Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Local flap procedures can offer patients excellent options for the repair of soft tissue defects. After proper design and execution, the long-term results can be very satisfactory. Regardless of the local flap selected, proper soft tissue techniques are vital to the success of the procedure. A well-designed local flap may be compromised if wound closure is poorly performed. In contrast, even lengthy suture lines can result in favorable scars with the implementation of judicious skin undermining and proper eversion of wound edges. In addition, the use of newer dissolvable sutures and fibrin-based tissue sealants has broadened the options for repair of skin incisions.
Proper wound closure techniques aid the body’s natural process of wound healing. Thus it is important to understand the physiology of wound healing. The wound healing process occurs in three distinct phases: (1) the inflammatory phase, (2) the proliferative or granulation phase, and (3) the differentiation or maturation phase.
The inflammatory phase (days 0–5) begins immediately after wounding and within 1 to 2 hours; there is a release of vasoactive substances including histamine, serotonin, and cytokines. These substances cause an increase in local vessel permeability, leading to an increased exudation of plasma. The leaky capillaries allow cells such as T lymphocytes, leukocytes, neutrophil granulocytes, monocytes, and macrophages to reach the wound area. Neutrophils (polymorphonuclear leukocytes) predominate in the wound during the first 48 hours after wounding. The polymorphonuclear leukocytes provide a nonspecific cellular defense that is the initial protection against wound infection. The nuclei of neutrophils contain proteolytic enzymes that facilitate cleaning of the wound and phagocytosis of bacteria. After the neutrophils, macrophages start to migrate into the wounded area and predominate by day 4. The macrophages’ main role is to phagocytose debris and to digest bacteria. During the fourth day of wound healing, fibroblasts begin to appear. Throughout this early phase of wound healing, the tensile strength of the wound is minimal and is the result of the fibrin coagulum in the wound bed.
In parallel with the changes occurring deep within the wound, the superficial epithelium also undergoes changes. The epidermis adjacent to the wound edge begins to thicken within 24 hours after injury. Basal cells at the margin of the wound release their attachment to the dermis, enlarge, and migrate across the wound surface to cover the wound. This is possible because the cells undergo a series of rapid mitotic divisions, resulting in migration of the new cells by moving over one another until the wound bed is completely covered by epithelium. Re-epithelialization is usually complete in less than 48 hours in the case of well-approximated wounds.
When a wound becomes infected, there is a failure to progress from the first to the second stage of wound healing. Bacteria or foreign bodies within the wound promote the persistence of neutrophils. With continued neutrophil degranulation and prolonged phagocytosis, there is an accumulation of partially digested material, which is the origin of pus. Furthermore, there is delayed synthesis of critical structural proteins such as collagen. The infected wound will therefore not progress to maximum structural integrity until the infection has resolved.
In the absence of significant infection or wound contamination, the second stage of wound healing begins. The proliferative or granulation phase (days 6–14) is characterized by the formation of granulation tissue in the wound. Granulation tissue consists of a mixture of cellular elements, including fibroblasts and inflammatory cells, along with newly formed capillaries contained within a matrix of collagen, fibronectin, and hyaluronic acid. Early in the second phase, there is a rapid increase in the numbers of fibroblasts. The increase is secondary to an influx, as well as in situ production of fibroblasts. Structural proteins required for wound repair are primarily synthesized by fibroblasts. Most importantly, fibroblasts produce large quantities of collagen, which make up the majority of the extracellular wound matrix. It is this matrix that is responsible for the tensile strength of scar tissue. The collagen is initially deposited in a disorganized fashion. Through cross-linking, the individual collagen fibrils are subsequently reorganized into regularly organized bundles. The new collagen array is oriented along the lines of mechanical stress in the healing wound. The process of fibroblast proliferation and synthetic activity is termed fibroplasia . Revascularization of the wound proceeds in parallel with fibroplasia. Capillary buds sprout from blood vessels adjacent to the wound and extend into the wound space. With continued growth, the new vessels eventually branch at their tips and join to form multiple capillary loops. With the formation of capillary loops, blood flow recommences. New sprouts then extend from these loops to form an active capillary plexus.
