) to alleviate dissection-induced branch vessel compromise and malperfusion modified the traditional indications for surgical treatment, with more patients now being treated medically and interventionally despite the presence of complications that earlier would have prompted emergency operation. The advent of TEVAR in patients with complicated acute type B aortic dissections since 1999 has been associated with promising results. Although TEVAR is now widely used in patients with acute type B dissection following U.S. Food and Drug Administration (FDA) approval of the Gore C-TAG and Medtronic Valiant stent grafts for acute or chronic dissections in 2013-2014, until the long-term durability and effectiveness of TEVAR are confirmed in randomized prospective trials comparing this therapy with standard medical and surgical therapy, the exact role of this new modality in the treatment of uncomplicated acute type B dissection remains unclear.
As described in Chapter 70 , various classification methods have been applied to aortic dissection. During the past 45 years, the Stanford functional approach, based on whether the ascending aorta is involved, regardless of the site of primary intimal tear, has gained broad acceptance. If only the descending thoracic aorta is involved, the dissection is called a Stanford type B, DeBakey type III, University of Alabama “descending,” Massachusetts General Hospital “distal,” or Najafi “posterior” dissection. Examples of various types and extents of dissections are illustrated in Figure 70-1 in Chapter 70 . This consensus has made it easier to interpret and to compare outcomes of various therapeutic strategies reported from various institutions. Aortic dissections diagnosed within 14 days of the onset of presenting symptoms are arbitrarily termed acute, whereas those diagnosed more than 14 days after onset are classified as chronic dissections on the basis of the expected biological behavior of the disease process. The term subacute has reemerged in the TEVAR era meaning dissections 14 to 90 days old based on the rationale that the dissection flap still remains moldable and dynamic enough that a stent graft usually can remodel the aorta successfully. This term, however, is too ambiguous to convey meaningful clinical discriminatory information and should not be adopted. Intramural hematoma (IMH) and penetrating aortic ulcers (PAUs) are now recognized as distinct pathologic variants of classic aortic dissection. These lesions are characterized by the absence of an intimal flap dividing the aorta into true and false lumens and more commonly involve the descending thoracic aorta. It is important to distinguish IMH and PAU from classic type B aortic dissection, but these entities constitute a continuum of pathophysiologic changes that can evolve rapidly from one to the other. Furthermore, management of these lesions can differ in certain clinical circumstances.
Acute type B aortic dissection more commonly affects middle-aged to older men. Aortic dissection is seen in all age groups, although the peak incidence is found between the ages of 50 and 69 years. Patients with type B dissections are older than those with type A dissections. In the Stanford 30-year experience with aortic dissection, the mean age of patients with acute type B dissection was 64 years, compared with 56 years for those with acute type A dissections. The estimated male-to-female ratio is between 2 : 1 and 3 : 1. The prevalence of arterial hypertension ranges from 45% to 80% and is highest in patients with type B dissections. Associated atherosclerotic vascular disease is also found more frequently in patients with type B dissections. Between 2% and 4% of patients presenting with acute type B dissections have Marfan syndrome.
The incidence of aortic dissection is estimated to be between 5 and 20 cases per million population per year, which is higher than the incidence of ruptured abdominal aortic aneurysms or ruptured thoracic aortic aneurysms. In a review of the Swedish national health care registers during a 15-year period, the incidence of aortic dissection increased substantially between 1987 and 2002. Approximately two thirds of all acute aortic dissections involve the ascending aorta (Stanford type A), with one third limited to the descending aorta (Stanford type B).
Untreated, acute aortic dissection can be highly lethal. In the 1967 report by Lindsay and Hurst, one third of the patients suffering from acute aortic dissection died within 24 hours, 50% within 48 hours, 80% within 7 days, and 95% within 1 month. In patients presenting with chronic dissection, only 15% were still alive after 5 years. These figures, however, should be interpreted only as approximations because none of the retrolective observational or autopsy investigations was able to capture the true denominator of how many patients sustained a dissection. Patients with dissection involving the descending thoracic aorta (Stanford type B), however, had a somewhat less ominous early prognosis; 75% were alive 1 month after onset. Anagnostopoulos and coworkers, in a large collected series of 963 cases of untreated aortic dissection (type A or B, acute or chronic), reported a cumulative mortality of 70% at 1 week and 90% at 3 months.
