Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
▪ Hemangioma of infancy ▪ Hemangioma
The most common benign soft tissue tumor of infancy
Demonstrate a typical growth pattern characterized by early proliferation followed by gradual, spontaneous involution
Distinct histopathologic and immunohistochemical features that differentiate them from other vascular anomalies in children
May be associated with extracutaneous findings when occurring at certain anatomic sites, e.g. the face, neck, lumbosacral area
Treatment choice, if any, depends on multiple factors and must be tailored to each individual patient
Systemic β-blockers have become the first-line therapy for most children with complicated hemangiomas
Infantile hemangiomas (IHs) are benign proliferations of endothelial cells and their supporting tissues and represent the most common tumors arising in the neonatal period. They are characterized by significant growth during the first several months of life, followed by slow spontaneous involution over the ensuing years. This natural history differentiates them from vascular malformations ( Table 103.1 ). Studies highlighting the role of a specific IH stem cell have provided new insights into their pathophysiology.
| DIFFERENCES BETWEEN INFANTILE HEMANGIOMAS AND VASCULAR MALFORMATIONS | ||
|---|---|---|
| Infantile hemangioma | Vascular malformation | |
| Clinical |
|
|
| Epidemiology | More common in:
|
No gender or gestation predilection |
| Pathology | Proliferating : endothelial cell hyperplasia, lobule formation, mast cells, prominent basement membrane Involuting : fibrofatty tissue replacement, decreased mast cells when fully involuted |
Dependent upon type, often irregular vascular channels |
| Immunohistochemistry | Positive for GLUT1, Lewis Y antigen, merosin, and FcγRII, Wilms tumor protein 1 (WT1) | Negative for GLUT1, Lewis Y antigen, merosin, and FcγRII, WT1 |
The terms “infantile hemangioma” and “hemangioma of infancy” describe a specific group of vascular tumors that arise during infancy and demonstrate characteristic clinical and histologic features. IHs have been recognized in the medical literature for centuries, and they have been given various names such as nevus maternus , angioma simplex , angioma cavernosum , angiodysplasia , strawberry nevus , and capillary hemangioma . Despite early attempts to make distinctions among different types of vascular lesions of infancy, the term “hemangioma” was frequently applied in a generic manner to a wide variety of congenital and acquired vascular anomalies including vascular malformations, without consideration of differences in their biologic behavior.
One of the most important developments in the study of vascular “birthmarks”, including lesions that become apparent during early infancy, was the acceptance of a biologic classification scheme. In 1982, Mulliken and Glowacki first proposed that vascular birthmarks should be categorized according to their biologic and clinical behavior. The International Society for the Study of Vascular Anomalies modified this classification system in 1996 and subsequently in 2014 to reflect new insights, including the genetic bases of several types of vascular malformations ( Table 103.2 ) .
| BIOLOGIC CLASSIFICATION OF VASCULAR BIRTHMARKS |
|---|
| Vascular tumors |
Benign
Locally aggressive or borderline
|
| Vascular malformations |
Simple
Combined
Of major named vessels
|
| Provisionally unclassified |
|
Under this organization scheme, vascular birthmarks are divided into two main categories, vascular tumors and vascular malformations, as well as a third group of “provisionally unclassified” lesions for which the biologic behavior has not yet been well characterized. Vascular tumors are characterized by cellular proliferation and include IH, pyogenic granuloma, and less common neoplasms that may arise during infancy or early childhood, including tufted angioma and kaposiform hemangioendothelioma (see Table 103.2 and Ch. 114 ). Vascular malformations, on the other hand, are believed to represent errors in vascular morphogenesis. They are frequently noted during the neonatal period, but, unlike IHs, they do not rapidly proliferate in the first year of life nor do they resolve spontaneously (see Table 103.1 ). Vascular malformations are characterized by the type of dysplastic vessels they contain and by their flow properties (see Ch. 104 ).
Adopting specific terminology has allowed investigators to better categorize vascular birthmarks and predict their clinical behavior and prognosis. The biologic classification was expanded to include histopathologic, immunohistochemical, and genetic features that aid in distinguishing IHs from other vascular lesions. Despite the wide acceptance of the biologic classification scheme, nosologic confusion still persists in the medical community as well as in the literature.
IHs arise during the first year of life and are the most common tumor of infancy. They develop in 4–5% of infants, with lesions usually noted within the first several weeks of life . IHs may occur more commonly in Caucasian infants than in other racial groups, but this predilection has not been consistently observed . A female : male ratio of 2–5 : 1 has been noted in multiple retrospective studies as well as prospective studies conducted in dermatology practices; however, a recent hospital-based prospective birth-cohort study found no female predominance among infants who developed an IH . A higher female : male ratio of 7–9 : 1 has been reported for patients with severe, complicated IHs.
IHs also develop more frequently in premature infants. Low birth weight represents an independent IH risk factor, and IHs affect 25–30% of infants with a birth weight <1000 g and 15% of those with a birth weight between 1000 and 1500 g . Factors associated with placental insufficiency (e.g. preeclampsia, placenta previa) are also associated with IH development, while prematurity and multiple gestation pregnancies are linked with the development of multifocal IHs . One study found a threefold increased incidence in infants born following chorionic villus sampling compared to those born following amniocentesis or without a history of prenatal instrumentation . A higher maternal age has also been associated with hemangioma development .
IHs typically arise sporadically, although Margileth and Museles reported a 10% incidence of familial cases in their 1965 series. The frequent occurrence of hemangiomas in the general population makes it difficult to assess the true familial incidence, and no specific genes have been consistently implicated.
IHs represent localized or regional areas of abnormal vascular development and proliferation. Several hypotheses have been proposed to explain their pathogenesis, but no single theory accounts for all their features. It is likely that several mechanisms under the control of multiple genes, in addition to local effects, play a role in the development, growth, and involution of hemangiomas.
Hemangioma-derived stem cells (HemSCs) and endothelial progenitor cells have both been isolated from IH specimens. HemSCs can differentiate into endothelial cells, pericytes, and adipocytes. Recent evidence supports derivation of IHs from multipotent stem cells, implying that IH development involves de novo formation of vessels from progenitor/stem cells (vasculogenesis) as well as formation of new vessels from existing ones (angiogenesis) .
