Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Colon cancer is a major public health problem in the United States. As a cause of cancer mortality, it ranks third to prostate and lung cancer in men and breast and lung cancer in women. The American Cancer Society estimates that in the year 2019 about 145,600 persons will be diagnosed with colorectal cancer and that about 67,100 will die of the disease. The distribution of this disease varies widely throughout the world. It is common in North America, Europe, and New Zealand, but the incidence is low in South America, Africa, and Asia. The United States has one of the highest incidences of colorectal cancer in the world. Colorectal neoplasms account for about 9% of new cancer diagnoses in the United States. From 1975 to 2013, the incidence rates (per 100,000) per year declined overall in the United States but have increased in the under age 50 group, especially for distal colon and rectal cancers. ,
The distribution of cancer in the large bowel is significant because it affects the diagnostic approach. Approximately 50% of these cancers occur in the rectum and sigmoid colon, within reach of the short flexible sigmoidoscope ( Fig. 43.1 ). The remainder are scattered throughout the proximal colon. The overall incidence of colorectal tumors increases with age and the increase is greatest for proximal neoplasms beyond the reach of the sigmoidoscope. , Also, race and gender can independently predict the location of the cancer. Generally, distal cancers are found more frequently in Asians followed by whites and then blacks.
The wide variation and distribution of colorectal cancer throughout the world is probably related to dietary differences. In general, diets low in fiber, high in saturated fat and animal protein (especially high and prolonged consumption of red or processed meat), and excessive alcohol consumption are associated with a higher prevalence of colorectal cancer, which is inversely related to the incidence of gastric cancer.
Heredity probably plays a role in 5% to 6% of all cases of colorectal cancer. Adenomatous polyposis coli (see Chapter 45 ) is transmitted by the classic mendelian dominant inheritance pattern, and almost all patients with this condition eventually develop colorectal carcinoma. They account for approximately 1% of colorectal cancers. Another 5% occur in patients with hereditary nonpolyposis colorectal cancer (HNPCC), possibly from an abnormality in the genetic material that may be associated with the development of colorectal cancer. DNA testing is advised in families with suspected HNPCC. In both these syndromes, carcinomas tend to occur in the proximal part of the colon and are found at a relatively young age.
Several factors place individuals at a higher-than-normal risk. These include older age, a personal or family history of colorectal polyps or cancer, certain hereditary conditions, certain diets (see above), sedentary lifestyle, obesity, and inflammatory bowel disease (IBD). The incidence of colorectal cancer increases markedly in those older than 50 and peaks in those about 70 years old. First-degree relatives of patients with colon cancer have a twofold to threefold increase in cancer risk, as do patients who have previously been treated for colon cancer.
Patients with chronic ulcerative colitis (UC) have a higher incidence of colorectal cancer. The incidence starts to increase after the disease has been present for about 10 years, and approximately 10% develop cancer for every decade after 10 years of disease activity. These cancers tend to be more uniformly distributed throughout the colon than in patients without UC, often toward a more proximal location and with a scirrhous appearance. Patients with pancolitis are predominantly affected. Sclerosing cholangitis is associated with a strong risk of developing colon cancer in patients with IBD; consumption of nonsteroidal anti-inflammatory drugs (NSAIDs) exerts a protective influence. The incidence, characteristics, and prognosis of colorectal carcinoma complicating Crohn’s disease are similar to the features of cancer in UC, including young age, multiple neoplasms, long duration of disease, and more than a 50% 5-year survival rate.
At least 70% of colorectal carcinomas start as benign adenomas that undergo malignant transformation (see Fig. 43.1 ). Up to 30% of cancers arise from a de novo sequence—that is, carcinoma developing in normal mucosa without an antecedent adenoma. Larger adenomas (>1 cm) pose a greater cancer risk and need to be removed to prevent subsequent development of cancer. Cancers that develop in patients with IBD generally arise from areas of high-grade mucosal dysplasia rather than from adenomatous polyps. Therefore, in the surveillance of these patients, blind biopsy specimens are often taken throughout the colon in an attempt to detect severe and persistent dysplasia.
Colorectal cancer is a slow-growing malignancy, requiring a period of 7 to 10 years for a benign adenoma to undergo malignant transformation. Colorectal cancer is sufficiently common in the Western world that routine screening is recommended before symptoms appear. The American Cancer Society recommends that starting at age 45 (“qualified” recommendation), men and women at average risk for developing colorectal cancer should follow one of the screening options listed below.
