Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Spinal vascular diseases consist of spinal vascular malformations, spinal aneurysms, and spinal cord arterial ischemia. They are relatively rare but should be kept in the differential diagnosis for spinal tumors, demyelinations, or compressive myelopathies. As for spinal vascular malformations, there have been various nomenclatures or classifications. Spetzler proposed a classification based on anatomic and pathophysiologic characteristics, and he subdifferentiated extradural arteriovenous fistulae (AVF), intradural dorsal/ventral AVFs, extradural-intradural arteriovenous malformations (AVMs), intramedullary AVMs, and conus medullaris AVMs. The Bicetre group proposed a classification based on genetic and hereditary points of view that comprised three main groups: genetic hereditary lesions, genetic nonhereditary lesions, and single lesions reflecting an incomplete expression of a genetic condition. The majority of spinal vascular malformations fall into this third group and can be subclassified, depending on their feeding artery, into dural AVFs, spinal cord pial AVMs (nidus or fistulous), and paravertebral AVFs. Clinical manifestations may be due to hemorrhage, space-occupying effects, venous congestion, and (rarely) arterial steal.
SDAVFs refer to a shunting lesion between dural branches of the segmental artery and a radicular vein. They are the most common form (70%) of spinal vascular malformation. SDAVFs are presumed to be acquired lesions, similar to their cranial counterpart, the cranial dural AVF. SDAVFs are most frequently found in the thoracolumbar region of elderly men. Most SDAVFs develop in the lateral epidural space near the dural root sleeve. Venous drainage is directed retrogradely into perimedullary veins, which is responsible for the following hemodynamic sequence: spinal venous pressure increase due to arterialization, spinal cord congestion, reduced tissue perfusion, and finally cord ischemia. Patients with SDAVFs usually present with progressive myelopathies, and additional autonomic symptoms (e.g., bladder, bowel, and erectile dysfunction) are seen in the later course of the disease.
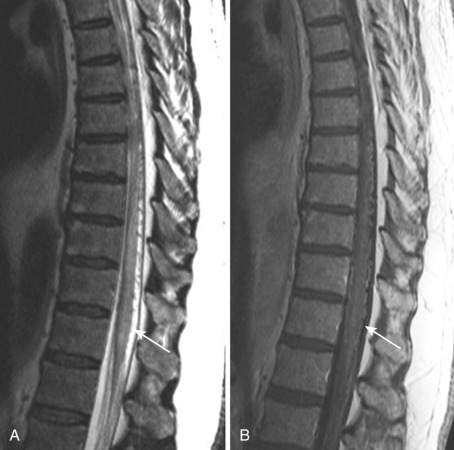
On computed tomography (CT), spinal cord swelling and dilated perimedullary venous enhancement may be noted; however, CT is not a comprehensive tool for the diagnosis of spinal vascular diseases. Magnetic resonance imaging (MRI) is the imaging method of choice because it can demonstrate the congestive edema of the spinal cord, breakdown of the blood–spinal cord barrier due to chronic venous ischemia, and the dilated perimedullary vessels. Thus on MRI the typical imaging findings are an enlargement of the spinal cord with central confluent T2 hyperintensity over multiple segments, patchy contrast enhancement over multiple segments, and dilated perimedullary veins on the dorsal surface of the spinal cord, with multiple serpentine flow voids on T2 that enhance on postcontrast T1. First-pass contrast-enhanced MR angiography may show the level of fistula and feeders, which is very useful guidance in catheter angiography. Most importantly, in equivocal cases this technique can identify with fairly high sensitivity and specificity the presence of a shunt due to early venous filling. Selective spinal catheter angiography is still the gold standard for evaluation of SDAVFs regarding confirmative diagnosis, identification of the exact level, precise ascertainment of the location of the anterior and posterior spinal arteries, and feasibility for embolization. Venous congestion can be demonstrated on angiogram as a delayed washout of contrast from the anterior spinal artery. MRI differential diagnosis includes other spinal vascular malformations (perimedullary and filum terminale AVFs may mimic dural AVFs), CSF pulsation artifact, hypervascularized spinal cord tumors (hemangioblastomas or paragangliomas), and serpentine nerve roots and venous engorgement related to spinal stenosis.
Both endovascular embolization and open surgery are available for treating these lesions, with the aim being to obliterate the most distal part of the feeding artery and the proximal draining vein. In our institution, embolization is usually attempted before surgery, because the confirmatory diagnosis requires spinal angiography. Liquid embolic agents, mostly glue, are used as embolizing agents, and penetration of glue into the proximal vein is necessary for complete occlusion; otherwise (i.e., in ligation types of embolization) the fistula will recur. In most cases, myelopathic symptoms stabilize after successful treatment. However, despite the resolution of cord edema, neurologic improvement is not always complete, especially when the treatment was delayed, because treatment outcome depends on symptom duration. Autonomic dysfunctions rarely improve, even after successful treatment ( Figs. 33-1 and 33-2 ).
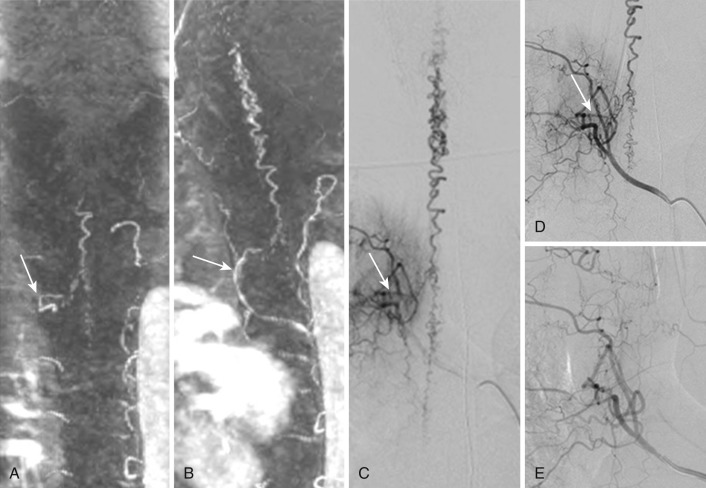
SCAVMs are intradural shunting diseases comprising about 20% to 25% of all spinal vascular malformations. They can be subdivided into spinal cord (pial) perimedullary or intramedullary, nerve root, and filum terminale lesions according to their anatomic location and resultant feeding artery. The gross angioarchitecture at their transition from artery into vein can be either of a nidus or fistulous type. The AVMs are fed by radiculomedullary arteries or radiculopial arteries and drained by intramedullary spinal veins, perimedullary veins, and/or radicular veins.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here