The overlapping immunohistochemical phenotype of hemangioma cells and human placental endothelium implies that the former may be of placental origin or undergo differentiation toward a placental microvascular phenotype. North and co-workers reported that glucose transporter protein-1 (GLUT1) is expressed by IHs during all phases of their development (proliferating, involuting, involuted) as well as by the placenta, but not by other vascular tumors or malformations. Additional placenta-associated vascular antigens, including merosin, FcγRII, and Lewis Y antigen, are present in hemangioma specimens and placental chorionic villi but absent in microvessels of the normal skin and subcutis . However, hemangiomas do not express placental trophoblastic markers (e.g. human placental lactogen) and are no longer thought to represent placental emboli.
Several signaling pathways play a role in IH development. Studies have confirmed the importance of vascular endothelial growth factor (VEGF; also known as VEGF-A) signaling, with a shift from expression of VEGF receptor 1 (VEGFR-1), which binds VEGF with high affinity but transmits a very weak signal, to VEGFR-2, which strongly stimulates endothelial cell proliferation upon VEGF binding . VEGFR-2 signaling activates the phosphatidylinositol 3-kinase (PI3K)-mammalian target of rapamycin (mTOR) pathway, leading to upregulation of hypoxia inducible factor-1α (HIF-1α) and increased levels of VEGF. Accordingly, treatment of hemangioma endothelial cells in vitro with the mTOR inhibitor rapamycin results in reduced proliferation . Hemangiomas also have increased expression of the endothelial cell-specific Tie-2 tyrosine kinase receptor and dysregulated production of angiopoietin-2, which may promote angiogenesis and inhibit vessel maturation (see Ch. 102 ) . The Notch pathway is thought to mediate differentiation of HemSCs into pericytes/vascular smooth muscle cells , and the latter appear to have pro-angiogenic properties and support IH development .
A variety of genetic factors may be involved in the development of IH. There have been reports of somatic mutations in genes that encode proteins involved in VEGF signaling (e.g. VEGFRs) and other pathways that affect vascular development within hemangioma tissue . Heterozygous germline mutations in VEGFR2 and ANTXR1 (anthrax toxin receptor 1), which encodes an integrin-like endothelial cell receptor, have been identified in a small subset of IH patients and may represent hemangioma predisposition factors . In addition, familial hemangiomas in several kindreds have been linked to chromosome 5q , and loss of heterozygosity of 5q has been noted in sporadic hemangiomas , suggesting that gene(s) at this locus may be involved in hemangioma formation.
A role for hypoxia in the pathogenesis of IHs is supported by their association with hypoxic placental changes, prematurity/low birth weight (often caused by placental insufficiency), retinopathy of prematurity (GLUT1-positive, hypoxia-induced neovascularization), and regional arterial insufficiency in PHACE[S] and LUMBAR syndromes (see below) . Hypoxia upregulates expression of GLUT1 and VEGF, leading to mobilization of endothelial progenitor cells. Moreover, the combination of hypoxia and estrogen has a synergistic effect on hemangioma endothelial cell proliferation in vitro , potentially explaining the predilection of IHs for female infants. Investigators have also found that HIF-1α and its downstream effectors are upregulated in IHs during their proliferative phase .
Other cell types in the hemangioma's environment, including monocytes, fibroblasts, pericytes, mesenchymal cells, adipocytes, and mast cells, can influence endothelial cell proliferation and may have a role in hemangioma formation and/or involution.
Hemangiomas have been studied during different phases of their clinical course to gain insights into the mechanisms that govern their growth and involution. In the proliferative phase, hemangiomas express markers of proliferation such as proliferating cell nuclear antigen (PCNA) and increased levels of pro-angiogenic molecules such as VEGF and basic fibroblast growth factor (bFGF) . In addition, other angiogenesis mediators, proteins found within the perivascular extracellular matrix, and enzymes involved in matrix remodeling are produced at higher levels during proliferation ( Table 103.3 ) .
| MARKERS OF PROLIFERATING AND INVOLUTING HEMANGIOMAS |
|---|
| Proliferative phase |
|
| Involution |
|
* VEGF and bFGF, but not the angiogenesis inhibitor interferon-β, are also expressed by the hyperplastic epidermis overlying proliferating hemangiomas.
The factors that govern the transition of hemangiomas from proliferation to involution and the mechanisms of the associated decrease in pro-angiogenic factors are not fully understood . Increased expression of angiogenesis inhibitors and apoptosis promoters has been observed in involuting hemangiomas (see Table 103.3 ) .
