Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Benign, borderline, and malignant neoplasms can arise from exocrine, endocrine, intraductal, and stromal elements within the pancreas. Additionally, secondary (metastatic) neoplasms and non-neoplastic tumor-like conditions (e.g., mass-forming chronic pancreatitis) can occur. The World Health Organization (WHO) recognizes over 50 types of pancreatic neoplasms. However, only a few of these (adenocarcinoma, cystic neoplasms, pancreatic neuroendocrine tumors [PNETs], and metastases) are encountered with any frequency in clinical practice. This chapter will focus on multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI) as primary diagnostic tools for detection and staging of pancreatic tumors. The roles of additional imaging such as positron emission tomography (PET) and endoscopic ultrasound (EUS) are briefly considered.
Pancreatic ductal adenocarcinoma (PDAC) is the fourth most common cause of cancer deaths for both men and women in the United States. The American Cancer Society estimates that in 2019 approximately 56,770 new cases with 45,000 deaths will occur ( https://www.cancer.net/cancer-types/pancreatic-cancer/statistics ) representing a continued true increase in incidence. Established risk factors for developing PDAC include cigarette smoking, male, African-American, BRCA2 gene positivity, hereditary pancreatitis, cirrhosis, diabetes mellitus, chronic pancreatitis, hypercholesterolemia, obesity, Peutz-Jehgers syndrome, Lynch syndrome, vitamin D deficiency, certain occupations, and carcinogens. Common symptoms include weight loss, jaundice, floating stool, pain, dyspepsia, nausea, and depression. New-onset type 2 diabetes in patients aged older than 50 years may trigger investigation for PDAC. Despite improvements in imaging, surgery, radiation, and chemotherapy, and rapidly emerging molecular understanding, mortality remains high; 23% of patients will be alive at 1 year from diagnosis, whereas 4% will be alive at 5 years.
Ductal adenocarcinoma accounts for 85%–90% of all pancreatic tumors. Of these, 60%–70% arise in the head, 5%–10% in the body, and 10%–15% in the tail. , Up to 22% of tumors can affect multiple regions (diffuse). By the time the tumors are discovered, they usually measure 2–3 cm in diameter, the vast majority being locally advanced. Pancreatic adenocarcinoma elicits an intense desmoplastic response within the stroma surrounding the tumor that encases intrapancreatic blood vessels, explaining why 90% of these masses are hypodense compared with background pancreas and adding to the difficulty of delivering chemotherapeutic agents to the mass. The upstream main pancreatic duct (MPD) may be obstructed, and the surrounding parenchyma is atrophic. In some cases, MPD dilation may be the only finding signaling neoplasm. Tumors in the head of the pancreas will also eventually obstruct the common bile duct (CBD). The tumor spreads through lymphatics, peripancreatic vessels, and nerves. Tumors may also grow along tissue planes into the duodenum, and posterior wall of the stomach. Lesions in the tail of the pancreas infiltrate into the splenic hilum and into the left renal hilum. Distant metastases are most frequently found in the liver and peritoneal cavity. These can be present even in cases where the tumor does not appear locally advanced.
The major blood vessels involved by PDAC are the superior mesenteric artery (SMA), celiac axis (CA), and common hepatic artery (CHA). The major veins involved include superior mesenteric (SMV), splenic, and portal veins (PV). Involved lymph nodes are most often not enlarged. Only distant lymph node disease is a contraindication to resection.
For patients with suspected or newly diagnosed pancreatic adenocarcinoma, the goal of the imaging study is to classify the disease status into one of three categories: resectable, borderline resectable, or locally advanced. Accurate detection of extrapancreatic metastases is essential, and changes the status of the patient regardless of local disease status.
The quality of imaging directly impacts the accuracy of staging; patients deemed unresectable based on non-pancreatic protocol imaging have been shown to actually be surgical candidates. Combined pancreatic and portal venous phase acquired with thin (<1 mm) section intravenous (IV) contrast-enhanced MDCT is the imaging modality of choice. Multiparametric MRI, including noncontrast T1-, T2-, and diffusion-weighted images, followed by gadolinium-enhanced 3D-T1 acquisitions in the pancreatic and portal phases, is reserved for patients with allergy to iodinated contrast. Magnetic resonance cholangiopancreatography (MRCP) is also performed using a heavily weighted T2 acquisition either in 2D with thick slab or a 3D respiratory-triggered acquisition. Biopsy of suspected lesions is achieved with EUS.