Fecal occult blood test (FOBT) or fecal immunochemical test (FIT) every year
Multitarget stool DNA test every 3 years
FOBT or FIT every year, plus flexible sigmoidoscopy every 5 years
Colonoscopy every 10 years
Computed tomography colonography (CTC) every 5 years
Flexible sigmoidoscopy every 5 years
The American Cancer Society no longer recommends double-contrast barium enema (DC-BE) as an acceptable screening option, but the American College of Radiology lists the DC-BE and magnetic resonance colonography (MRC) as “may be appropriate” for average-risk and moderate-risk but not for high-risk individuals. The European Society of Gastrointestinal Endoscopy and European Society of Gastrointestinal Radiology consider CTC as the examination of choice for the diagnosis of colorectal cancer. Patients at increased risk for colon cancer (see risk factors above) should be tested at an earlier age than the average-risk patients. For these patients, optical colonoscopy is preferred because of its ability to obtain biopsies to detect dysplasia. After incomplete colonoscopy, CTC is usually appropriate for colorectal cancer screening for individuals at average, moderate, or high risk.
Symptomatic colorectal cancer most often presents as bleeding. The severity of bleeding may range from the presence of occult blood in the stool to the passage of bright red blood per rectum to the development of iron deficiency anemia. The patient’s symptoms are usually related to the location of the tumor. An examining finger or a rigid proctoscope can reach approximately one-third of carcinomas in the rectum. About 50% of large bowel cancers are in the left half of the colon and can be reached with a flexible sigmoidoscope. Left-sided tumors often present with bright red rectal bleeding or constipation caused by obstruction. The remaining tumors are scattered throughout the colon. Cecal carcinomas constitute about 10% of the entire group and are most likely to present as iron deficiency anemia caused by chronic blood loss. Other frequent symptoms of colorectal cancer include abdominal pain, which may be related to the development of obstruction or to tumor invading adjacent tissues. Patients may also present with a change in bowel habit or nonspecific symptoms such as weight loss or fever.
Radiology plays a critical role in the diagnosis and management of patients with colorectal cancer. Single-contrast barium enema or DC-BE is no longer considered a primary screening tool. , It is a time-consuming and technically demanding procedure not well suited to screening. It may be used as a method for diagnosing symptomatic colorectal cancer, and single-column studies should be considered only for detecting complications, such as