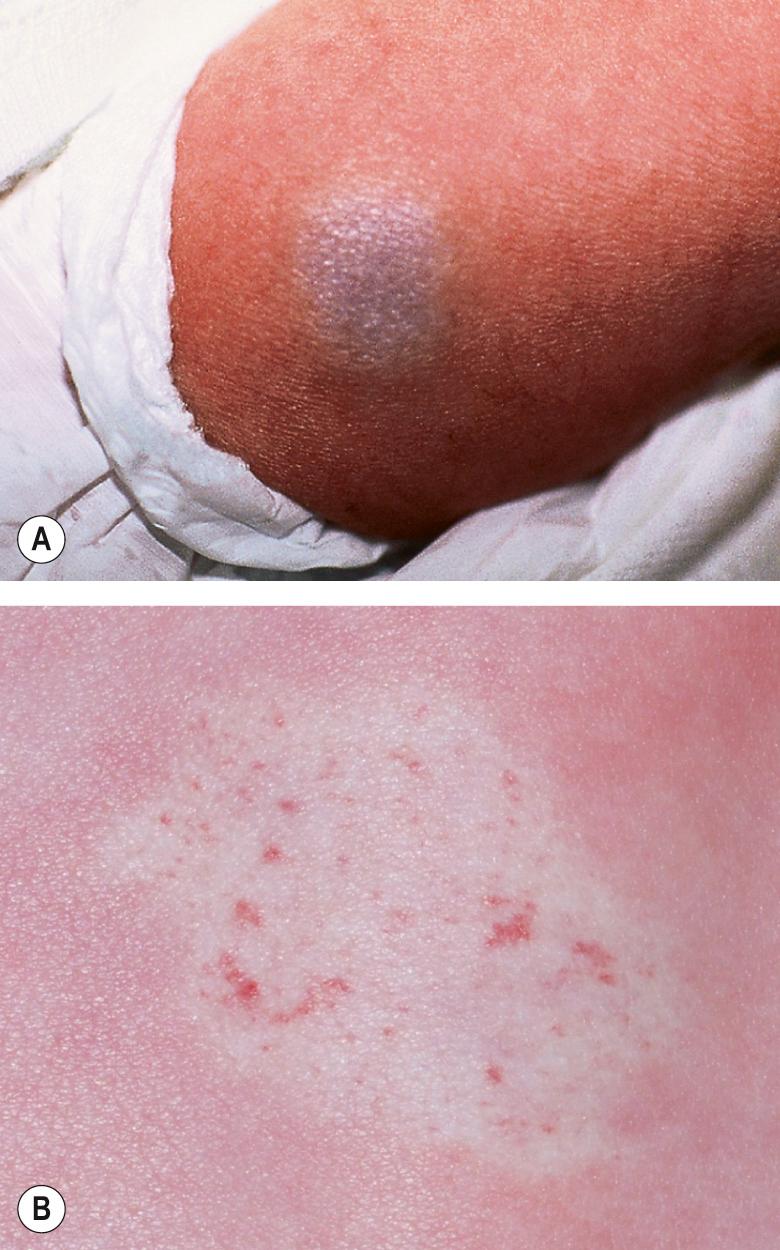
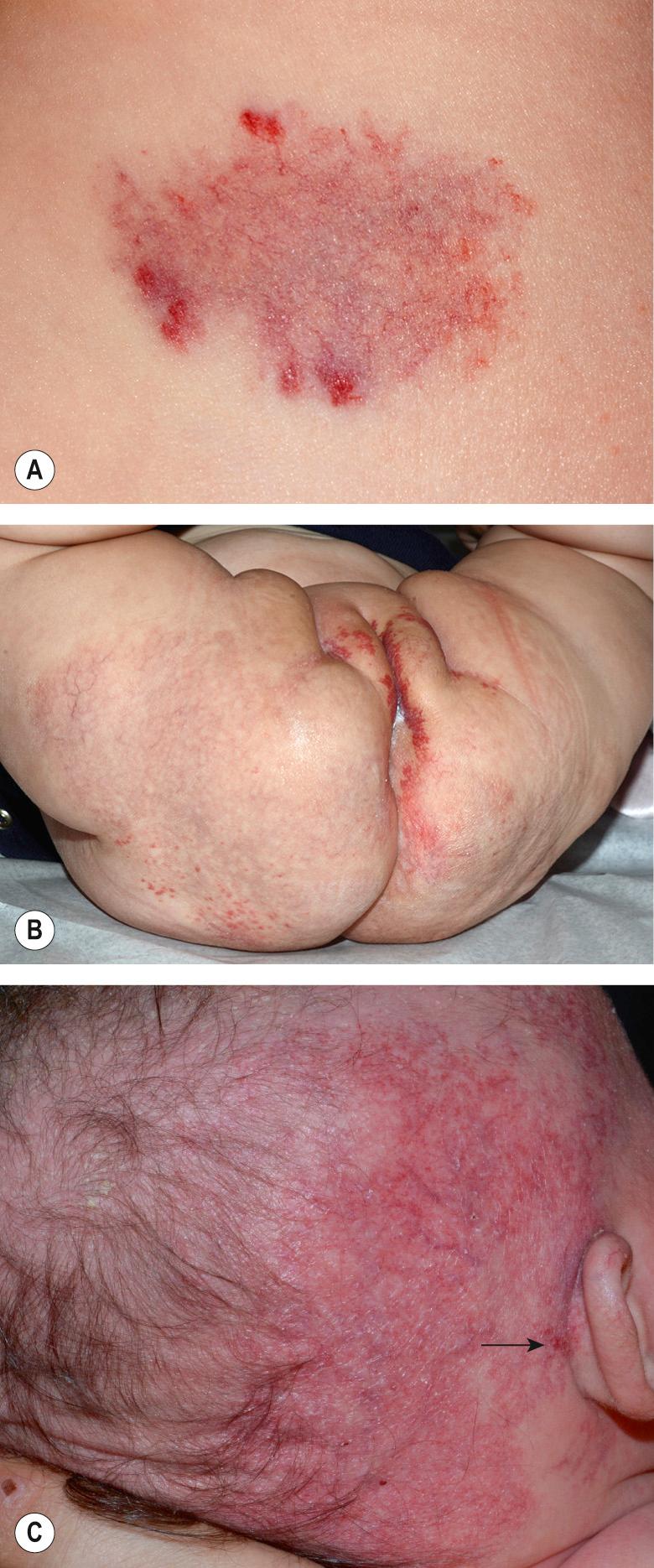
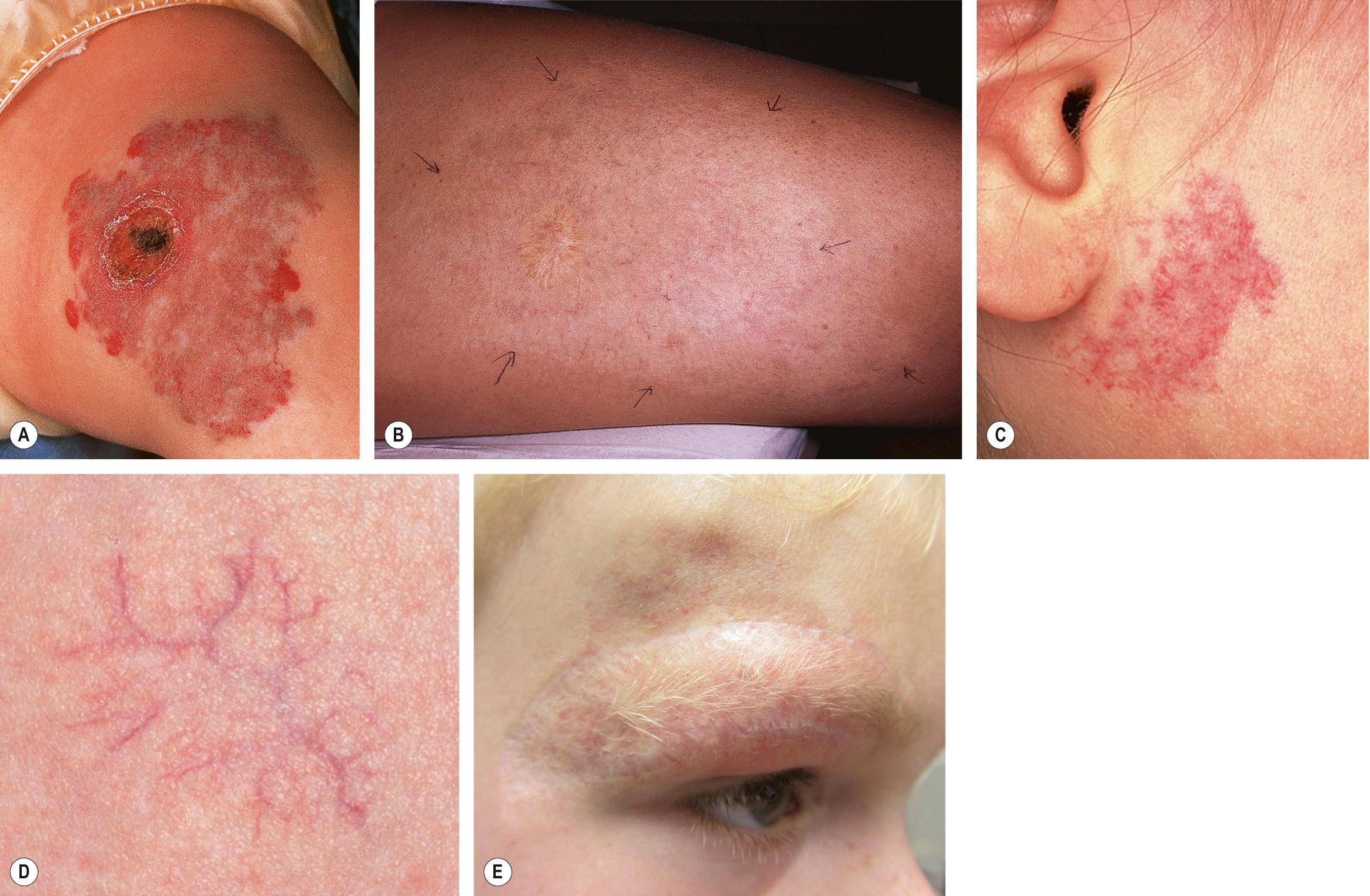
Most IHs have a typical presentation and growth pattern. Lesions usually become apparent during the first few weeks of life, although the proportion of hemangiomas that are “congenital” in published series has ranged from 15% to 60% . IHs evident at birth most often appear as precursor lesions but occasionally they present as relatively well-formed typical IHs that subsequently demonstrate variable proliferation then slow involution; rapidly involuting and non-involuting congenital hemangiomas (RICH/NICH) represent separate entities that are discussed below. Precursor IH lesions include telangiectasias surrounded by a vasoconstricted halo, areas of pallor, pink macules, and blue bruise-like patches ( Fig. 103.1 ). Pink macules and patches may mimic a capillary malformation, and repeat examination over the ensuing weeks is essential to