The differentiation, or maturation, phase is the third segment of wound healing. This occurs from day 15 to 1 year postoperatively and beyond. During this period, there is a gradual egress of fibroblasts and macrophages. Overall, the wound becomes less vascular. Collagen fibers progress on the continuum from disarray to organization. As the collagen becomes more organized, wound strength continues to improve; 45% of normal wound strength is achieved by day 70 and 50% by day 120. In general, with optimal wound healing, scar tissue strength approaches 80% of the original tissue strength before wounding. Many factors contribute to normal wound healing; these are listed in Table 4.1 . An understanding of these factors helps with successful primary wound closure.
| Local Factors | General Factors |
|---|---|
| Blood supply | Age |
| Denervation | Endocrine function (pancreas, thyroid) |
| Fluid collection | Drug therapy (anti-inflammatory, cytotoxic) |
| Infection | Sepsis |
| Previous or concurrent irradiation | Major organ failure (pulmonary, cardial, hepatic, renal) |
| Mechanical stress | Obesity |
| Surgical technique | Malignant disease |
Investigators have used their understanding of wound healing science to guide novel therapies. Lee et al showed that the use of laser therapy (intense pulsed light) or microneedle techniques could increase collagen deposition throughout the wound healing response. An increase in collagen deposition tended to result in improved scar appearance and overall wound healing. Other investigators have shown a decrease in the incidence of scar hypertrophy and an improvement in wound healing by the use of lasers to treat scars after surgery. Kim et al showed that three postoperative treatments of suture line scars with the erbium; glass laser (1550 nm) dramatically improved the appearance of thyroidectomy scars.
The pharmacologic action of local anesthetics is to block nerve impulses by disrupting the permeability to sodium during an action potential. The interval between an anesthetic injection and its influence on the action potential is dependent on the pharmacokinetics of the drug used and the dosage administered. There are significant differences in potency and duration between various anesthetic agents. An agent’s potency and length of action depend on its level of hydrophobicity. When the agent is mixed with epinephrine, the resulting vasoconstriction increases the time required for clearance of the agent. This reduction in clearance rate increases the agent’s duration of action and decreases the total dosage required to achieve an effective nerve block. Many surgeons use anesthetics containing epinephrine for the added benefit of improved hemostasis.
The core chemical structure of local anesthetics is an amine connected to an aromatic end. The amine end is hydrophilic, and the aromatic end is lipophilic. Changing the amine or aromatic end alters the pharmacokinetics of the drug. There are two classes of local anesthetics based on variations at the amino end: amino amides and amino esters. The class of amino amides includes lidocaine, mepivacaine, and bupivacaine. The amino esters group includes tetracaine, cocaine, and benzocaine.
Lidocaine is the most frequently used infiltrative local anesthetic. It is available in a variety of concentrations. A 1% solution is commonly used with or without epinephrine. With a rapid onset of action, it is an ideal choice for simple soft tissue surgery. Bupivacaine has a longer duration of action than lidocaine and thus may be more appropriate for complex wounds requiring prolonged repair times. The author prefers a 1:1 mixture of 0.5% bupivacaine (Marcaine) and 1% lidocaine with a total concentration of 1:100,000 epinephrine. If a surgical procedure is to occur without general anesthesia, 10% by volume of an 8.4% bicarbonate solution is included in the anesthetic mixture. This mixture produces an excellent rapid-onset anesthesia with long-term analgesia. The addition of bicarbonate buffers the solution and decreases injection discomfort.
The method of infiltration will vary by the site of injection. Wherever possible, selective nerve blockade should be employed to reduce the discomfort of diffuse injection. Infraorbital, supraorbital, and mental nerve blocks provide excellent regional anesthesia for facial soft tissue procedures. For diffuse infiltration, several factors can reduce injection discomfort: slow injection rate, use of small-bore needles (27-gauge or higher), room-temperature injection fluid, and injection with use of small-volume syringes.
During infiltration, the surgeon must avoid intravascular injection. This complication can be minimized by frequent aspiration during introduction of the local anesthetic. Inadvertent intravascular administration can lead to lidocaine toxicity. Central nervous system lidocaine toxicity occurs first. The initial manifestations are excitatory, such as tingling, numbness, mental status changes, and eventually seizures. As lidocaine blood levels increase, there is a progression to central nervous system depression with somnolence and increasing respiratory depression. Higher serum concentrations have cardiovascular effects, such as myocardial depression and arrhythmia.
A wide variety of scalpel blades are commercially available for performing skin incisions ( Fig. 4.1 ). For soft tissue surgery, most surgeons prefer the No. 15 blade. This scalpel blade provides a sharp tip for precise angulation and a moderately rounded belly for an efficient cutting surface. A No. 11 blade is often helpful for complex incisions, such as those required for a geometric broken-line scar revision. This blade has an elongated tip with a straight belly that allows an accurate incision of acute angles with short sides. A newer scalpel blade, the 15C, is a modification of the traditional No. 15 blade. This design is a hybrid of the No. 11 and No. 15 designs. An elongated tip with a rounded but low-profile belly allows traditional scalpel use with improved accuracy for complex geometric incisions.