Patients with untreated type B dissection usually die of aortic rupture in the left pleural space or distal malperfusion with or without occlusion of major distal aortic branches resulting in ischemic injury to vital organs. An autopsy study by Roberts and Roberts of 40 patients with acute or chronic type B dissections illustrated that the dissection or its vascular complications caused at least 84% of deaths in the 31 patients who were treated medically. Some fortunate individuals with acute dissections survive untreated; in almost all these cases, distal reentry sites are found, allowing decompression of the false lumen. The patent false lumen remains prone to progressive expansion over time, resulting in the potential formation of a thoracic or thoracoabdominal false aneurysm.
The typical pathologic lesion found in older adult patients with type B aortic dissection is smooth muscle degeneration within the aortic media, in part a normal manifestation of the aging process. These findings are distinct from the elastic tissue medial degeneration of the aorta observed in younger patients presenting with type A dissection, which is more frequently associated with heritable connective tissue disorders.
The initial event in aortic dissection is tearing of the intima. Type B dissections can infrequently also arise from rupture of an atherosclerotic plaque, but this usually represents a localized dissecting process that does not propagate and produces a characteristic “mushroom cap” appearance on computed tomography (CT) scans; this is a different process from classic type B dissection, which frequently involves most or all of the descending and abdominal aorta. After a primary intimal tear occurs, blood flow within the aortic wall separates the layers of the media and creates the false channel. Propagation of the dissection occurs within the outer third of the aortic media, usually in an antegrade direction, but it may also propagate proximally, or retrograde, to involve the transverse arch. As in type A aortic dissections, distal reentry sites are usually multiple and commonly are located in regions of sheared-off ostia of arterial branches. Factors influencing dissection propagation include the rate of increase of aortic systolic pressure or aortic dP/dt, aortic diastolic elastic recoil pressure, mean arterial pressure, and aortic wall stiffness and intrinsic integrity. In the descending aorta and abdominal aorta, the false lumen often spirals along the left posterolateral wall, with the false lumen frequently incorporating the left renal artery. The false lumen ordinarily remains patent, especially if reentry fenestrations are present, but it may occasionally thrombose partially or completely if there is a “non-reentering” false lumen; the pressurized and partially thrombosed false lumen can then extrinsically compromise aortic true lumen blood flow distally. Indeed, most distal malperfusion problems can be traced to a non-reentering false lumen. In the chronic phase of dissection, progressive dilation of the patent false lumen results in overall enlargement of the aorta and formation of a false aneurysm. This usually is a diffuse process involving the entire length of the dissection from the arch to the iliac arteries.
For aortic dissections involving the descending thoracic aorta, the primary intimal tear is located in the proximal descending thoracic aorta just beyond the origin of the left subclavian artery in approximately 80% of cases, as illustrated in Figure 71-1 . In 10% to 20% of patients, the primary intimal tear is located in the transverse aortic arch, and the dissection extends in an antegrade direction to involve variable lengths of the descending aorta or in a retrograde fashion to involve the ascending aorta. When it propagates backwards across the arch and involves the ascending aorta, the dissection is called a retro-A, type A, or Ruel-Cooley-DeBakey type III-D dissection. In less than 5% of aortic dissections, a distinct primary intimal tear cannot be identified; these dissections are usually confined to the descending thoracic aorta. In rare cases, the dissection can be limited to the aortic arch without either antegrade or retrograde propagation (isolated arch dissection). It is also estimated that 2% to 4% of aortic dissections may originate in the abdominal aorta.
IMH originates from spontaneous rupture of the vasa vasorum within the outer third of the aortic media, allowing accumulation of blood within the aortic wall in the absence of a large intimal defect. Alternatively, IMH can follow rupture of an atheromatous plaque through the internal elastic lamina, leading to the formation of a PAU, with subsequent extravasation of blood into the aortic wall. In the past 2 decades, major advances in cardiovascular imaging techniques have led to increasing recognition of IMH with or without associated PAU in patients with acute aortic syndromes. Several investigators reported that these lesions can stabilize with medical therapy, but IMH with or without associated PAU can also rupture or evolve quickly into a classic aortic dissection. It is now recognized that IMH involving the descending aorta (i.e., type B IMH) may have a natural history different from that of classic aortic dissection and a higher propensity for aortic rupture, especially in patients with severe acute symptoms or when the IMH is associated with a deep or large PAU.