determine the diagnosis ( Fig. 103.2 ). Occasionally, an area of ulceration in the diaper area or on the lip may herald the onset of an IH, and in a newborn infant these lesions may be confused with bacterial or viral infections. Biopsy of the ulcerated lesion may not be clearly diagnostic of an IH, but continued observation usually clarifies the diagnosis .
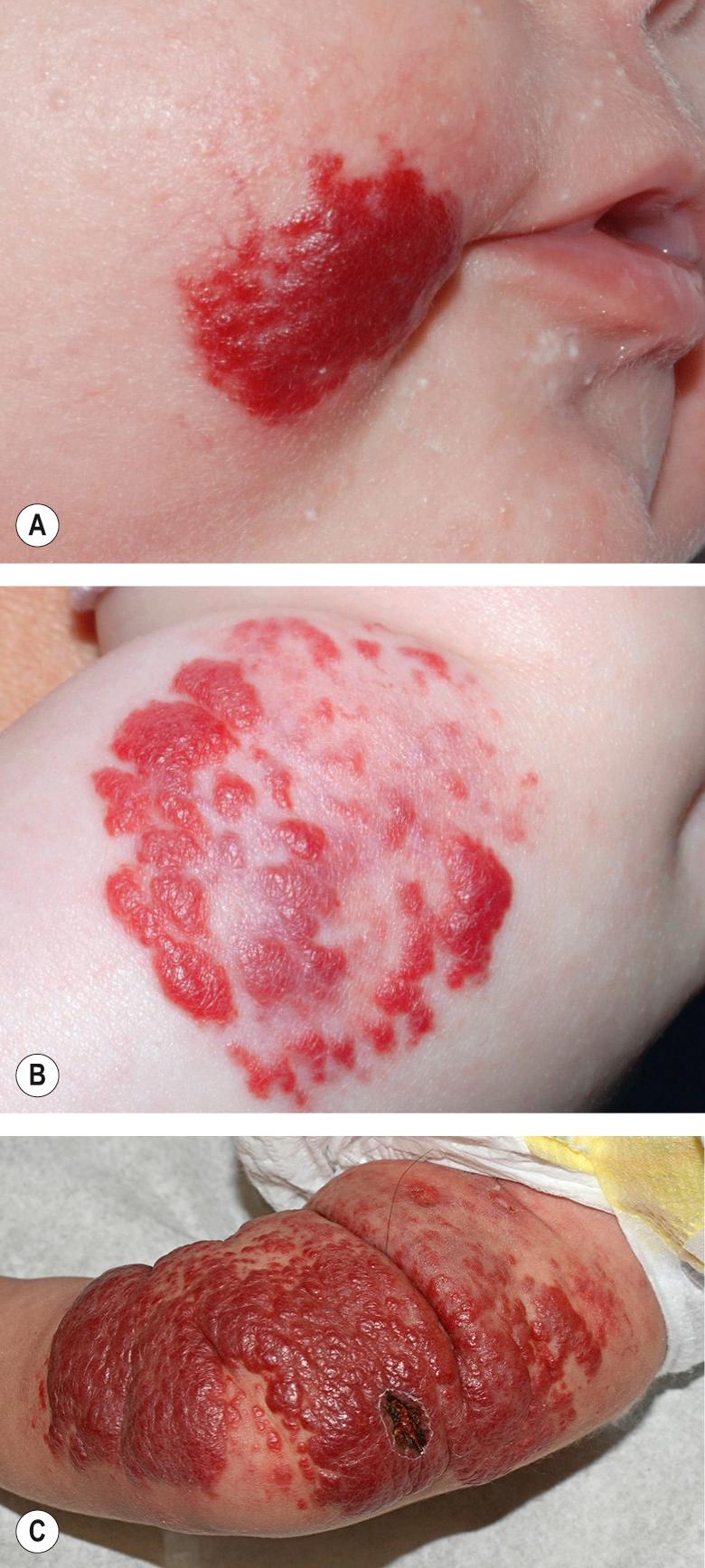
Hemangiomas may occur anywhere on the skin and mucosal surfaces. Although overall they most commonly occur on the trunk , ~50% of the IHs that present to a pediatric dermatologist are located on the head and neck . The clinical appearance of an IH is influenced by its location within the skin and subcutaneous tissues. Superficial hemangiomas are situated in the superficial dermis and are bright red in color during their proliferating phase. The surface is finely lobulated, and the term “strawberry hemangioma” has been used to describe them ( Fig. 103.3 ).