The Society of Abdominal Radiology and the American Pancreatic Association called together a panel of experts in imaging, gastroenterology, and surgery to create a template for reporting imaging findings in patients with pancreatic adenocarcinoma. This has been incorporated into the NCCN guidelines for pancreatic adenocarcinoma and supported in a review by the Society of Abdominal Radiology’s disease-focused panel for pancreatic carcinoma. , Specific details of imaging protocols, and staging can be found in those documents.
PDAC most frequently appears as a hypodense mass compared with the background pancreas ( Fig. 60.1 ); 11% of cases may be isoattenuating. Secondary findings include dilation of the upstream pancreatic duct, parenchymal atrophy, loss of “acinar” pattern, and dilation of the CBD when the tumor is located within the pancreatic head.
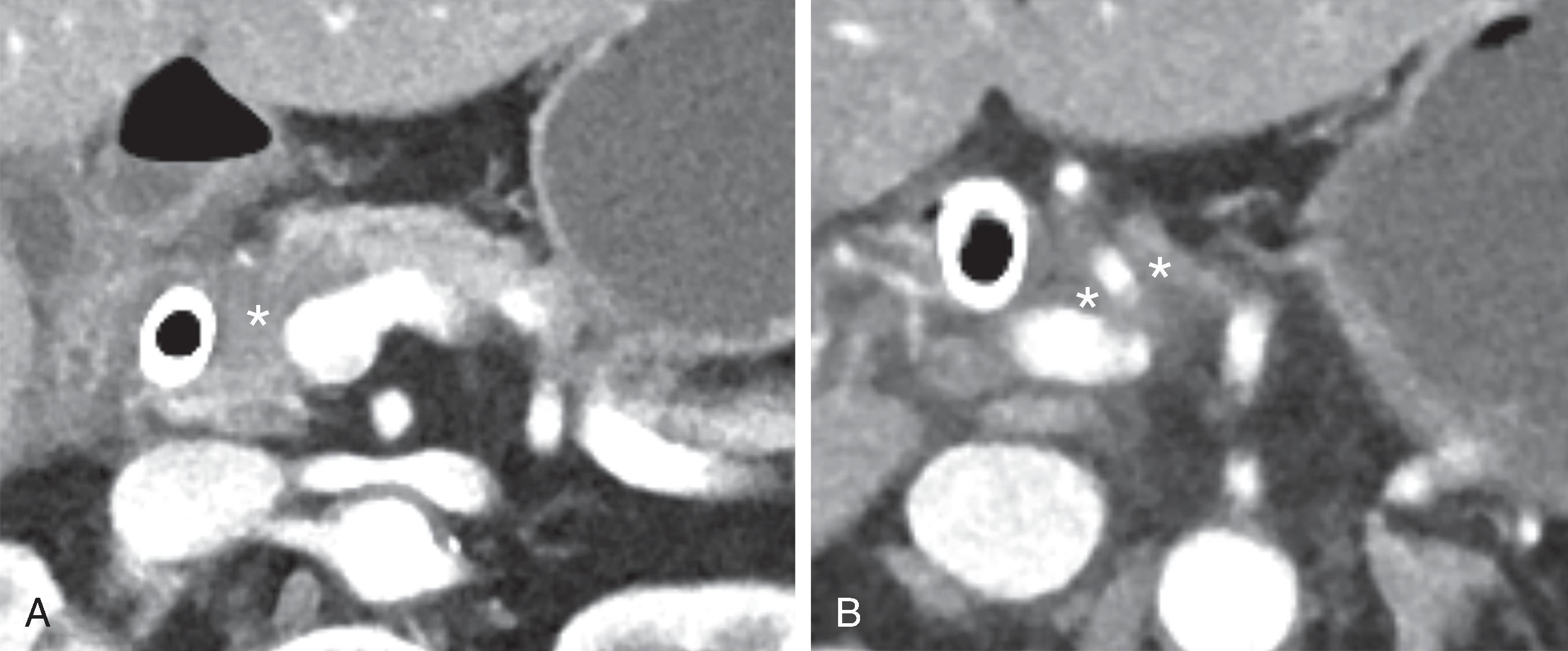
The findings of arterial and venous involvement are well described in the literature and detailed in several publications. , Radiologists today must be able to translate these findings into accurate assessment of resections status. A lesion is considered “resectable” when there is no tumor contact with either CA, SMA, or CHA and there is no contact with the SMV, PV, or contact of <180 degrees without deformity. Pancreatic head lesion is considered “borderline resectable” if there is CHA contact without involvement of the hepatic artery bifurcation, <180-degree contact with the SMA, solid contact with variant arterial anatomy (e.g., replaced right hepatic artery) or SMV or PV contact >180 degrees with deformity but sufficient vein for resection and vein reconstruction, or contact with the inferior vena cava. For body/tail lesions, solid contact with CA of <180 degrees, solid contact >180 degrees without aortic contact and intact gastroduodenal artery is considered borderline resectable. A tumor is considered “locally advanced” when there is >180-degree contact with SMA or CA whether in body or tail or aortic contact, or unreconstructable SMV/PV ( Fig. 60.2 ).