Aortic branch vessel involvement or thoracoabdominal malperfusion results when the dissection compromises blood flow to important downstream aortic tributaries. As illustrated in Figure 70-4 of Chapter 70 , the most common mechanisms producing aortic branch compromise are extrinsic compression of the aortic true lumen by the false lumen and an intimal flap compromising the orifice of the branch artery. As defined by Williams and associates, static branch compromise is extension of the dissection flap into a branch vessel with subsequent mechanical obstruction of flow; conversely, in dynamic branch compromise, the dissection flap prolapses into the vessel origin or the true lumen is narrowed above it because the bulk of flow is in the aortic false lumen. Compression by the large false lumen can thus result in near-obliteration of the true channel (true lumen collapse or “obliteration”). With extension of the dissection, some aortic tributaries may be spared and continue to be perfused by the true lumen; others may be perfused exclusively from the false lumen after being sheared off and eventually become permanently dependent on flow from the aortic false lumen. Thus, clinical presentation is dependent on which aortic branches are involved and on the severity of compromised perfusion, which can be variable, thus confounding and delaying the correct diagnosis. Simultaneous occurrence of a variety of acute clinical problems without a readily apparent unifying cause should prompt consideration of acute aortic dissection.
Patients with acute type B dissection can present with symptoms and physical findings that suggest almost any other acute medical or surgical disease process. These numerous, nonspecific manifestations are the main reason that the rapid, correct diagnosis of aortic dissection remains such a formidable clinical challenge. Indeed, aortic dissection occurs more frequently than ruptured abdominal aortic aneurysm, but it is diagnosed correctly less frequently antemortem.
Most commonly, the clinical hallmark of acute type B aortic dissection is the acute onset of severe, lancinating chest or back pain. The initial pain can be in any location, but it usually originates in the interscapular region with later migration to the lower back or abdomen. Pain in acute dissection is thought to be secondary to stretching of the aortic adventitia caused by the dissecting hematoma. Abrupt onset of symptoms and description of sharp, ripping, or tearing pain are also characteristic of acute dissection. Persistence or further migration of pain suggests continuing expansion or distal extension of the dissecting process. In rare cases, acute dissection can be painless; vigilance is essential to recognize other manifestations of aortic dissection in these cases. In a summary from the International Registry of Acute Aortic Dissection (IRAD), 98% of 175 patients with acute type B dissection reported some pain, pain was of sudden onset in 84%, and 63% reported chest pain (anterior in 44%, posterior in 41%) that was significantly different from acute type A dissection (chest pain in 79% and anterior in 71%). Back pain was observed in 64% and abdominal pain in 43% of patients with acute type B dissections, much more frequently than in patients with type A dissection. Moreover, the pain was described as the “worst ever” in 90% of patients, sharp in 68%, and tearing in 52%. Radiating pain was observed in 30% of cases, whereas migration was reported in only 19%.
Despite clinical signs of poor peripheral perfusion, elevated blood pressure is usually observed. In the IRAD report, 70% of patients with acute type B dissection were hypertensive at initial presentation; only 4% were hypotensive and in shock, compared with 25% of patients with acute type A dissection. If the patient is hypotensive, aortic rupture should be suspected. Cardiac tamponade is rare in acute type B dissection; only 2% of patients with acute type B dissection in the 30-year Stanford experience had tamponade, which was thought to be the result of leakage of blood and fluid into the pericardial sac from a large, high-pressure mediastinal hematoma.