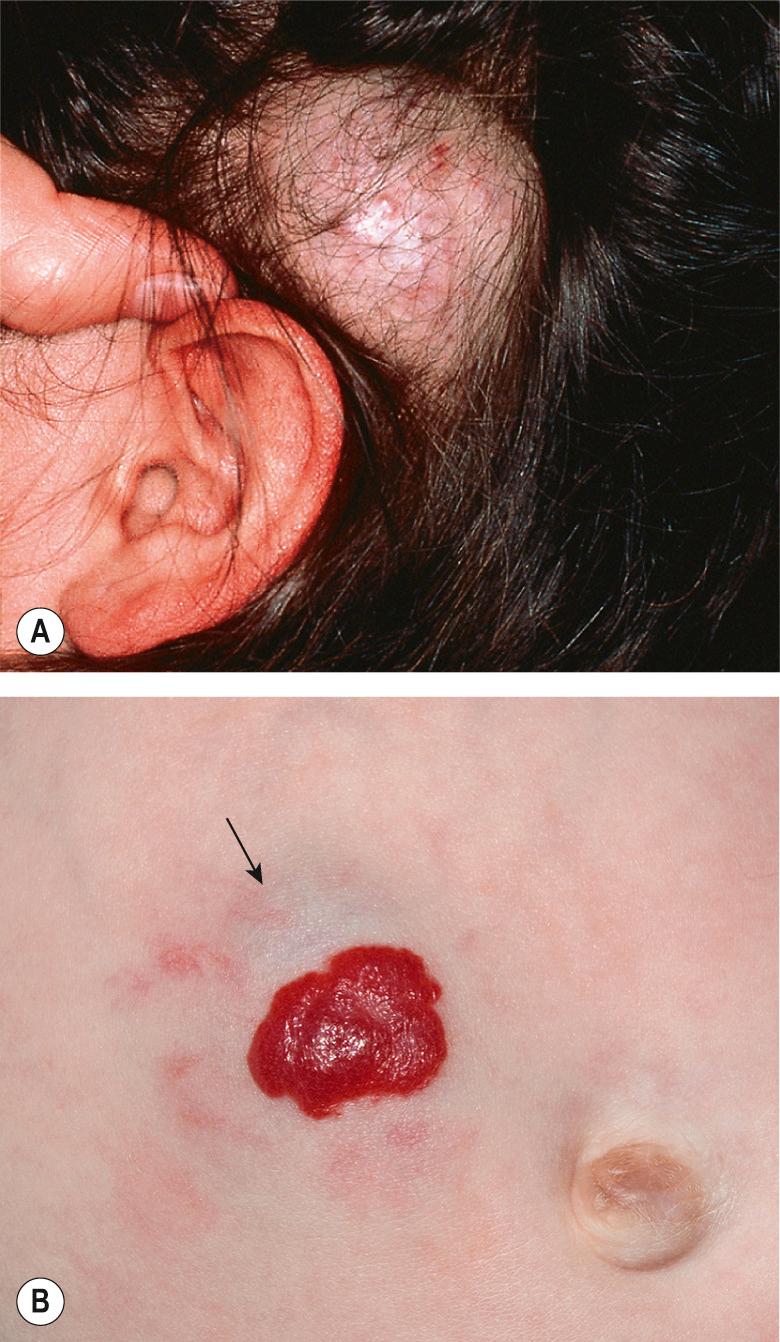
Deep hemangiomas are located in the deep dermis and/or subcutis. They usually are not apparent in the immediate newborn period, often becoming evident a few weeks or even months after birth. They present as warm, ill-defined, light blue–purple masses with minimal or no overlying skin changes, making them more difficult to diagnose than superficial or mixed hemangiomas ( Fig. 103.4 ). The presence of dilated veins or telangiectasias overlying a deep hemangioma provides a clue that the lesion is of vascular origin. Larger deep hemangiomas often have significant arterial blood supply during the proliferative phase, and detection of high flow by Doppler ultrasonography can help to confirm the diagnosis. Mixed hemangiomas have both superficial and deep components, often presenting during the proliferative phase as a well-delineated red vascular plaque overlying a larger, poorly circumscribed violaceous or light blue nodule.