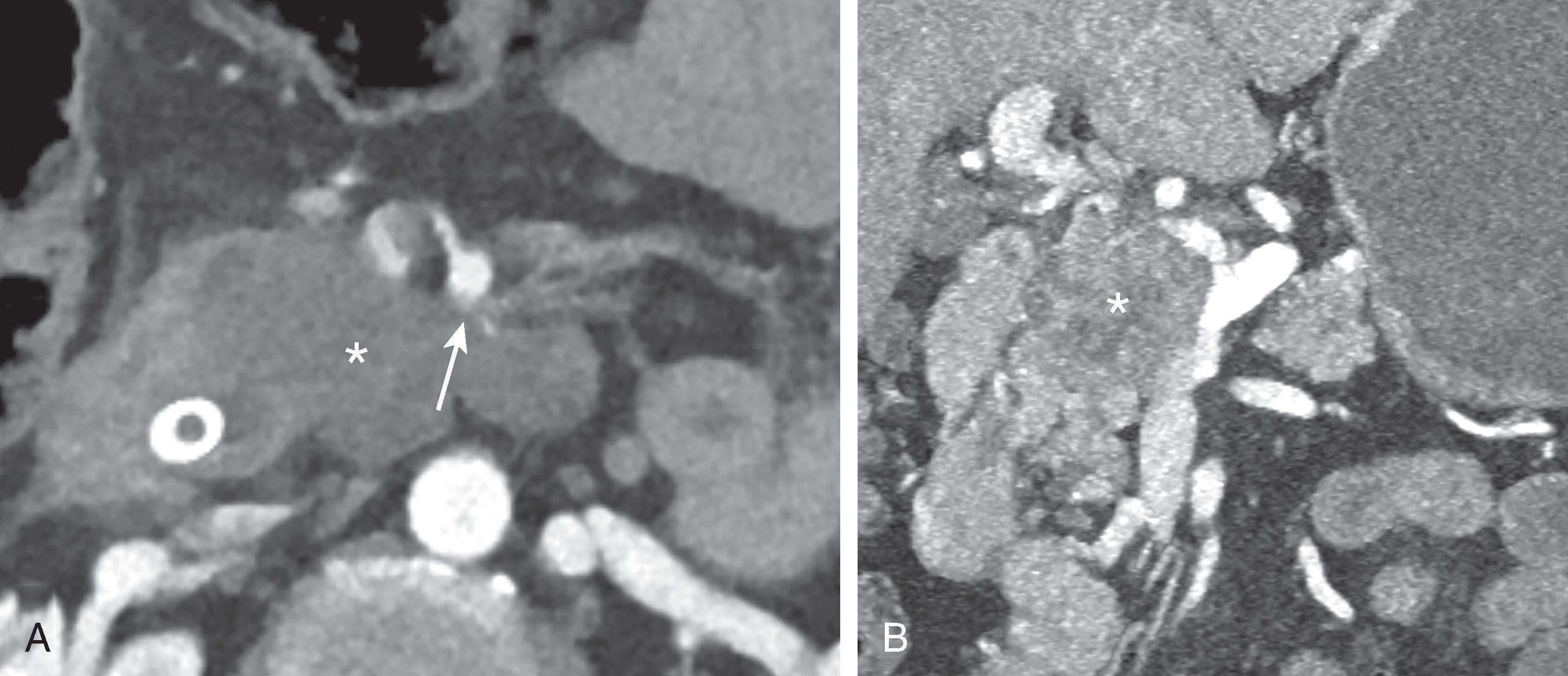
PDAC will frequently invade nerves and lymphatic channels before it encases major vessels. Predictable pathways for perilymphatic and perineural tumor infiltration have been correlated with detailed anatomic dissections. High-quality thin-section imaging is needed to view these changes. When spread along these pathways is suspected, the surgeon should be alerted and neoadjuvant therapy may be appropriate before attempt at resection. , Currently, perineural or perilymphatic extension does not impact current resectability definitions.