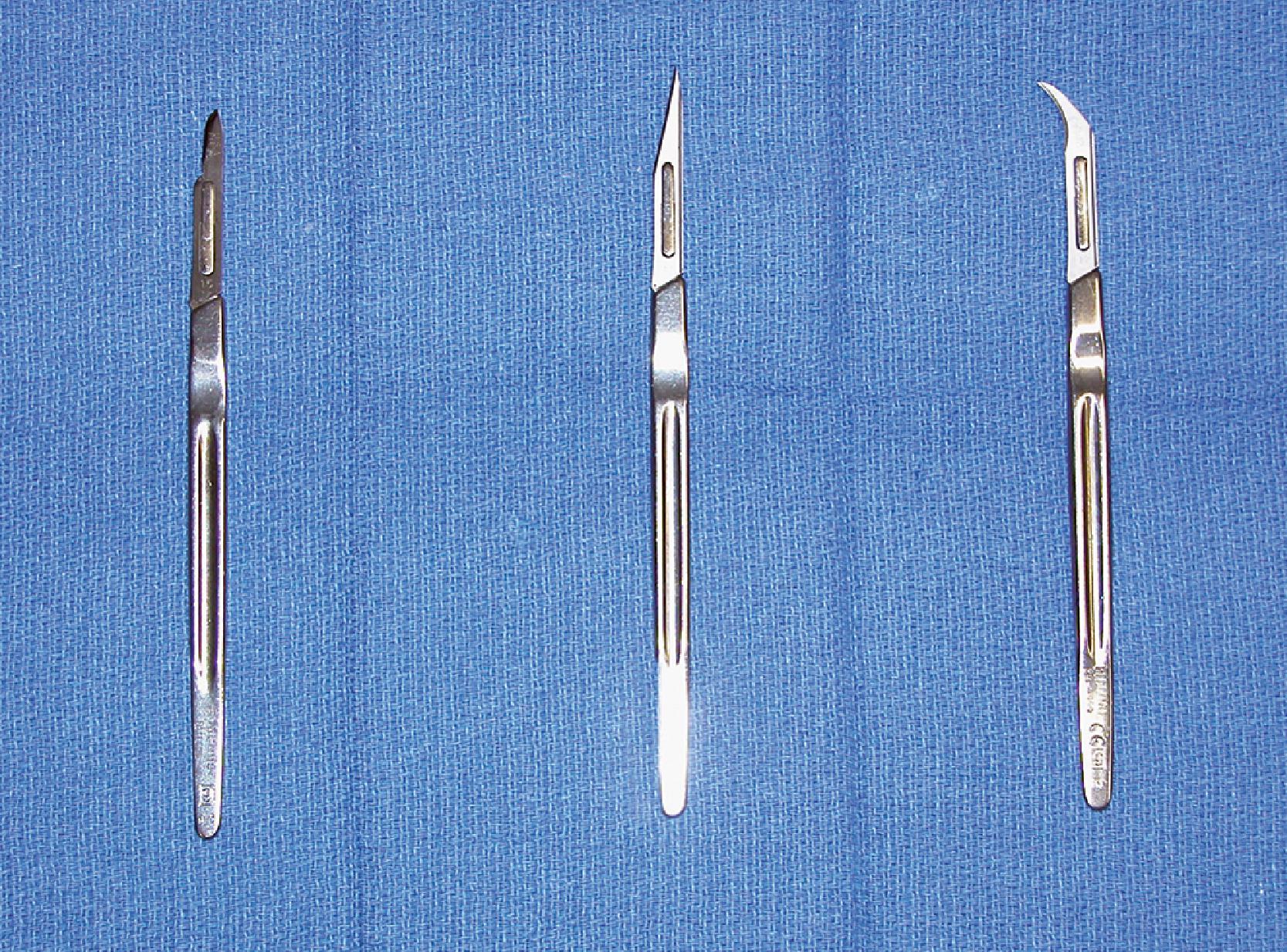
Once the proper scalpel blade is selected, several simple steps should be employed to create wound edges that are optimal for approximation. This is especially true for more precise geometric incisions. At the start of the incision, the tip of the blade is used in a stabbing motion. The cutting surface of the blade is then transitioned to the belly. The blade is simultaneously angled to create a slight bevel. The opposing side of the wound or flap is incised in a similar fashion but with an opposite bevel. At the end of the incision, the cutting surface of the blade is once again transitioned to the tip. The use of the blade tip at either end of the incision promotes precision and well-defined angles to the wound. The bevel created by the angled bias of the blade facilitates tissue eversion when the wound margins are approximated.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here