Superficial hemangiomas are the most common type, accounting for ~50–60% of IHs. An additional 25–35% of hemangiomas are mixed and 15% are deep . Approximately 25% of patients have multiple lesions, which are occasionally associated with visceral hemangiomatosis (see below).
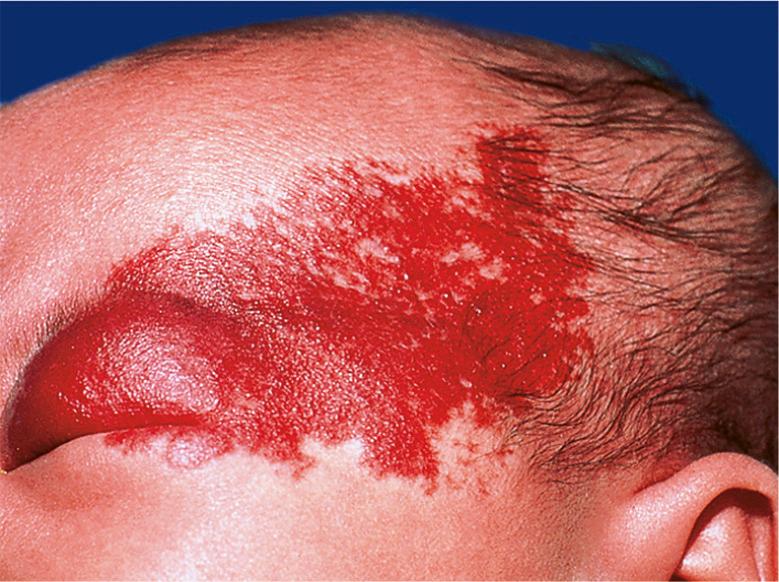
IHs can also be categorized based upon the pattern of involvement, which may help to predict prognosis . The two main pattern subtypes are: (1) focal – arising from a single localized nidus (see Fig. 103.3A, B ); and (2) segmental – covering a broad area or developmental unit in a “plaque-like” manner (see Fig. 103.3C ). Lesions that are difficult to classify are designated as indeterminate . The majority of IHs are focal lesions, and those on the face may show predilection for embryonic fusion lines . The distribution patterns of segmental hemangiomas on the face correlate somewhat with classic embryonic facial prominences, differing the most on the upper face. Investigators have identified four primary segments for facial hemangiomas, S1 to S4 ( Fig. 103.5 ) . Involvement of these segments may be incomplete, and some hemangiomas encompass more than one segment.
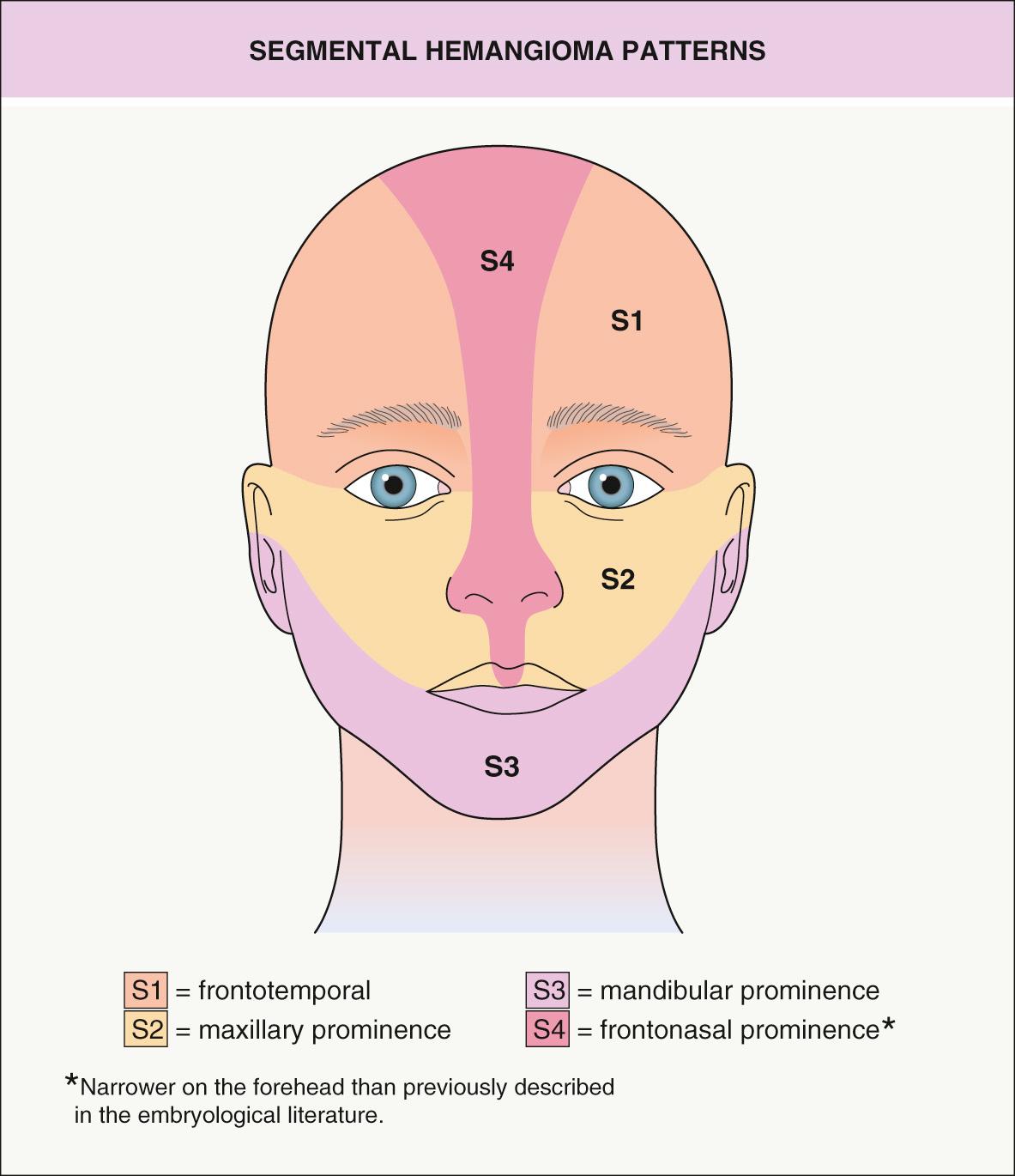
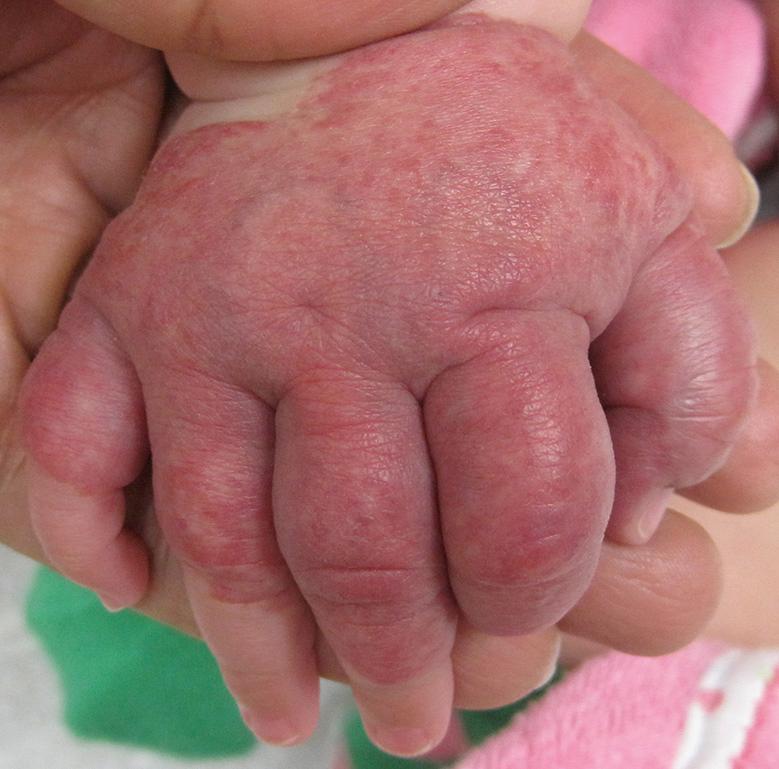
Segmental hemangiomas often begin as broad patches of confluent or reticulated erythema and/or telangiectasias. Within a few weeks, bright red papules and plaques arise within this “field”. Segmental hemangiomas are more likely to be associated with regional extracutaneous anomalies, including PHACE(S) and LUMBAR