obstruction or perforation. Imaging using multidetector-row CT (MDCT), CTC, magnetic resonance imaging (MRI), and/or transrectal ultrasound (TRUS) plays an important role in tumor detection and staging. Treatment decisions are often based on results from positron emission tomography and CT (PET/CT). Radiology plays a significant role after treatment for colorectal cancer in the detection of recurrent disease, local and distant metastases, and metachronous colon tumors.
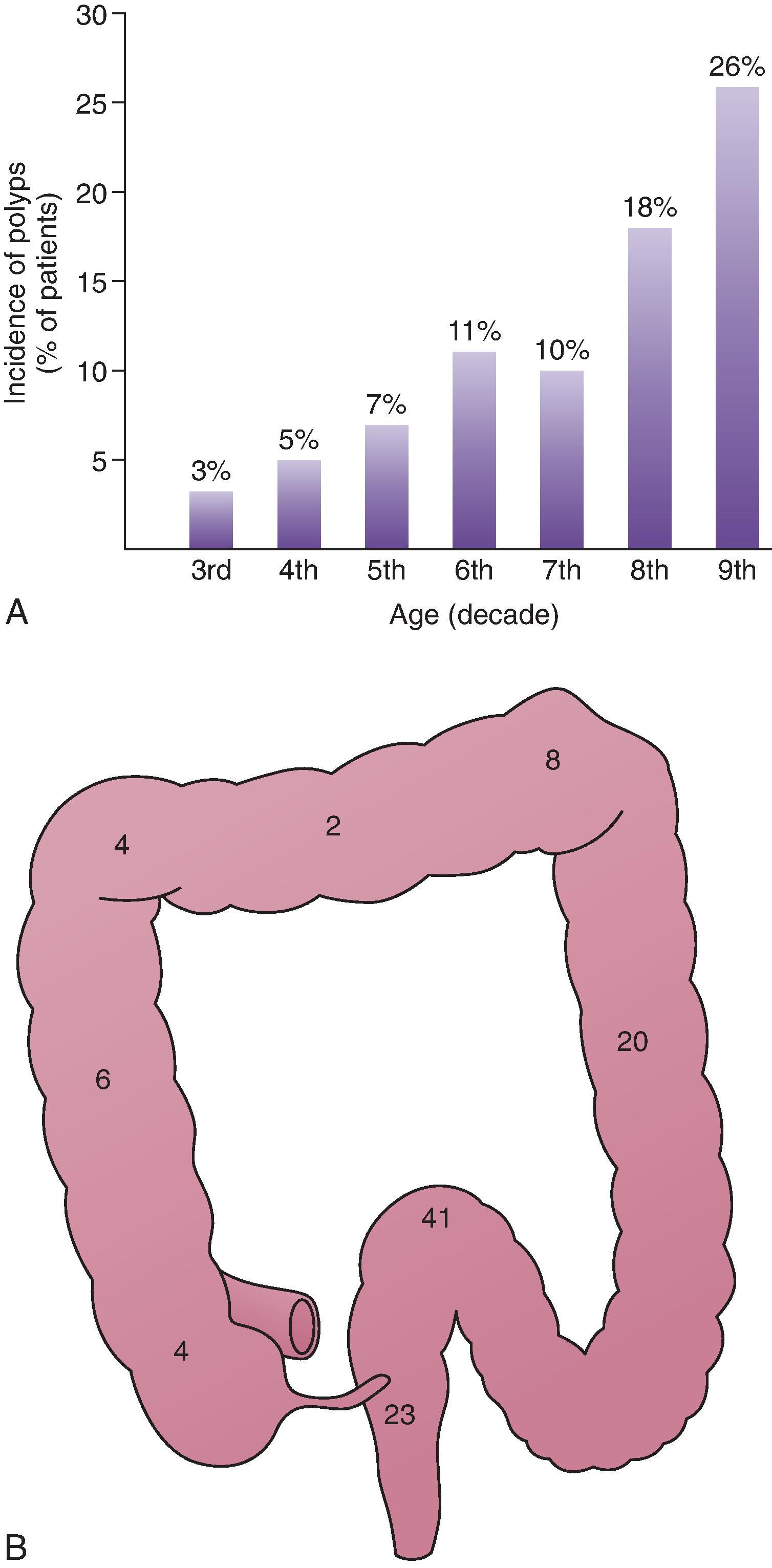
Benign tumors of the colon are extremely common and usually asymptomatic. These polyps are found in 10% to 12.5% of patients studied with DC-BE, and the incidence rises dramatically with increasing age ( Fig. 43.2A ). Polyps occur most frequently in the left colon and are scattered throughout the remainder of the transverse and right colon ( Fig. 43.2B ). As people age, there is a shift in the incidence of polyps to the right side of the colon, coinciding with a shift in the distribution of colon carcinoma to the right. ,
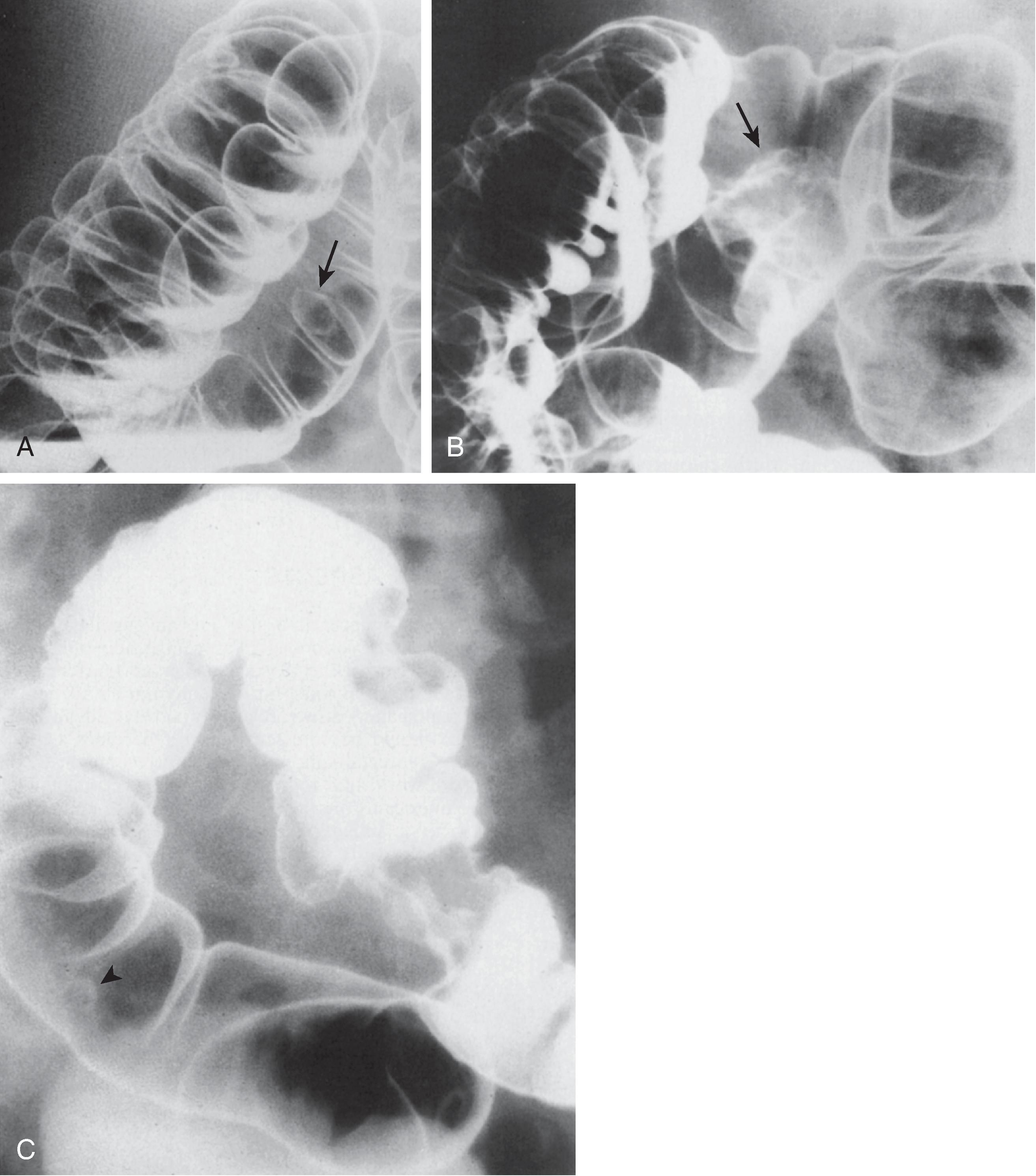
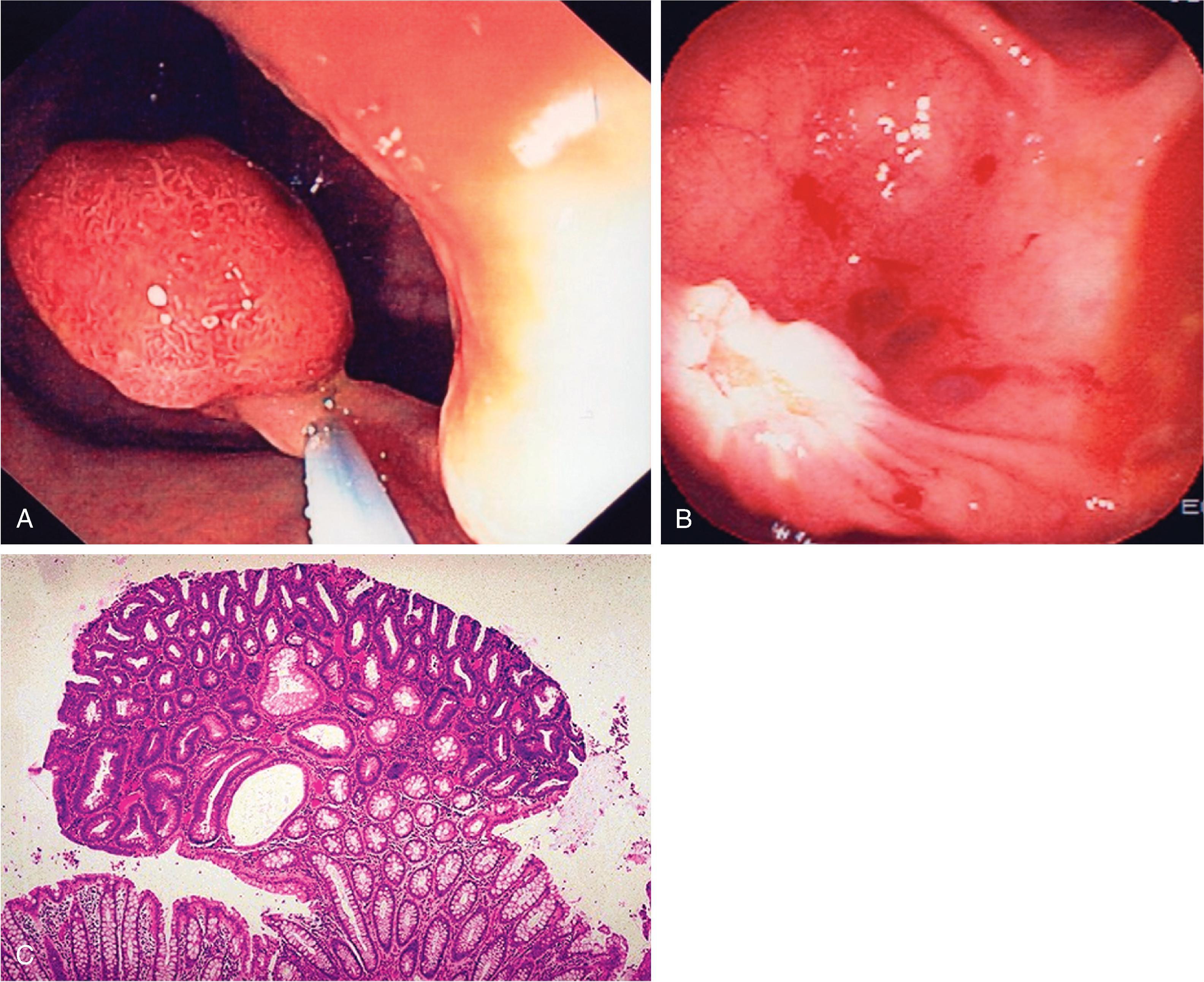
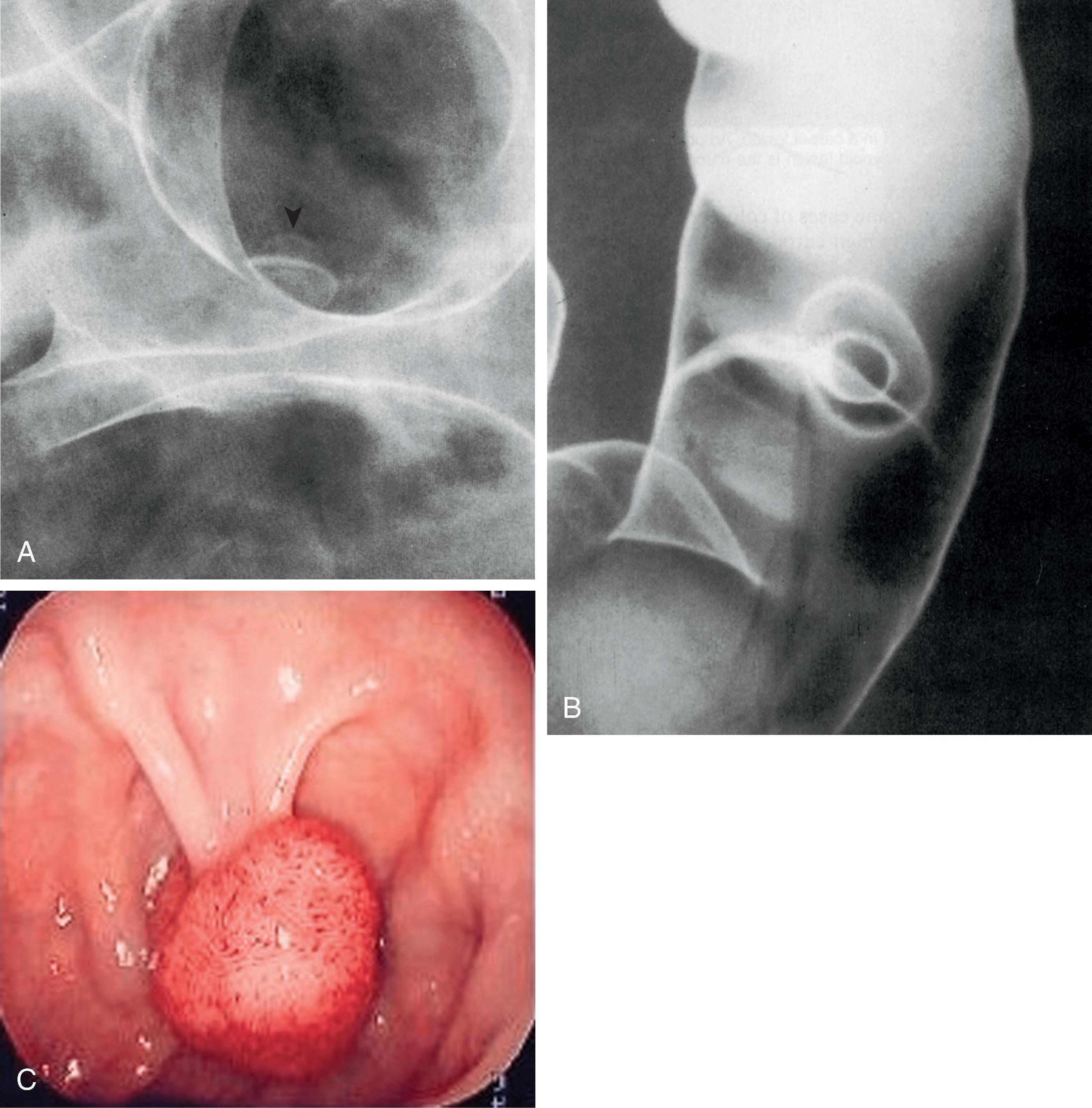