The constellation of other symptoms and signs relates largely to which distal aortic branches are involved in the dissection. Approximately 25% of patients present with symptoms related to aortic branch compromise or develop such symptoms early in the course of their illness ; alternatively, loss of a peripheral pulse may be clinically asymptomatic. In a review of the Stanford experience with peripheral vascular complications of aortic dissection by Fann and colleagues, 85 (31%) of 272 patients with all types of dissections sustained at least one peripheral vascular complication, whereas 20% of patients with type B dissections had such complications ( Fig. 71-2 ). Of the 85 patients with a vascular complication, 18 individuals (21%) suffered two complications, and 7 (8%) had three or more vascular problems. Among the 40 patients with acute type B dissection, no patient presented with a stroke, 3% had acute paraplegia at presentation, 20% sustained loss of one or more peripheral pulses, 8% had impaired renal perfusion demonstrated angiographically, and 5% had compromised visceral perfusion by angiography. The incidence of these complications with the attendant operative mortality rate after surgical graft replacement of the descending thoracic aorta is summarized in Table 71-1 . The distribution of specific sites of peripheral pulse loss in these patients is summarized in Table 71-2 . Others authors have reported similar figures, with the prevalence of peripheral vascular manifestations ranging from 10% to 30%. As a general rule, morbidity and mortality rates are higher in patients presenting with branch vessel involvement.
| Peripheral Vascular Complication | Prevalence (n) | Mortality (n) |
|---|---|---|
| Stroke | 0% (0) | — |
| Paraplegia | 3% ± 3% (1) | 100% (1) |
| Pulse loss | 20% ± 8% (8) | 50% ± 18% (4) |
| Renal ischemia | 8% ± 4% (3) | 67% ± 28% (2) |
| Visceral ischemia | 5% ± 3% (2) | 50% ± 37% (1) |
| Location | Type A ( n = 128) | Type B ( n = 40) |
|---|---|---|
| Right carotid | 6 | 0 |
| Left carotid | 6 | 0 |
| Right arm | 25 | 0 |
| Left arm | 10 | 2 |
| Right leg | 21 | 4 |
| Left leg | 14 | 3 |
| Total | 82 | 9 |
The clinical course of peripheral limb ischemia can vary; up to one third of patients may experience spontaneous resolution of the peripheral pulse deficit or a fluctuating course, often because of the reentry of flow into the distal true lumen from the false lumen. Stroke and transient ischemic attack can complicate acute type A dissection but are seen only rarely in patients with type B dissections. Neurologic findings can vary from minor sensory deficits to frank paraplegia resulting from spinal cord ischemia caused by interruption of intercostal artery blood supply to peripheral ischemic neuropathy. Abdominal pain out of proportion to the physical abdominal findings must be considered potentially to reflect mesenteric ischemia or infarction, which must be confirmed or ruled out expeditiously. Oliguria or anuria suggests renal perfusion compromise; flank pain or hematuria caused by renal malperfusion or infarction can mimic symptoms usually associated with ureteral colic or kidney stones.
Physical examination of patients with suspected aortic dissection should include measurement of blood pressure in both upper and lower extremities. A complete evaluation of peripheral pulses is imperative, in addition to a comprehensive motor and sensory neurologic examination. The patient should be reexamined periodically because new vascular or neurologic deficits may come and go. The remainder of the physical examination is often normal.
Most patients with chronic type B dissection are asymptomatic. However, progressive enlargement of the aortic false lumen may eventually produce compression, obstruction, or erosion into adjacent thoracic structures, producing symptoms such as chest pain, dyspnea, wheezing, hoarseness, dysphagia, hemoptysis (aortobronchial fistula or erosion into the lung), and hematemesis (aortoesophageal fistula) and symptoms secondary to compromised flow in an important distal aortic branch.
Definitive diagnostic procedures should be performed as expeditiously as possible to confirm the diagnosis of acute type B aortic dissection. Before the widespread availability of newer imaging techniques, the diagnosis was generally made by conventional catheter aortography. Today, much better options include multislice computed tomographic angiography (CTA), transesophageal echocardiography (TEE), and magnetic resonance imaging (MRI). Chest radiography is neither sensitive nor specific. The imaging modality chosen should determine the type of dissection and its extent, the site of the primary intimal tear, and the presence or absence of major aortic branch compromise. More than one imaging study may be necessary to confirm the diagnosis or to identify additional pathoanatomic details; in IRAD, multiple imaging studies were needed in 76% of patients, and an average of 2.2 imaging studies were carried out in patients with acute type B dissection before definitive treatment.