syndromes (see below) . On the extremities, smaller segmental hemangiomas typically spare the distal digits in a “biker glove” pattern ( Fig. 103.6 ), while larger lesions that encompass the distal digits are associated with a higher risk of extracutaneous anomalies .
Studies have documented the typical growth pattern of IHs. The phases include early proliferation with a rapid increase in size, late proliferation with continued growth at a slower rate, plateau (existence as a distinct phase debated), and involution . Hemangiomas tend to “mark out their territory” early on, subsequently undergoing primarily volumetric rather than centrifugal growth . During the proliferative phase, hemangiomas frequently become warmer and firmer in texture, and the surface of superficial hemangiomas may appear tense. Mixed and deep hemangiomas in the proliferative phase often feel firmer and look larger with crying or activity. Deep hemangiomas tend to proliferate for a longer period of time than do superficial hemangiomas, and the deep component of mixed lesions often continues to grow even after the superficial component has plateaued ( Fig. 103.7 ).
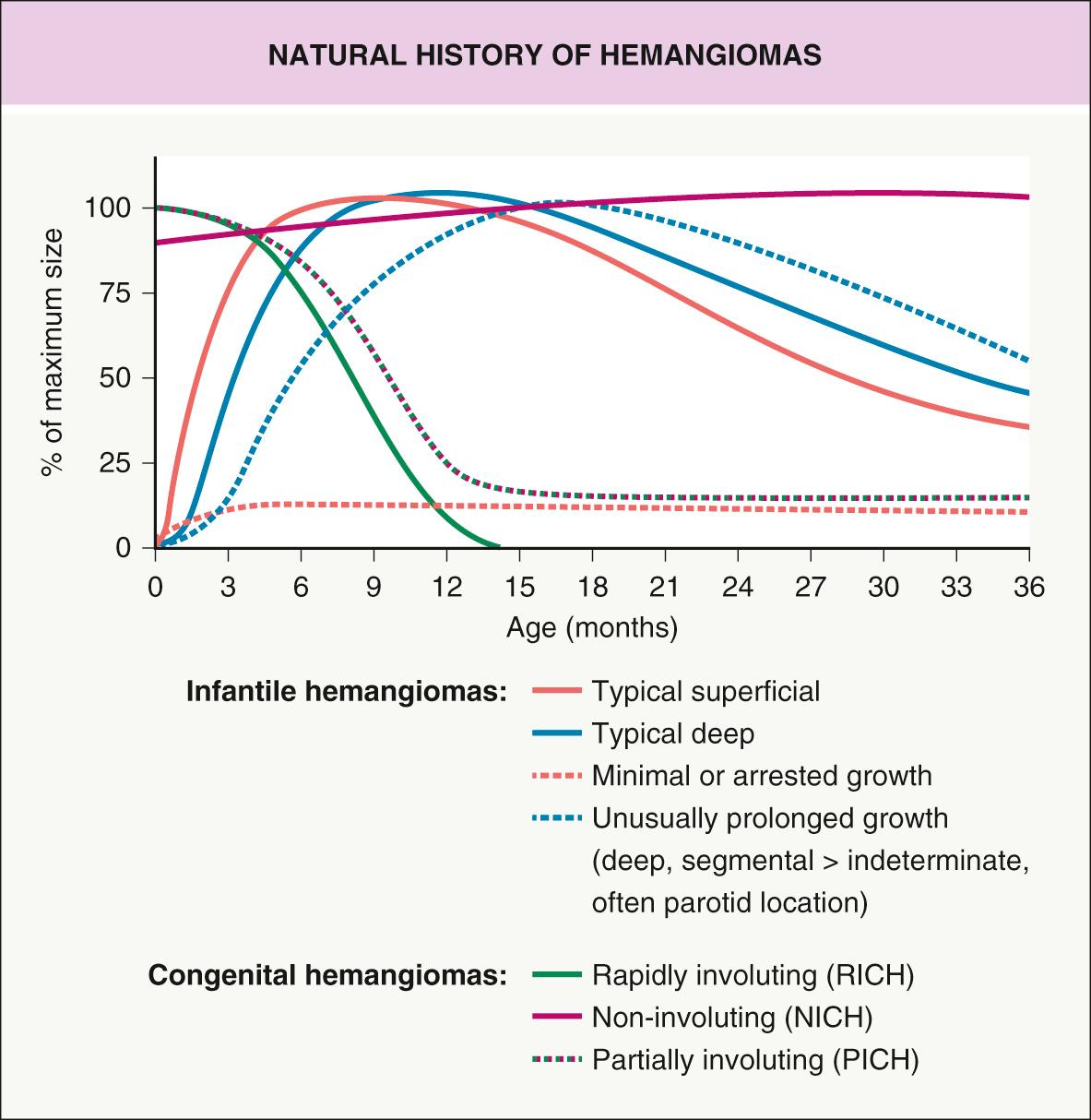
The majority of hemangiomas reach 80% of their final size by the end of the early proliferative phase, which occurs at a mean age of 3 months, and growth is usually most rapid from 5 to 8 weeks of age . The small minority of hemangiomas that grow after 9 months of age tend to have a deep component and/or segmental morphology, and IHs in the parotid gland area are particularly prone to this behavior .