The term polyp refers only to a focal, protruded lesion within the bowel. In general terms, a polyp may be neoplastic or non-neoplastic. Most non-neoplastic polyps are inflammatory or hyperplastic. They are generally small and usually occur in the distal colon. Neoplastic polyps may represent true neoplasms of any component of the bowel wall. Epithelial neoplasms—adenomas—are the most important because they serve as precursors to colorectal carcinoma. The demonstration of a polypoid lesion on a DC-BE does not necessarily provide information regarding its pathologic nature. Whenever possible, polyps larger than 1 cm in diameter must be removed, preferably by colonoscopic polypectomy ( Fig. 43.3 ), because of the increased risk of harboring a malignancy. Many gastroenterologists also remove polyps larger than 5 mm.
Most colonic adenomas are tubular adenomas. However, tubular adenomas have various degrees of villous change and, as a polyp becomes larger, the degree of villous change increases. At the other end of the spectrum, some polyps are villous adenomas because the surface consists of frondlike structures arising from the base of the mucosa. Thus, there may be a transition from tubular adenoma to tubulovillous adenoma to villous adenoma. Any adenoma with more than 75% villous change should be referred to as a villous adenoma. In general, the risk of carcinoma is related to the proportion of villous change in an adenoma.
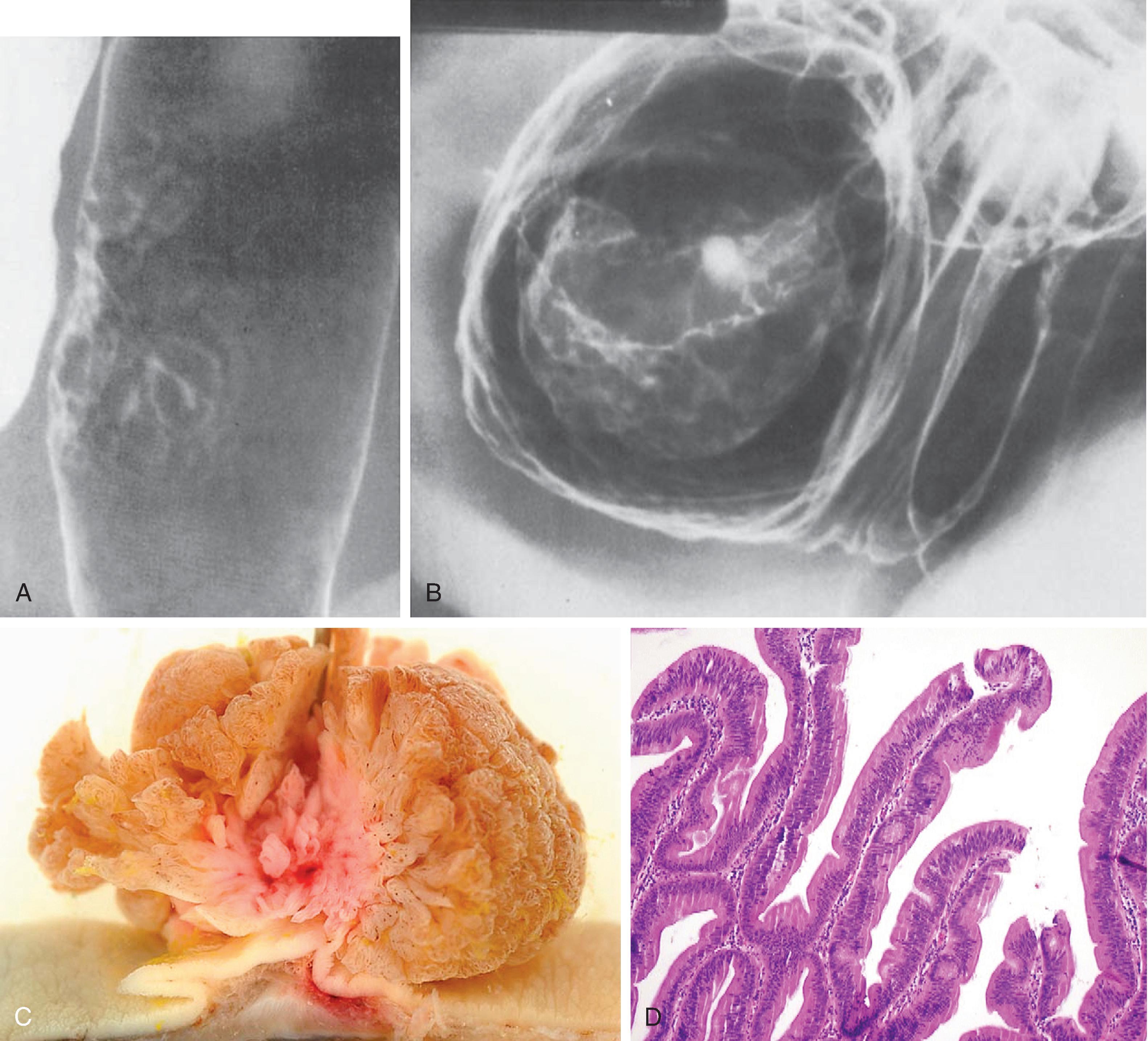