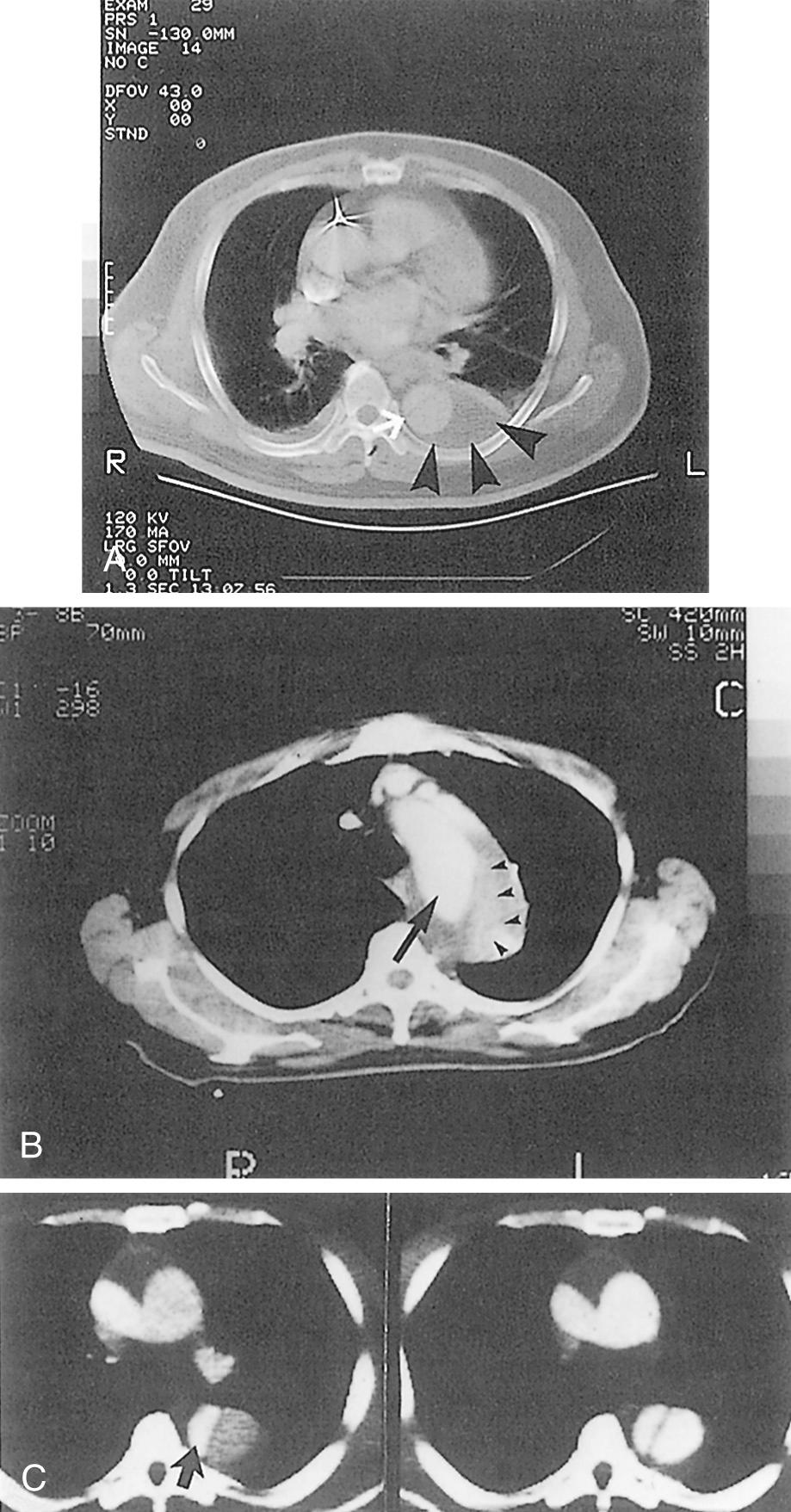
CT scanning has markedly facilitated the rapid and accurate diagnosis of acute aortic dissection. In most cases, a thin-slice spiral CTA with intravenous administration of contrast material can determine rapidly and noninvasively the dissection type (type A or B), as illustrated in Figure 71-2 . The extent of dissection, the perfusion status of individual aortic branches, and the size of the true and false lumens in all aortic segments can also be assessed accurately. Identification of two distinct lumens in the descending thoracic aorta separated by an intimal flap confirms the diagnosis of type B aortic dissection. Other important signs include compression of the true lumen by the false lumen, displaced intimal calcification, thrombosed false lumen, nonopacified crescent-shaped area along the aortic wall (IMH), and ulcer-like projection of contrast material within the aortic wall indicating a PAU. The presence or absence of pericardial or pleural effusions is also defined. The sensitivity and specificity, respectively, of CTA in making the diagnosis of acute type B aortic dissection are between 82% and 100% and 89% and 100%. * In the 1993 study by Nienaber and coworkers
* References
† References
that prospectively evaluated noninvasive modalities in 110 patients with suspected acute dissection, the sensitivity and specificity of CT scanning were 96% and 89% in patients with type B dissection; the positive and negative predictive values were 80% and 98%, respectively. A drawback associated with CT scanning is the requirement for administration of intravenous contrast material in patients with impaired renal function.