A subset of hemangiomas referred to as infantile hemangioma with minimal or arrested growth (IH-MAG) , “ abortive ”, or “ reticular ” display little or no growth beyond patches of reticulated erythema with coarse and/or fine telangiectasias, often superimposed on a background of pallor ( Fig. 103.8 ) . If present, the proliferative component involves <25% of the surface area, often presenting as small red papules at the periphery . These hemangiomas favor the lower body and may develop recalcitrant ulceration or (if larger and segmental) be associated with LUMBAR or PHACE(S) syndrome (see below) . They are GLUT1-positive and involute at a pace similar to classic IHs, although larger ectatic vessels may persist.
Involution of IHs may begin as early as the first year of life and continues for several years. A color change from bright red to gray–purple ( Fig. 103.9 ) and flattening of the surface are often the earliest signs of involution in superficial lesions. The latter often break apart into smaller islands before clearing. As the tumor involutes, deeper lesions become less firm and the superficial portion may develop a wrinkled appearance. During involution, larger lesions demonstrate less fluctuation of size with crying and activity .
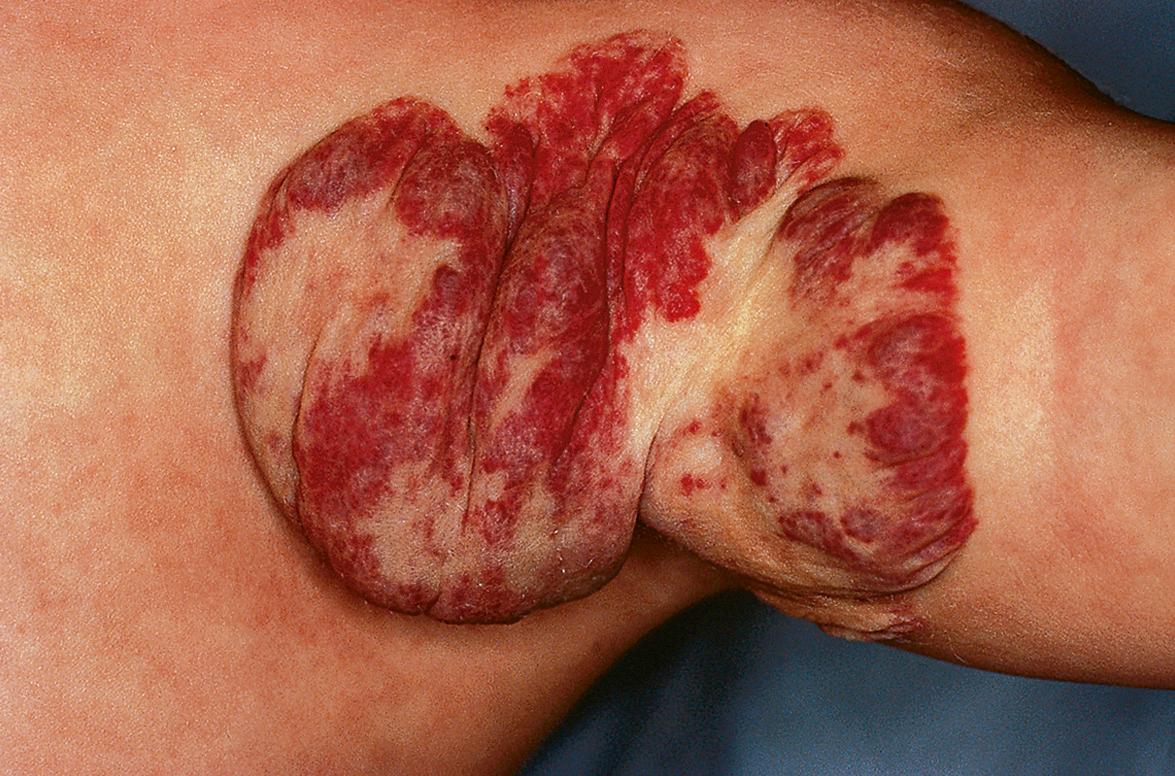
Classic studies on the natural history of untreated hemangiomas demonstrated that 30% of lesions involute fully by 3 years of age, 50% by 5 years, 70% by 7 years, and >90% by 9 years . However, recent studies have concluded that the median age of completed involution is 36 months, with >90% of children showing no further improvement after 4 years of age . Some hemangiomas involute completely, while others leave atrophic, fibrofatty, or telangiectatic residua ( Fig. 103.10 ). Predicting whether the residual lesion will be of cosmetic significance is one of the most challenging aspects of hemangioma management.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here