In small adenomas, it may be impossible to distinguish villous change radiologically. However, as an adenoma becomes larger and the proportion of villous change increases, a reticular or granular mucosal surface may be seen ( Fig. 43.4A ). Large villous adenomas have an atypical radiologic appearance. They are large, bulky polypoid masses with barium caught between the frondlike protrusions, producing a lacy surface pattern ( Fig. 43.4B ). Large villous tumors should be removed completely because malignant degeneration may occur in only a portion of the tumor, and random biopsy sampling is unreliable for the detection of malignant change.
Other non-neoplastic polyps may also be found in the colon. These include juvenile polyps, which may be single or multiple and may be found in adults and in children. Hamartomatous polyps are found in patients with Peutz-Jeghers syndrome, and inflammatory polyps are found in patients with Cronkhite-Canada syndrome (see Chapter 45 ).
A polyp may be demonstrated as a radiolucent filling defect, contour defect, or ring shadow because it is simply a protrusion of the mucosa into the bowel lumen (see Chapter 36 ). Polyps can be distinguished from fecal residue by the fact that the latter is mobile and usually found on the dependent surface in the barium pool. In addition, several features may suggest that the filling defect represents a true polyp. These include the bowler hat sign ( Fig. 43.5A ) and Mexican hat sign ( Fig. 43.5B ). , Villous adenomas may have a reticular or granular surface because of barium trapped between the fronds of the tumor. Some hyperplastic polyps may not appear as smooth sessile lesions measuring 5 mm or less, but as larger lobulated lesions that cannot be distinguished morphologically from adenomatous polpys. Rubesin and colleagues have used the term carpet lesion to describe a flat lobulated lesion that is manifest primarily as an alteration in the surface texture of the bowel. These lesions may be large and are mostly tubular adenomas with varying degrees of villous change. In some cases of colorectal carcinoma, the background of a benign carpet lesion can be recognized.
Benign lesions of the colon, such as hyperplastic polyps or adenomatous polyps, are usually not examined by CT or MRI unless CTC or MRC is used (see Chapter 38 ). They are demonstrated by barium examinations or by an endoscopic technique and are seen during CT or MRI examinations only incidentally. These techniques are used only if these benign tumors become large enough to have a high likelihood of harboring a malignancy. Benign polyps appear as sessile soft tissue masses that protrude into the lumen of the bowel on axial images ( Fig. 43.6 ). They are usually detected only if the patient has undergone colonic cleansing or if the polyp is large.