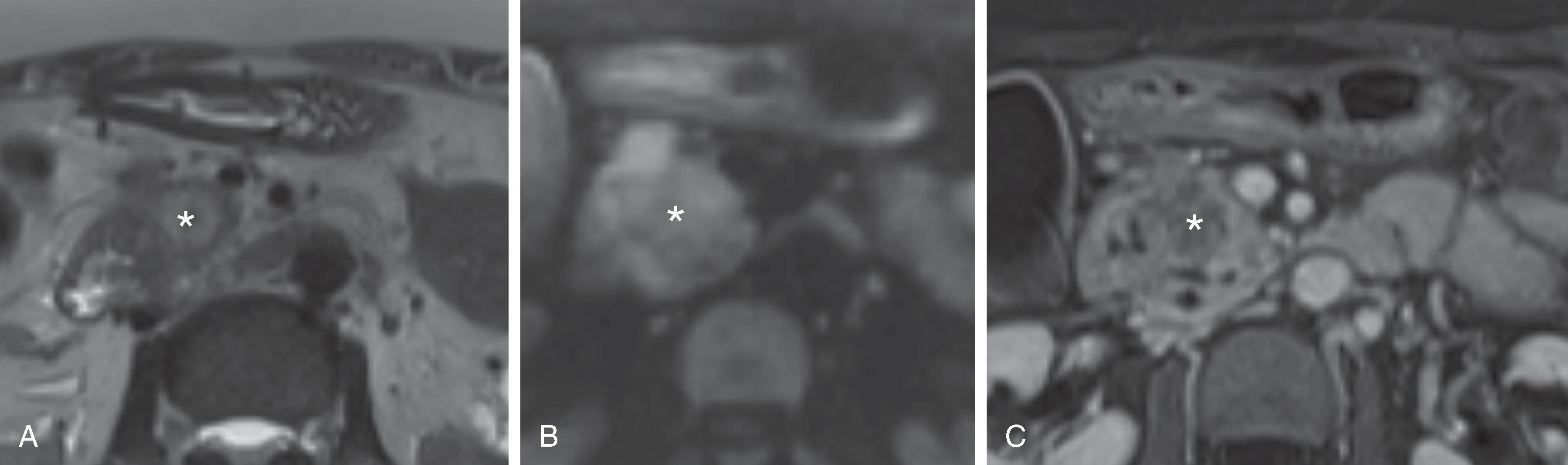
The appearance of the pancreatic mass will depend on the acquisition sequence utilized ( Fig. 60.3 ). As outlined in the preceding section, a multisequence approach is used for a comprehensive evaluation. Because the pancreatic mass elicits a dense desmoplastic stromal response, most masses will have a shorter relaxation time than the surrounding normal parenchyma and therefore appear as a “dark” region. Contrast-enhanced pancreatic phase T1-weighted sequences are most effective in delineating the mass. Fat suppression will increase the conspicuity of the mass, heightening contrast between normal parenchyma, the mass, and the pancreatic duct. Similar to MDCT, PDAC is hypointense compared with background pancreatic parenchyma. Liver metastases are seen as hypointense in the portal phase, but also as brighter than background on T2- and diffusion-weighted sequences. The degree of brightness will be visually less than the spleen. .
Because the gadolinium-enhanced imaging sequences are acquired as a 3D volume, the arterial and venous anatomy can be displayed as MR angiographic images with similar imaging features of vascular involvement and tumor extension as displayed on MDCT. Familiar 3D techniques, such as maximum intensity projection (MIP) and volume rendering, can be employed to create displays of the pathology in any useful plane.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here