In most centers worldwide, TEE and/or CTA are the diagnostic modalities of choice in patients with suspected type B aortic dissection, and many patients do not require additional corroborative studies. † TEE is rapid, convenient, and noninvasive and can be performed in the emergency department, in the intensive care unit, or in the operating room with minimal risk. Undesirable blood pressure elevation is a potential risk of TEE, mandating adequate sedation of the patient. Multiplanar, phased array TEE with color flow imaging can accurately demonstrate flow in both aortic channels and the flap separating the true and false lumens; real-time three-dimensional TEE imaging can provide spectacular surface-rendered images. The most important finding is identification of an intimal flap, ideally seen in more than one view, oscillating independently of the motion of the aortic wall. Frequently, the primary intimal tear and secondary fenestrations in the descending thoracic aorta can also be identified. Overall, the sensitivity and specificity of TEE in the evaluation of suspected type B aortic dissection are between 97% and 100% and 94% and 98%, respectively. ‡
‡ References
Limitations of TEE include dependence on an experienced interpretation of the findings and limited capability to assess abdominal branch vessel involvement and extent of the dissection below the diaphragm; if the patient is very small or very thin, as in Japan, the takeoff of the celiac axis, superior mesenteric artery, and occasionally one or both renal arteries can be visualized with TEE.
Currently, MRI does not play a major diagnostic role in patients with acute dissection because these individuals are often critically ill and connected to various monitoring devices, infusion pumps, or respirators. In the acute setting, the limited 24-hour availability, the relatively long time necessary for image acquisition, and the limited access to the patient during the procedure make MRI much less practical than other diagnostic modalities. Nevertheless, MRI can delineate noninvasively the entire thoracoabdominal aorta and demonstrate the intimal flap, both aortic channels, and involvement of major aortic branches. As with CTA, the most important criterion for the diagnosis of acute aortic dissection with MRI is the identification of two distinct flow lumens separated by an intimal flap. Many investigators have reported that MRI is associated with high sensitivity and specificity in the evaluation of suspected aortic dissection, both in the range of 95% to 100%. § For suspected acute type B dissection, Nienaber and coworkers
§ References
observed that MRI had a sensitivity of 97% and a specificity of 100%. Today, magnetic resonance scans are most useful for serial, long-term follow-up of patients with chronic aortic dissections, including postoperative patients and those initially treated medically.