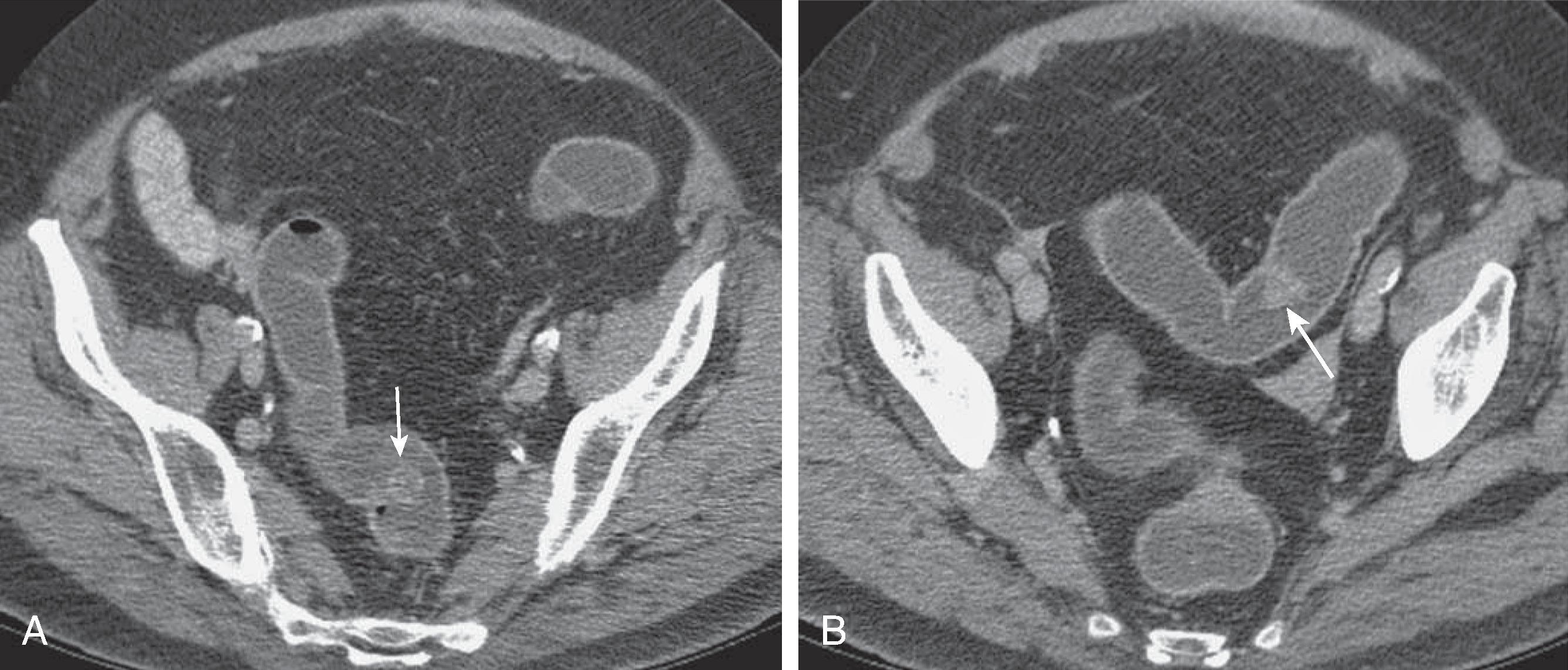
Kim and colleagues have suggested that villous adenomas have a characteristic CT appearance based on their high mucus content. The CT features of villous adenomas include homogeneous water density (<10 HU) occupying more than 50% of the lesion and an eccentric location on the luminal side of the mass. No air-fluid level is seen, and the lesion should not have a round cystic configuration. Because of their malignant potential, larger villous adenomas should always be staged by MRI or TRUS if located in the rectum.
On MRI scans, benign polyps appear as low-intensity structures on T1-weighted spin-echo sequences. If there are many mucin-producing cells, the signal intensity of the polypoid mass with extracellular mucin increases on T2-weighted sequences significantly. TRUS is used for assessment of sessile polyps large enough to have an increased risk for malignancy. Because TRUS can best demonstrate the various layers of the colonic wall, it can reveal the depth of invasion. If the intraluminal component is large, false tumor measurements and erroneous depictions of the surface of the mass can occur because of compression of the tumor by the transrectal probe.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here