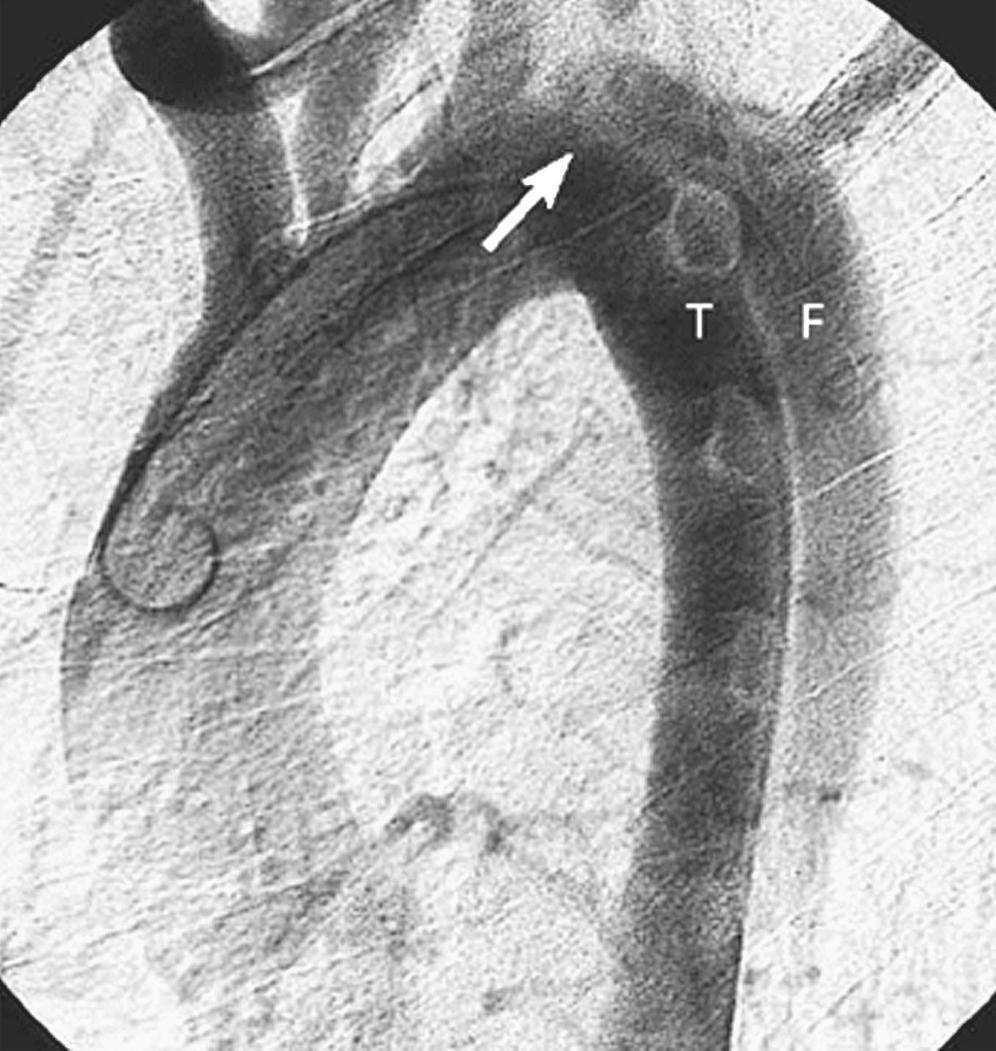
Contrast aortography historically was the “gold standard” in the diagnosis of aortic dissection ( Fig. 71-3 ). Angiography, however, is invasive, is time-consuming, and necessitates the use of contrast material; moreover, it is not infallible, and the technique carries a risk of morbidity and mortality, but it can provide detailed information about perfusion status of important aortic branches. Angiographic diagnosis of acute dissection requires identification of a double lumen or an intimal flap; indirect signs that are suggestive of an acute dissection include compression of the true channel by an expanding false lumen, thickening of the aortic wall, ulcer-like projection in the aortic wall (in cases of penetrating atherosclerotic ulcer), and abnormal position of the guidewire or catheter in the aorta. Biplane angiographic studies of the thoracic aorta are mandatory because single-plane aortography can miss important findings; false-negative results can also occur when the false lumen is thrombosed and in cases of IMH. The sensitivity and specificity of aortography in the evaluation of aortic dissection range between 80% and 90% and 85% and 95%, respectively. Currently, aortography is reserved for patients with acute type B dissection presenting with clinical evidence of malperfusion or those with persistent peripheral vascular complications after proximal aortic repair to delineate the mechanism of aortic branch vessel compromise and guide subsequent appropriate endovascular interventions to restore distal perfusion. ║
║ References
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here